-
植物内生真菌与宿主植物共生,可调节植物应对多种生物和非生物胁迫,促进植物产次生代谢产物[1-4]。除了对自身宿主植物的有益影响,当前已有许多研究表明内生真菌对非宿主植物同样具有重要的生态功能,如分离自短芒大麦草、麦草、醉马草的内生真菌asexual Epichloë gansuensis可显著改善老芒麦种子萌发并促进其生长[5];分离自沙漠植物的印度梨形孢(Piriformaspore indica)可促进药用植物丁香罗勒生长并减少叶和茎中熊果酸和齐墩果酸的积累[6];分离自重阳木的内生真菌拟茎点霉(Phomopsis sp.)可加快药用植物苍术凋落物木质素的降解,有助于减小连作障碍效应[7]。这些现象为内生菌资源的新生态功能研究,及其对非宿主药用植物的活性成分的研究提供了新的思路,促使越来越多国内外的学者在这一领域开展相关研究。
中药丹参为唇形科植物丹参Salvia miltiorrhiza的干燥根和根茎[8],是一种被广泛应用于心脑血管疾病治疗的重要中药材[9]。现代药理学研究表明,丹参的主要活性成分为脂溶性的丹参酮类化合物和水溶性的丹参酚酸类化合物[10]。本课题组前期获得一种可促进模式植物拟南芥生长的内生真菌Epichloë bromicola SH09。E. bromicola是一种与冷季型牧草长期、永久共存的内生真菌,可导致禾草香柱病[11-13],其对双子叶药用植物的生长和代谢是否有影响尚不明确。因此,本文主要探究内生真菌E. bromicola SH09是否可以促进模式药用植物丹参生长,增加其活性成分的积累,研究成果有助于理解内生真菌对非宿主植物产生的生态功能,并有助于丹参生物菌肥的栽培应用。
-
内生真菌E. bromicola SH09由本课题组保存。使用马铃薯葡萄糖固体培养基(PDA)活化E. bromicola SH09菌株,温度为(28± 1)℃。4周后,在PDA平板上使用打孔器打孔,将E. bromicola SH09菌块转移至马铃薯葡萄糖培养基(PDB)进行扩大培养,条件为25 ℃,140 r/min。本研究所用的固体发酵培养基由250 g麦麸,250 g棉籽壳,20 g葡萄糖,3 g KH2PO4,1.5 g MgSO4.7H2O,加1 L去离子水组成,经121 ℃,30 min高压灭菌后备用。接着将培养7 d的E. bromicola SH09培养液倒入含40 g固体培养基中(容器为250 ml培养瓶,图1 C),用接种针混匀后划成1 cm×1 cm方块,25℃条件下培养3周,得到表面长满菌丝的E. bromicola SH09固体菌肥(图1A菌株正面,图1B菌株背面)。
-
丹参种子来源于陕西省商洛市天士力丹参种植基地,经浙江中医药大学药学院院长秦路平教授鉴定。
-
二氢丹参酮Ⅰ、隐丹参酮、丹参酮Ⅰ、丹参酮ⅡA、迷迭香酸、丹酚酸B均购自成都曼思特生物科技有限公司,甲醇、乙腈为色谱纯。
-
将丹参种子播种至润湿的混合栽培基质(腐殖土:蛭石=1:1)中,于22~ 24 ℃,16/8 h光照周期条件培养1个月。选取长势相近的丹参苗,继续培养20 d。选取15株5 cm左右高的丹参苗,随机分成3组,分别为对照组、菌肥处理60 d组、菌肥处理120 d组,每组5株,菌肥处理组于根部均匀撒20 g E. bromicola SH09固体菌肥,对照组于根部均匀撒20 g不含菌的固体菌种培养基。隔天浇水。
共培养至第60天和第120天分别收获丹参苗,测定植株的分蘖数、株高(以分蘖的长度作为株高)、叶数、叶面积作为形态指标;冲洗丹参根,拭去表面的水,称量鲜重;将新鲜的丹参根置于50 ℃烘箱干燥1周,称量干重;研磨干燥的丹参根,过40目筛,用于测定丹参酮和丹参酚酸含量。
-
精密称取0.20 g丹参根粉末,加入4 ml甲醇浸泡过夜,500 W超声提取45 min,再用0.45 μm 微孔滤膜过滤备用。利用Agilent -1200 自动进样色谱仪,Agilent ZORBAX SB - C18 (4.6 mm×250 mm,5 μm)色谱柱进行丹参酮和丹参酚酸的含量测定。色谱条件:流速为0.8 ml /min ;柱温为30 ℃;进样量为20 μl;检测波长为280 nm;流动相为乙腈(A)-0.05%甲酸水(B);梯度洗脱:0~20 min,20 %~40 %A;20~21 min,40%~80% A;21~40 min,80%~ 90% A;40~45 min,90%~20% A。分别进行重现性试验、精密度试验、稳定性试验、加样回收率试验,以验证分析方法的可行性。
-
实验数据均以(
$\bar x $ ±s)的形式表示,利用SPSS 22.0 软件进行统计分析,采用独立样本t检验法分析菌肥处理组和对照组的差异显著性(P < 0.05)。 -
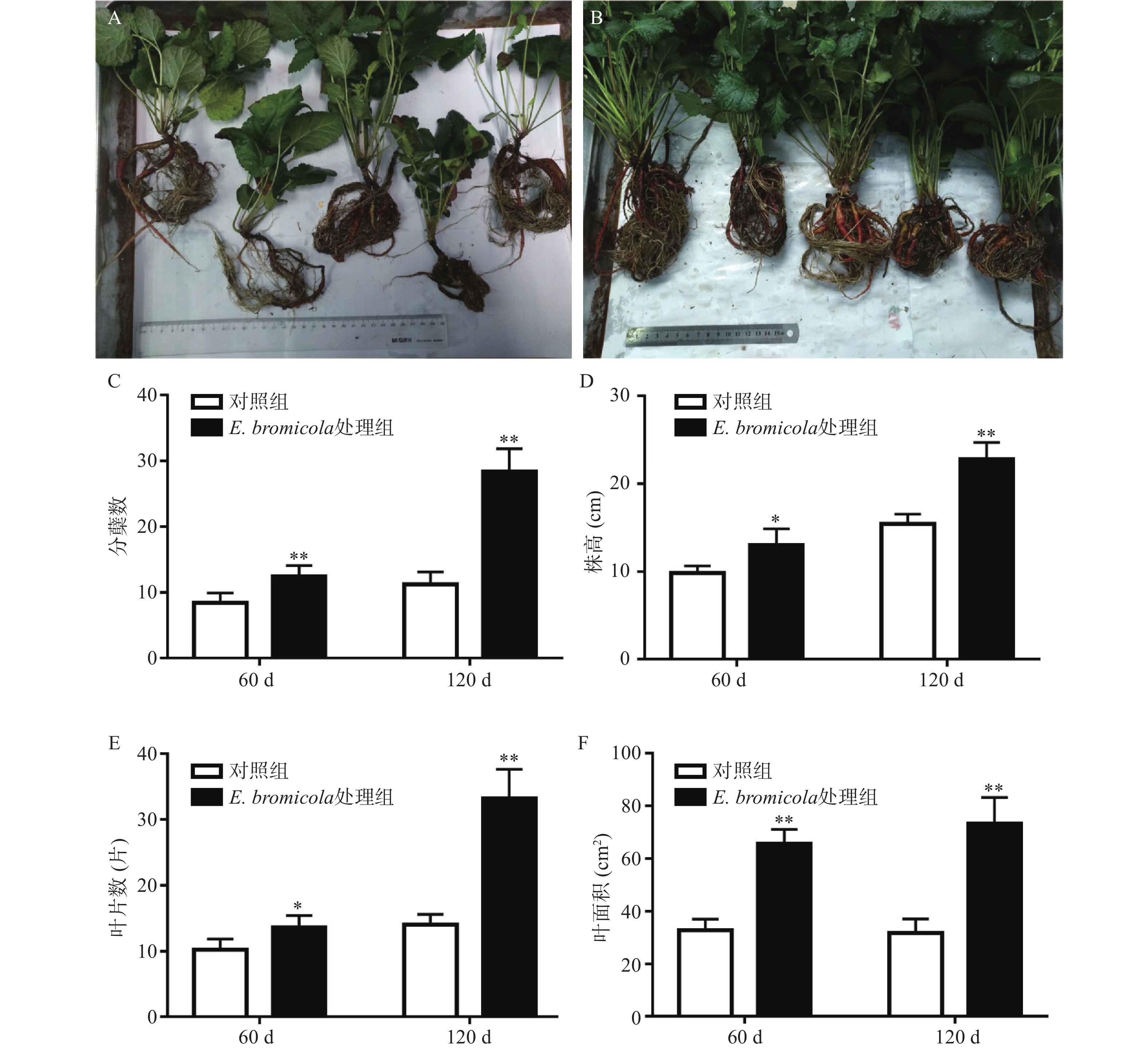
通过丹参苗的4个形态指标和丹参根的鲜重、干重2个指标,评价内生真菌E. bromicola SH09对丹参生长的影响。由图2可知,E. bromicola SH09与丹参共培养60 d和120 d后,共培养组丹参苗的分蘖数(图2C)、株高(图2D)、叶片数(图2E)、叶面积(图2F)与对照组相比均显著增加。其中分蘖数在共培养60 d和120 d后分别是对照组的1.48和2.29倍;株高分别是对照组的1.33倍和1.48倍;叶片数分别是对照组的1.33倍和2.37倍;叶面积分别是对照组的2.00倍和2.32倍。
由图3可得,E. bromicola SH09与丹参共培养60 d和120 d后,丹参根的生物量与对照组相比显著提高。其中,丹参根的鲜重在共培养60 d和120 d后分别是对照组的2.11和2.16倍(图3A);干重分别是对照组的3.06倍和2.73倍(图3B)。上述结果均表明内生真菌E. bromicola SH09可促进丹参苗的生长。
-
测定丹参干燥根中4种丹参酮类成分和2种丹参酚酸类成分的含量,以探究E. bromicola SH09对丹参活性成分积累的作用。由图4、图5可知,E. bromicola SH09与丹参苗共培养60 d和120 d可显著促进丹参根中4种丹参酮类成分和2种丹参酚酸类成分的积累。对于丹参酮类成分,在共培养60 d和120 d后,共培养组隐丹参酮含量分别是对照组的5.77倍和2.63倍(图4A);二氢丹参酮I含量在共培养60 d后是对照组的2.57倍,在共培养120 d后无显著差异(图4B);丹参酮I含量分别是对照组的2.49倍和2.29倍(图4C);丹参酮IIA含量分别是对照组的3.44倍和2.13倍(图4D)。对于丹参酚酸类成分,在共培养60 d和120 d后,共培养组丹酚酸B含量分别是对照组的 1.76倍和1.32倍(图5A);迷迭香酸含量在共培养60 d后是对照组的1.32倍,而共培养120 d后与对照组无显著差异(图5B)。综上可得,E. bromicola SH09可促进丹参根中丹参酮类和丹参酚酸类成分的积累。
-
由于丹参对心脑血管疾病有显著疗效,丹参相关制剂在全球范围有较高需求量。然而现产丹参多为栽培种,不同产区品质参差不齐,且生长周期长达2年,因此我们亟需对其传统培育技术进行改良,以得到高产、稳产的优质丹参[14-15]。植物内生真菌普遍存在于植物健康组织中[4],已有研究表明,内生真菌可以通过促进药用植物的生长发育和次生代谢产物积累,从而提高药用植物品质[16-18]。本研究探究了内生真菌E. bromicola SH09对丹参生长及其活性成分丹参酮和丹参酚酸积累的影响。
研究结果显示,E. bromicola SH09与丹参苗共生2个月和4个月后,分蘖数、株高、叶片数、叶面积以及鲜重、干重均较对照组显著提高,且分蘖数、株高、叶片数、叶面积、鲜重的促进程度(与对照组相比)均随着培养的时间延长而增加。已有研究表明内生真菌深绿木霉D16菌肥可有效促进药用植物丹参的生长及其活性成分丹参酮的积累[3],5、15、25 g D16菌肥分别培养丹参4个月后,3种浓度的菌肥对丹参干重的促进程度均不及本研究E. bromicola SH09对丹参干重的促进程度。但D16菌肥培养丹参6~8个月时,丹参苗快速生长,15 g D16培养丹参8个月后,其干重高达对照组的5.61倍。E. bromicola SH09与丹参苗共生2个月和4个月后,丹参酮(隐丹参酮、二氢丹参酮I、丹参酮I、丹参酮IIA)和丹参酚酸(丹酚酸B、迷迭香酸)含量均被显著增加,其中隐丹参酮、丹参酮I、丹参酮IIA和丹酚酸B的积累被持续促进。D16与丹参共培养后和E. bromicola SH09有类似作用,且该作用在共培养6个月后达到峰值。丹参是多年生植物,故E. bromicola SH09在4个月后如何影响丹参的生长和活性成分积累有待进一步研究。
目前,内生真菌对非宿主药用植物的作用研究仍不够深入。内生真菌可能通过促光合作用促进药用植物生长,通过介导植物体内信号分子、酶基因、植物激素等调节药用植物次生代谢,从而提高药用植物的品质。有研究表明,毛竹的3种内生菌均可提高叶绿素含量,促进光合作用,以促进毛竹生长[19];地黄内生真菌GG22通过诱导茉莉酸合成关键基因的表达上调地黄中茉莉酸类物质[20];丹参内生真菌U104通过上调丹参酮生物合成途径的关键酶基因促进丹参酮的积累[21];转录组和蛋白质组学共同揭示了丹参内生真菌制备的诱导子通过调节钙离子、氧化应激、乙烯、茉莉酸、水杨酸等信号转导过程影响丹参酮和丹参酚酸的代谢[22];并有研究推测药用植物龙胆的3种木霉属内生菌通过改变龙胆中超氧化物歧化酶、过氧化氢酶和过氧化物的酶活性提高植物抗病性[23]。然而,E. bromicola SH09具体通过怎样的途径促进丹参生长和活性成分积累,也有待更深层的研究。
Effects of endophytic fungus SH09 on plant growth and accumulation of active components in Salvia miltiorrhiza
doi: 10.12206/j.issn.1006-0111.202108055
- Received Date: 2021-08-12
- Rev Recd Date: 2021-12-10
- Available Online: 2023-11-06
- Publish Date: 2022-05-25
-
Key words:
- endophytic fungus /
- morphological index /
- tanshinone /
- phenolic acid
Abstract:
| Citation: | WU Sijia, XIE Xingguang, YANG Yang, ZHENG Chengjian, HAN Ting. Effects of endophytic fungus SH09 on plant growth and accumulation of active components in Salvia miltiorrhiza[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(3): 213-217, 269. doi: 10.12206/j.issn.1006-0111.202108055 |













 DownLoad:
DownLoad: