-
随着人类预期寿命的延长,人口老龄化问题日趋严重,阿尔兹海默症等神经退行性疾病的发病率也大幅上升[1]。学习记忆障碍作为阿尔兹海默症的主要临床表现之一,贯穿阿尔兹海默症疾病发展的全过程,并呈进行性加重,严重降低患者的生活质量,成为亟待解决的公共卫生问题[2]。海龙(Syngnathus)系为海龙科动物刁海龙Solenognathus hardwickii(Gy)、尖海龙Syngnathoides biaculeatus(Bloch)、拟海龙Syngnathus acus Linnaeus的干燥体,具有温肾壮阳、散结消肿的作用[3]。现代研究表明海龙富含多种脂肪酸、氨基酸以及甾体化合物,具有抗衰老、抗骨质疏松、性激素样等药理作用[4]。DHA作为海龙脂肪酸的重要成分之一,具有促进神经元细胞生长发育、抑制神经炎症及氧化应激的作用[5]。目前尚无治疗阿尔兹海默症的特效药物,海龙在改善学习记忆损伤方面的研究亦属空白。本研究拟探讨海龙对D-半乳糖诱导衰老小鼠学习记忆损伤的保护作用,并测定海龙中DHA含量,初步阐明海龙改善学习记忆损伤的作用机制。
-
3月龄雄性ICR小鼠,体重(28±2)g,清洁级,购自昭衍(苏州)新药研究中心有限公司,合格证编号:No.202009910;许可证号:SCXK(苏)2008-0006。动物饲养于海军军医大学药学系实验动物中心,室温控制在(24±0.5) ℃,12 h光照/12 h黑暗,自由饮水、饮食。
-
D-半乳糖、羧甲基纤维素钠(Sigma公司);脂质过氧化物丙二醛(MDA)试剂盒、总超氧化物歧化酶(SOD)试剂盒、BCA蛋白检测试剂盒、蛋白酶磷酸酶抑制剂混合物(上海碧云天生物技术有限公司);AKT、p-AKT、FOXO1、SOD2、GAPDH抗体(CST公司);二十二碳六烯酸(DHA,纯度≥98%)购自上海麦克林生化科技有限公司。
-
海龙(购自安徽亳州药材市场)经海军军医大学药学系生药学教研室辛海量教授鉴定为刁海龙。精密称取干燥海龙生药,剪碎,以料液比为1:10的80%乙醇浸泡12 h,80%乙醇冷凝回流提取2次,90%乙醇冷凝回流提取1次,每次回流提取2 h,过滤,合并滤液,减压浓缩干燥为浸膏。
-
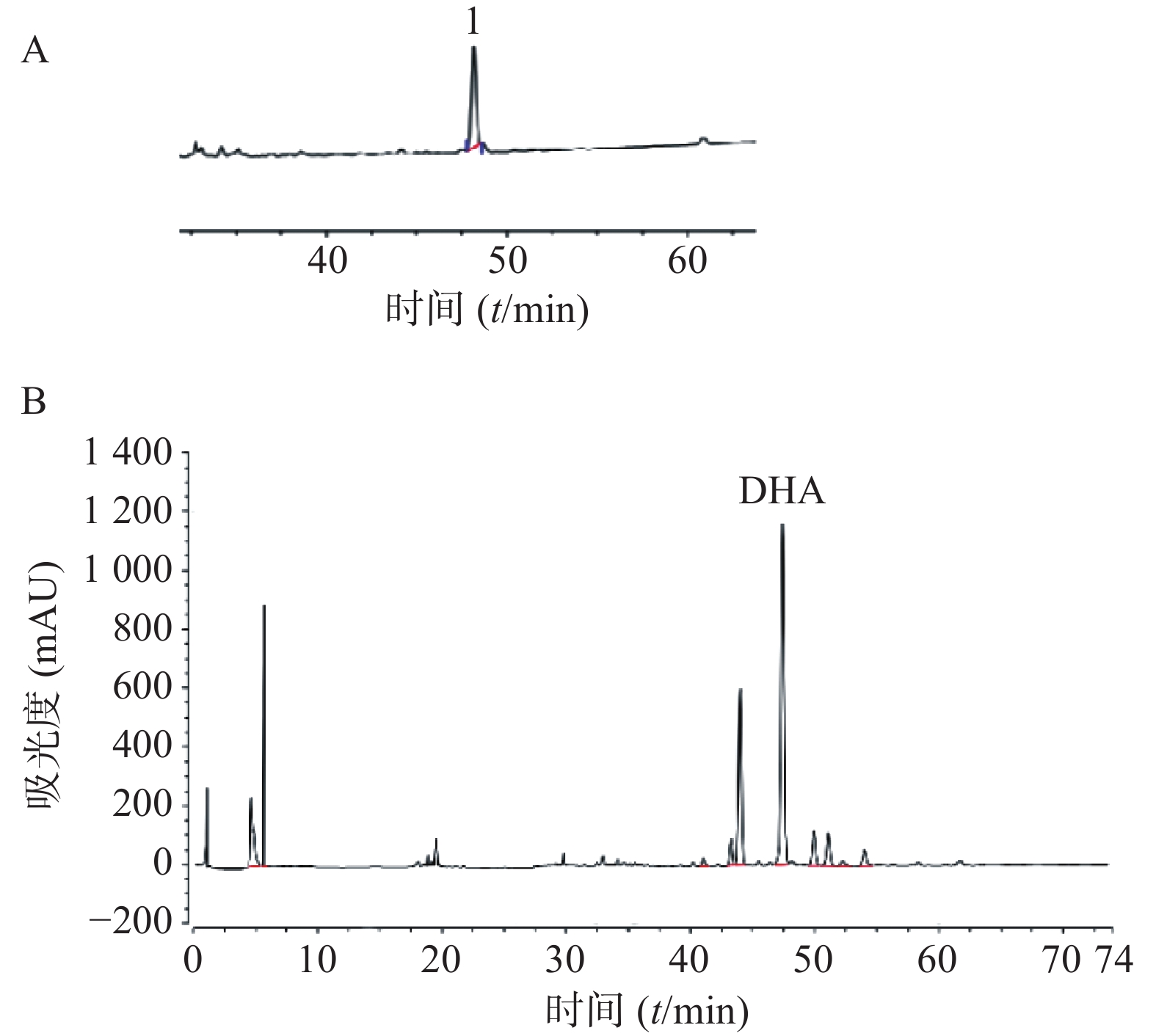
色谱柱:AcclaimTM120 C18(4.6 mm×250 mm,5 μm);流动相:乙腈-0.05%磷酸水溶液梯度洗脱;流速:0.6 ml/min;柱温:30 ℃;检测波长:203 nm;进样量:20 μl。
-
精密称定海龙乙醇提取物浸膏,配制成0.04 g/ml(以生药计)乙醇溶液;精密称取DHA对照品,溶解配置成0.5 mg/ml乙醇溶液。供试品溶液及对照品溶液均经0.45 μm微孔滤膜过滤除菌,备用。
-
将24只小鼠随机分为空白组、模型组、海龙低剂量组、海龙高剂量组,每组6只。对照组腹腔注射生理盐水,其余3组小鼠均腹腔注射D-gal(150 mg/kg),每周3次,复制衰老动物模型。海龙低、高剂量组给药剂量为1、2 g/kg(以生药计),空白组、模型组灌胃CMC-Na溶液,每周灌胃给药6 d。给药体积为0.1 ml/10 g,每周称重1次,给药量随体重变化增减,连续造模给药12周。
-
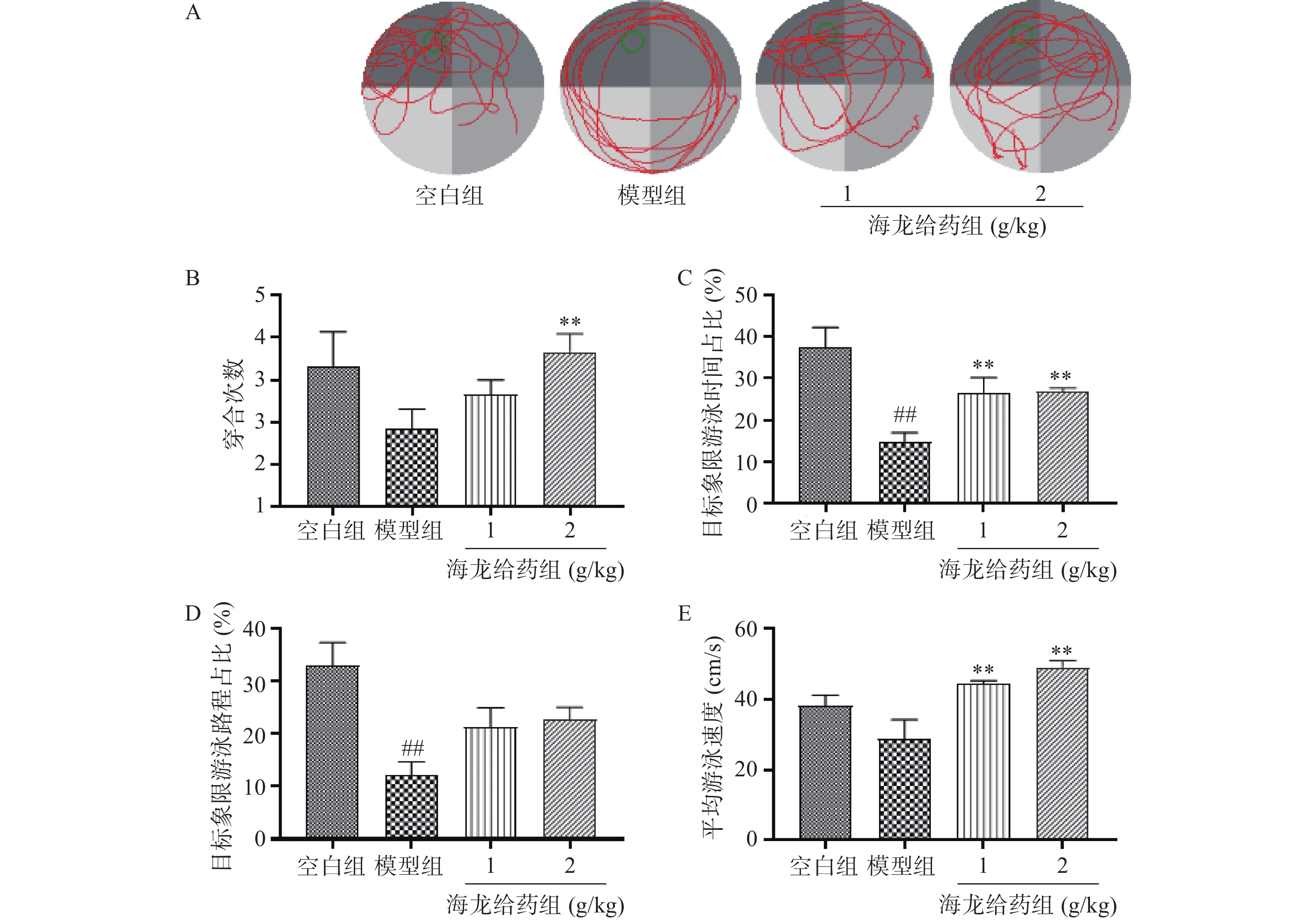
水迷宫实验的前1~4 d为定位航行测试,第5天为空间探索实验。定位航行测试:将各组小鼠从水池4个象限放入水中,记录60 s内小鼠从入水至达平台并停留超过3 s所需时间(即逃避潜伏期),测试4 d,观察各组小鼠逃避潜伏期时间变化。空间探索实验:Morris水迷宫测试第5天,移除平台后,将小鼠从原平台对侧象限放入水中,记录小鼠60 s内的活动轨迹,并分析数据。
-
水迷宫试验结束后处死小鼠,冰上迅速摘取小鼠海马组织。取部分海马组织加入生理盐水匀浆、离心,收集匀浆上清液,BCA法测定蛋白浓度后调整各样本蛋白浓度至一致,严格按照说明书检测小鼠海马组织匀浆上清液中MDA含量及SOD活性。
-
以细胞裂解液制备海马组织匀浆,离心后收集上清液,并以BCA法测定蛋白浓度。海马组织上清液蛋白加热变性后进行十二烷基硫酸钠(SDS)-聚丙烯酰胺凝胶电泳(PAGE),PVDF膜转印后,室温下5%脱脂牛奶封闭1 h。一抗(1∶1 000)4 ℃孵育过夜,次日TBST洗膜3次,每次10 min。二抗(1∶10 000)室温孵育1 h后,TBST洗膜3次,每次10 min。ECL化学发光法显影,ImageJ软件对目的条带进行分析。
-
实验结果以(
$ \bar x \pm s $ )表示,采用SPSS 21.0软件进行数据分析,选用单因素方差分析法进行组间变量分析,LSD-t法比较组间差异。以 P <0.05表示有显著性差异,以 P <0.01表示有极显著性差异。 -
取对照品溶液,按照“2.2.1”项下方法进样测定。以峰面积为纵坐标、对照品浓度为横坐标进行回归处理,得DHA回归方程:Y=1 085.8X+4.799 9,相关系数r=0.999 9,线性范围0.034~0.41 mg/ml;取供试品液,按照“2.2.1”项下方法进样测定,并计得含量为7.761 3 mg/g(以生药计),见图1。
-
小鼠定位航行实验结果:与空白组相比,模型组小鼠在定位航行实验第4天逃避潜伏期明显延长(P<0.01);与模型组比较,海龙低剂量组、高剂量组小鼠逃避潜伏期均显著缩短(P<0.01),见图2。
空间探索实验结果:与空白组比较,模型组小鼠目标象限游泳时间占比和游泳路程占比显著降低;与模型组比较,海龙高剂量组穿台次数显著增加(P<0.01);海龙低剂量组、海龙高剂量组目标象限游泳时间占比和游泳速度显著提高(P<0.01),见图3。
-
与空白组相比,模型组海马组织MDA含量显著升高,SOD活力显著降低(P<0.01);与模型组比较,海龙低剂量组、海龙高剂量组海马组织MDA含量显著降低(P<0.01),SOD活力显著升高(P<0.05, P<0.01),且高剂量组小鼠海马组织氧化损伤程度较低,见图4。
-
与空白组相比,模型组小鼠海马组织p-AKT/AKT比值、FOXO1、SOD2蛋白表达降低;海龙给药组可显著上调D-gal诱导记忆损伤小鼠海马组织中p-AKT、FOXO1、SOD2蛋白表达,激活AKT/FOXO1/SOD2通路(图5A-C),改善学习记忆损伤小鼠海马组织氧化损伤。
-
氧化应激损伤与衰老密切相关。研究发现,氧化应激致机体衰老的主要机制是因过量的ROS导致线粒体损伤、脂质过氧化、抗氧化酶活力降低,进而诱导细胞周期停滞甚至细胞凋亡[6-7];同时自由基也会导致氧化损伤,并且自由基清除酶活力随年龄增长而降低,进一步导致机体氧化损伤加剧[8-9]。海马组织作为负责短时记忆储存转换与空间导航的器官[10],与学习记忆能力密切相关。研究发现[11],过量D-gal可诱导小鼠体内氧化应激加剧,导致海马组织神经元细胞损伤,从而导致动物学习记忆能力降低。本研究发现,经海龙给药干预后,小鼠海马组织MDA含量显著降低,SOD活力显著提高,学习记忆能力显著提高。表明海龙可通过降低海马组织氧化损伤改善D-gal诱导衰老小鼠学习记忆损伤。
PI3K/AKT/FOXO1信号通路是经典的抗氧化通路,能拮抗多种原因引起的氧化应激。AKT信号通过磷酸化活化激活下游mTOR、FOXOs家族蛋白表达[12],从而发挥抗氧化作用。FOXO1作为PI3K/AKT信号通路的重要作用底物,在细胞抗氧化应激反应中发挥重要作用。研究发现,FOXO1激活可以促进其下游抗氧化蛋白SOD2、CAT表达[13]。此外,FOXOs转录因子表达的减少也增加了氧化应激诱导细胞凋亡的易感性[14]。本研究发现,D-gal模型组小鼠海马组织p-AKT/AKT比值、FOXO1、SOD2蛋白表达降低,海龙给药组小鼠海马组织p-AKT/AKT比值、FOXO1、SOD2蛋白表达显著提高。表明海龙可通过激活AKT/FOXO1/ SOD2信号通路改善小鼠海马组织氧化损伤,进而改善D-gal诱导衰老小鼠学习记忆能力。
海龙为海龙科硬骨鱼类,富含多种脂肪酸、氨基酸、甾体类成分。研究发现[15-16],鱼类所含的不饱和脂肪酸类代表成分DHA可促进大脑神经元细胞发育,并通过激活Nrf2/HO-1通路、FOXO3a/SOD通路,促进抗氧化相关蛋白及基因表达,降低活性氧水平,从而减轻神经元细胞氧化及炎症损伤,改善记忆损伤。此外,DHA可通过上调包括小胶质细胞和星形胶质细胞在内的神经胶质细胞AKT、Nrf2、HO-1等氧化应激相关蛋白表达,提高抗氧化酶活性从而抑制细胞凋亡,间接防止神经元细胞损伤[17-18]。本研究通过建立海龙HPLC图谱,发现DHA为海龙主要化学成分之一,约占总成分的47%,推测海龙改善小鼠学习记忆损伤作用可能与其富含DHA有关,其作用机制有待进一步研究。
Effect of traditional Chinese medicine Syngnathus on D-galactose-induced learning and memory impairment in aging mice
doi: 10.12206/j.issn.1006-0111.202201074
- Received Date: 2022-01-20
- Rev Recd Date: 2022-04-09
- Available Online: 2023-11-06
- Publish Date: 2022-05-25
-
Key words:
- Syngnathus /
- docosahexaenoic acid /
- D-galactose /
- learning and memory impairment /
- hippocampus /
- oxidative damage
Abstract:
| Citation: | ZHANG Jingwen, HE Xuhui, XIA Tianshuang, JIANG Yiping, XIN Hailiang. Effect of traditional Chinese medicine Syngnathus on D-galactose-induced learning and memory impairment in aging mice[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(3): 259-264. doi: 10.12206/j.issn.1006-0111.202201074 |












 DownLoad:
DownLoad: