-
夏枯草消瘤合剂源于著名中医肿瘤学专家钱伯文教授的经验方,是上海中医药大学附属岳阳中西医结合医院用于肿瘤治疗的特色院内制剂(批准文号:沪药制字Z05050340),已在临床应用30余年,对于肺癌、肝癌、甲状腺癌等癌症治疗效果显著,现已成为医院肿瘤内科的一线用药。该方由夏枯草、生地黄、牡蛎、煅牡蛎、莪术、麸炒白术、麸炒苍术等七味药组成,具有化痰、散结、解毒的功效[1-2]。
课题组前期采用高效液相色谱-飞行时间串联质谱(HPLC-TOF/MS)对夏枯草消瘤合剂中的37种主要药效成分进行了定性鉴别[3]。通过文献检索,明确夏枯草消瘤合剂中主要含有咖啡酸[4-6]、迷迭香酸[7-9]、丁香酸[10-12]、芦丁[13-15]、白术内酯Ⅱ[16-18]、白术内酯Ⅲ[19-20]等多种具有抗肺癌药理作用的活性成分。尽管夏枯草消瘤合剂临床疗效显著,抗癌活性成分明确,但作用机制尚不明确,因此,对夏枯草消瘤合剂开展体内药动学研究十分有必要。
笔者采用超高效液相色谱-串联质谱(UPLC-MS/MS)分析技术,利用正常大鼠,经口灌胃给药,对夏枯草消瘤合剂中6种主要的抗癌活性成分进行体内药动学研究,为其临床合理用药评价提供必要的科学支持。
-
Xevo TQ-S超高效液相色谱-串联质谱(UPLC-MS/MS);I-CLASS UPLC液相色谱(Waters );CP225D型电子天平(德国赛多利斯公司);Heraeus Fresco 21小型高速冷冻离心机(赛默飞)。
-
夏枯草消瘤合剂(上海中医药大学附属岳阳中西医结合医院);咖啡酸标准品(批号111871-201706,含量以99.7%计)、迷迭香酸标准品(批号110885-201703,含量以98.1%计)、芦丁标准品(批号100080-201811,含量以91.7%计)、白术内酯Ⅱ标准品(批号111976-201501,含量以99.9%计)、白术内酯Ⅲ标准品(批号111978-201501,含量以99.9%计),以上标准品均由中国食品药品检定研究院提供;丁香酸标准品(批号180919,北京北纳创联生物技术研究院,含量以97%计);内标对照品甲苯磺丁脲标准品(99.7%)、卡利拉嗪标准品(99.5%)均由实验室提供。
-
健康SD大鼠6只,雌雄各半,每只体重约250 g[动物使用许可证:SYXK(沪)2019-0027]。
-
色谱柱为 ACQUITY UPLC BEH C18 (2.1 mm×100 mm,1.7 μm)。正离子模式流动相为甲醇-水(0.1% 甲酸);流速为 0.3 ml/min;进样量为2 μl;柱温为 40 ℃,内标为卡里拉嗪。负离子模式流动相为乙腈-水(0.1% 甲酸);流速为0.4 ml/min;进样量为5 μl;柱温为50 ℃,内标为甲磺酸丁脲。离子源为电喷雾离子化源 (ESI);源温度为120 ℃,雾化气体温度为 500 ℃;毛细管电压为3.2 kV;扫描方式为多反应监测(MRM)。待测物与内标的质谱检测参数见表1。
化合物 检测方式 质谱扫描 锥孔电压
(P/V)碰撞电压
(P /V)母离子 碎片离子 咖啡酸 ESI- 178.94 135.04 28.00 16.00 迷迭香酸 ESI- 359.02 161.03 29.00 14.00 丁香酸 ESI- 196.95 181.96 15.00 12.00 芦丁 ESI- 609.09 299.98 39.00 32.00 甲苯磺丁脲(IS) ESI- 268.94 169.84 43.00 18.00 白术内酯Ⅱ ESI+ 233.07 187.13 32.00 16.00 白术内酯Ⅲ ESI+ 293.11 63.09 55.00 18.00 卡利拉嗪(IS) ESI+ 427.26 382.04 25.00 24.00 -
精密称取咖啡酸、迷迭香酸、芦丁、丁香酸、白术内酯Ⅱ、白术内酯Ⅲ标准品适量,甲醇溶解配制1 mg/ml的对照品储备液。前4种储备液按比例混合后使用50%甲醇稀释,得到混标对照品溶液a;另取白术内酯Ⅱ、白术内酯Ⅲ储备液按比例混合后使用50% 甲醇稀释,得到混标对照品溶液b。
-
分别精密称取甲苯磺丁脲、卡利拉嗪标准品,甲醇溶解配制1 mg/ml的对照品储备液,并用80%甲醇稀释至20 ng/ml及100 ng/ml,备用。
-
取“2.2”项下混标对照品溶液a 5 μl、大鼠空白血浆50 μl,混匀得到不同浓度的工作曲线样本。加入内标溶液 5 μl、1 mol/L盐酸30 μl,混匀后加入400 μl乙酸乙酯,震荡10 min后离心(13 000 r/min,10 min,4 ℃),取360 μl上清液,40 ℃条件氮气吹干。用50 μl 50%甲醇复溶,离心(13 000 r/min,3 min,4 ℃),取上清即得负离子质控样本。正离子质控样本制备则取“2.2”项下混标对照品溶液b 5 μl,操作同前,在加入内标溶液后,加入5‰氢氧化钠溶液30 μl 后,同负离子质控样品操作,即得。对照品工作曲线和质控样品浓度见表2。
待测物 线性范围(ng/ml) 质控样品浓度(ng/ml) 低 中 高 咖啡酸 2~400 5 40 320 迷迭香酸 1.5~300 3.75 30 240 芦丁 2.5~500 6.25 50 400 丁香酸 5~1000 12.5 100 800 白术内酯Ⅱ 1.5~240 3 24 192 白术内酯Ⅲ 3~480 6 48 384 -
精密吸取大鼠血浆样本50 μl,加入内标溶液5 μl、50%甲醇溶液5 μl后,按“2.4”项下处理,取上清液进行分析。
-
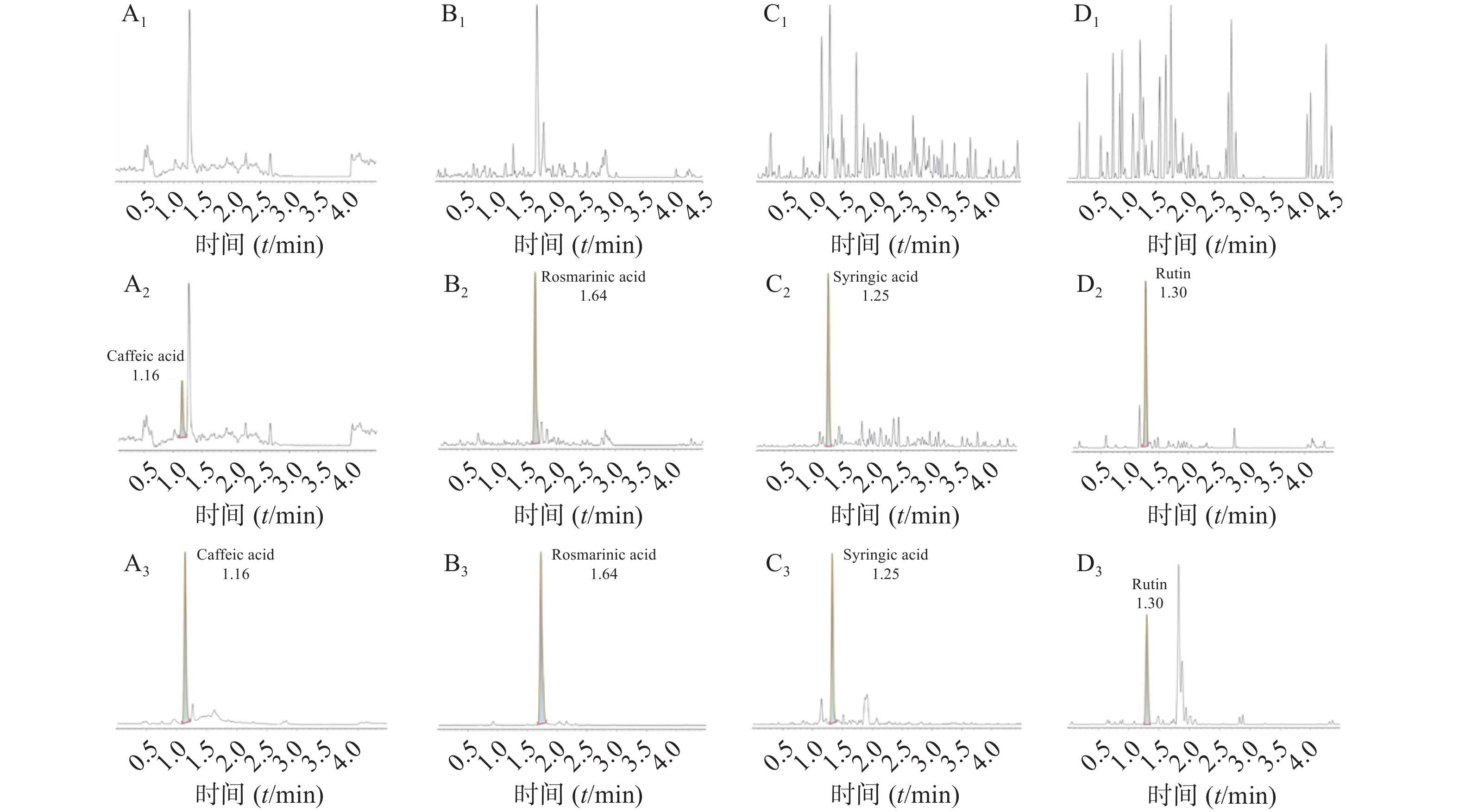
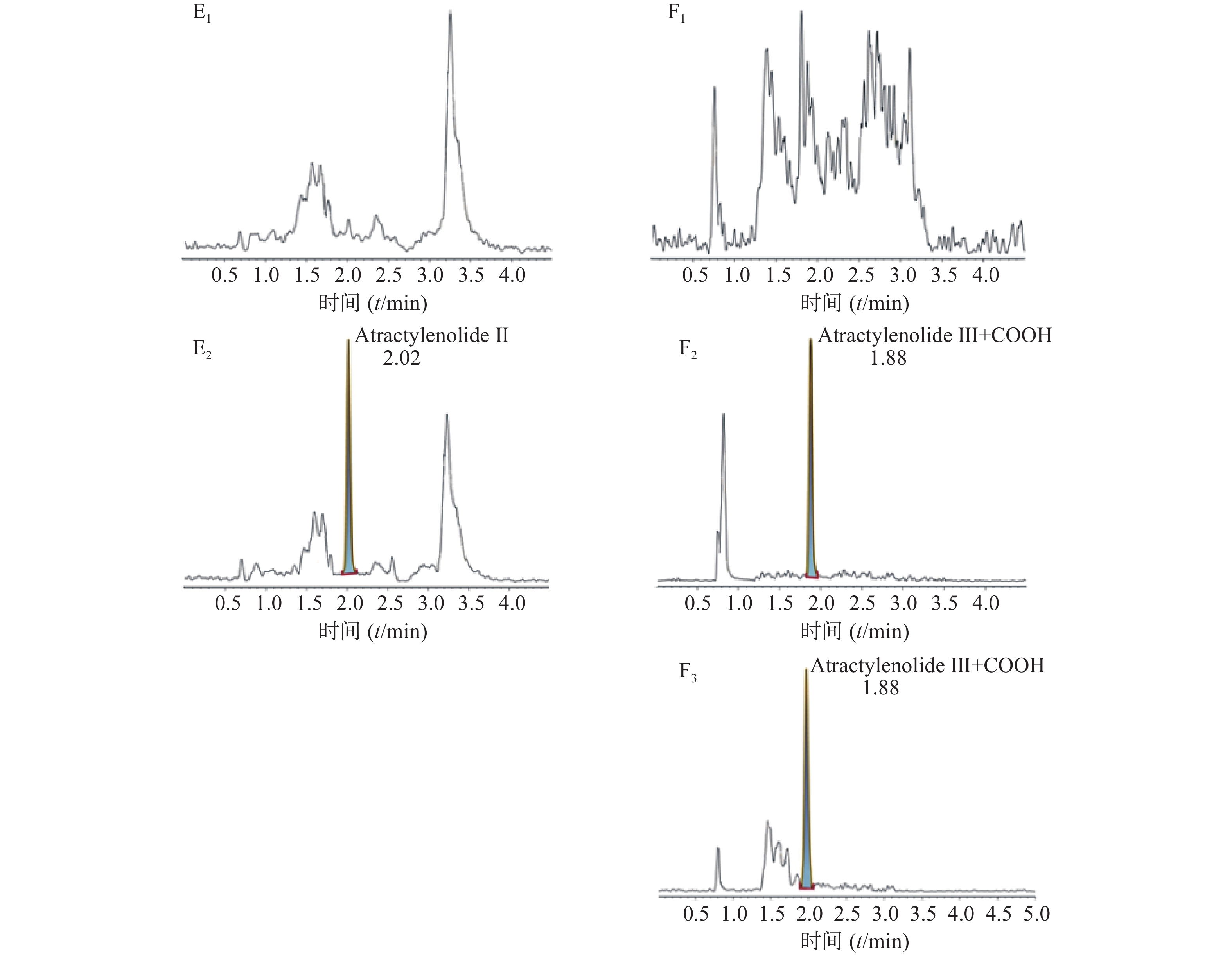
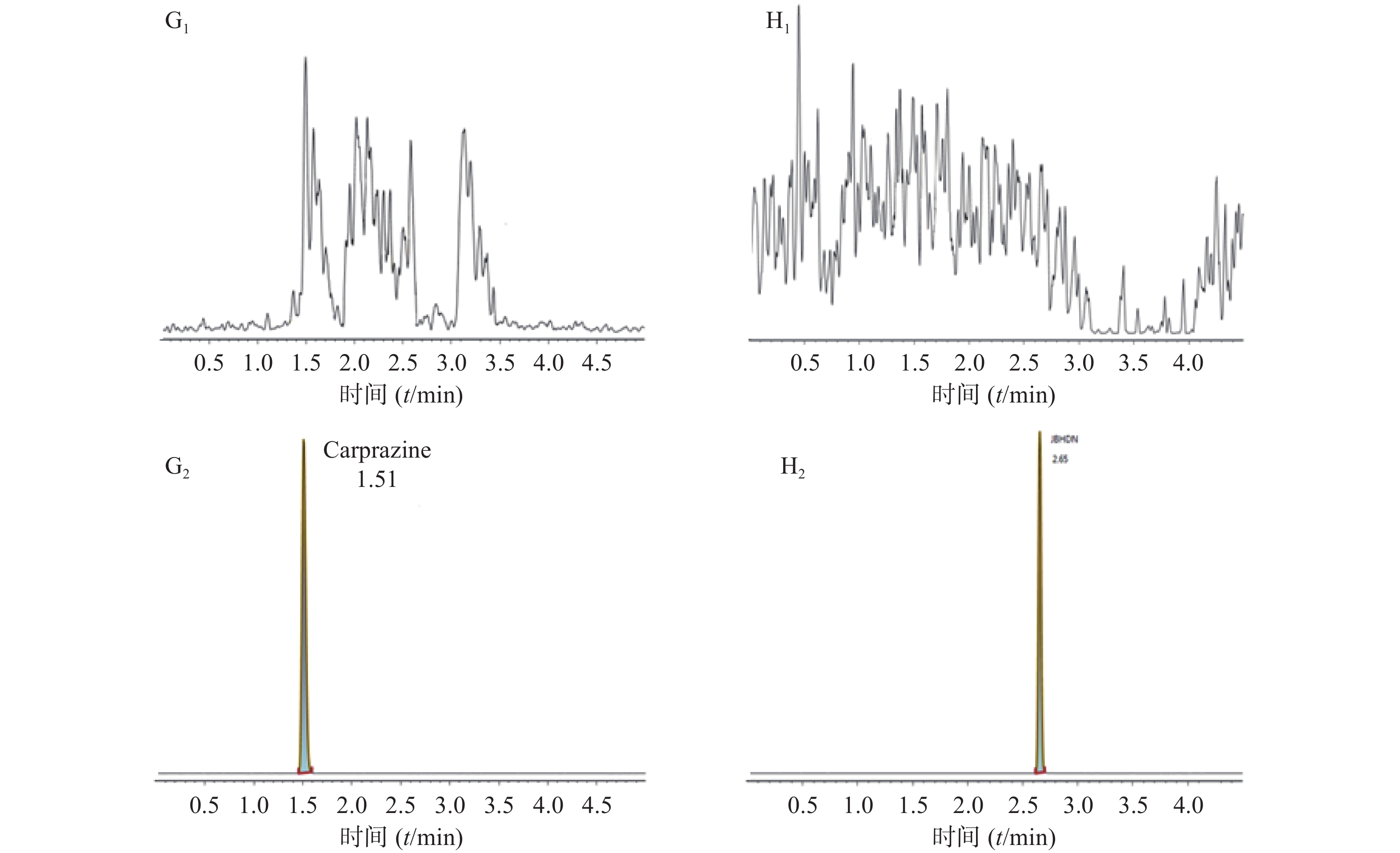
空白大鼠血浆、定量下限样品及给药后血浆样品中,咖啡酸、迷迭香酸、丁香酸、芦丁(负离子模式)的色谱图见图1,白术内酯Ⅱ、白术内酯Ⅲ(正离子模式)的色谱图见图2,内标甲苯磺丁脲、卡利拉嗪的色谱图见图3。实验结果可见,大鼠血浆中的内源性物质均不干扰各待测成分以及内标的测定。
-
按“2.4”项下制备系列标准血浆样品溶液,每个浓度平行3份。以各待测物峰面积与内标峰面积的比值 (AS/AIS,Y) 对相应的浓度 (C,X) 进行加权回归(权重为1/X2),得到各待测物在大鼠血浆中的标准曲线数据,见表3。相关系数r>0.9983,各待测物在浓度范围内呈线性,且线性关系良好。
待测物 线性范围(ng/ml) 标准曲线 相关系数r 咖啡酸 2~400 Y=7.31×10−4+1.28×10−3X 0.9987 迷迭香酸 1.5~300 Y=-4.79×10−4+8.11×10−4X 0.9988 芦丁 2.5~500 Y=5.96×10−4+8.98×10−5X 0.9987 丁香酸 5~1000 Y=1.13×10−4+3.55×10−5X 0.9984 白术内酯Ⅱ 1.5~240 Y=8.62×10−3+1.52×10−2X 0.9983 白术内酯Ⅲ 3~480 Y=2.05×10−5+1.98×10−3X 0.9983 -
按照“2.4”项下方法配制定量下限、低、中及高 4 个浓度的大鼠血浆质控样品各 5 份,连续测定3批。精密度与准确度结果见表4。
成分 理论浓度
(ng/ml)日内(n=5) 日间(n=5) RSD
(%)准确度
(%)RSD
(%)准确度
(%)咖啡酸 2 6.7 80.8 16.6 95.6 5 8.4 87.1 12.8 94.9 40 7.5 99.0 8.8 97.3 320 7.9 96.7 6.4 96.3 迷迭香酸 1.5 12.0 118.7 9 117.3 3.75 3.6 94.4 9 96.4 30 12.0 94.5 10.7 96.3 240 11.0 100.7 6.9 102.3 丁香酸 5 6.1 111.4 13.8 105.2 12.5 8.8 87.3 13 96.9 100 7.3 93.5 12.5 94.7 800 11.6 103.4 9.8 105.3 芦丁 2.5 11.3 87.2 12.2 91.8 6.25 3.4 93.9 12.6 95.4 50 9.0 98.6 11.3 92.1 400 8.0 102.0 9.9 96.9 白术内酯Ⅱ 1.5 11.9 95.7 12.5 100.9 3 9.3 100.9 12.4 101.3 24 10.9 97.8 11.8 99.8 192 8.7 99.7 8.9 93.9 白术内酯Ⅲ 3 14.8 95.9 15.4 101.4 6 12.4 108.0 12.8 105.9 48 8.2 102.9 7.7 100.2 384 3.5 103.1 9.2 99.1 -
按照“2.4”项下方法配制低、高两个浓度的大鼠血浆质控样品,平行制备6份,作为基质样品。取大鼠空白血浆50 μl,按“2.4”项下操作至复溶时加入混标溶液 5 μl、内标溶液5 μl、50% 甲醇40 μl混匀并离心取上清液,作为提取基质样品。通过基质样品峰面积与提取基质样品峰面积的比值求得方法的回收率,结果见表5,回收率在50%~110%,RSD小于15%,待测成分提取回收率良好。
成分 提取回收率(%) RSD(%) 咖啡酸 75.16±0.04 5.5 75.36±0.09 11.9 迷迭香酸 76.56±0.05 6.3 75.07±0.07 9.4 芦丁 60.62±0.08 12.7 60.62±0.05 9.0 丁香酸 103.9±0.07 6.3 92.97±0.13 14.1 白术内酯Ⅱ 53.48±0.07 12.5 50.04±0.04 7.5 白术内酯Ⅲ 58.47±0.06 9.9 62.54±0.06 9.1 卡利拉嗪(IS) 74.47±0.07 9.8 甲苯磺丁脲(IS) 98.77±0.05 4.6 -
按照“2.4”项下方法配制低、高两个浓度的大鼠血浆质控样品均平行制备 5份,根据当日随行的标准曲线计算样品浓度,考察处理前放置 4 h、冻融循环1次(−80 ℃)、放置1周(−80 ℃)、处理后放置 4 h和进样器内(4 ℃)放置 24 h的稳定性,结果准确度在85%~115%范围内,RSD小于15%,在上述条件下样品稳定。
-
取对照品工作溶液 5 μl、内标溶液5 μl、50% 甲醇40 μl 混匀后,作为无基质样品进行 UPLC-MS/MS分析。按照“2.4”项下方法制备提取基质样品。低、高两个浓度样品均平行制备6份。
通过提取基质样品中待测物峰面积与无基质样品待测物峰面积的比值比上提取基质样品中内标的峰面积与无基质样品中内标峰面积的比值即为基质效应,结果见表6,白术内酯Ⅲ基质效应较高,但高、低浓度对基质效应影响较为一致,不影响测量结果的准确性。基质效应RSD小于15%,样品中基质对方法无影响。
成分 基质效应(%) RSD(%) 咖啡酸 113.2±0.11 10.0 112.0±0.10 8.6 迷迭香酸 104.8±0.08 7.7 108.6±0.04 3.8 芦丁 109.0±0.10 9.2 104.1±0.06 5.9 丁香酸 114.5±0.12 10.5 110.0±0.10 9.4 白术内酯Ⅱ 99.7±0.10 9.9 107.8±0.15 14.0 白术内酯Ⅲ 166.7±0.08 13.7 169.8±0.04 11.5 -
SD大鼠6只,采取灌胃给药7.8 ml/kg,给药后分别于0、10、20、30、45 min,以及 1、2、3、4、6、8、10 h 眼眶采全血约100 μl(1% 肝素抗凝),低温(4 ℃) 离心 8000 r/min,8 min,分别取50 μl 上清液于对应离心管中,−80 ℃低温备用。
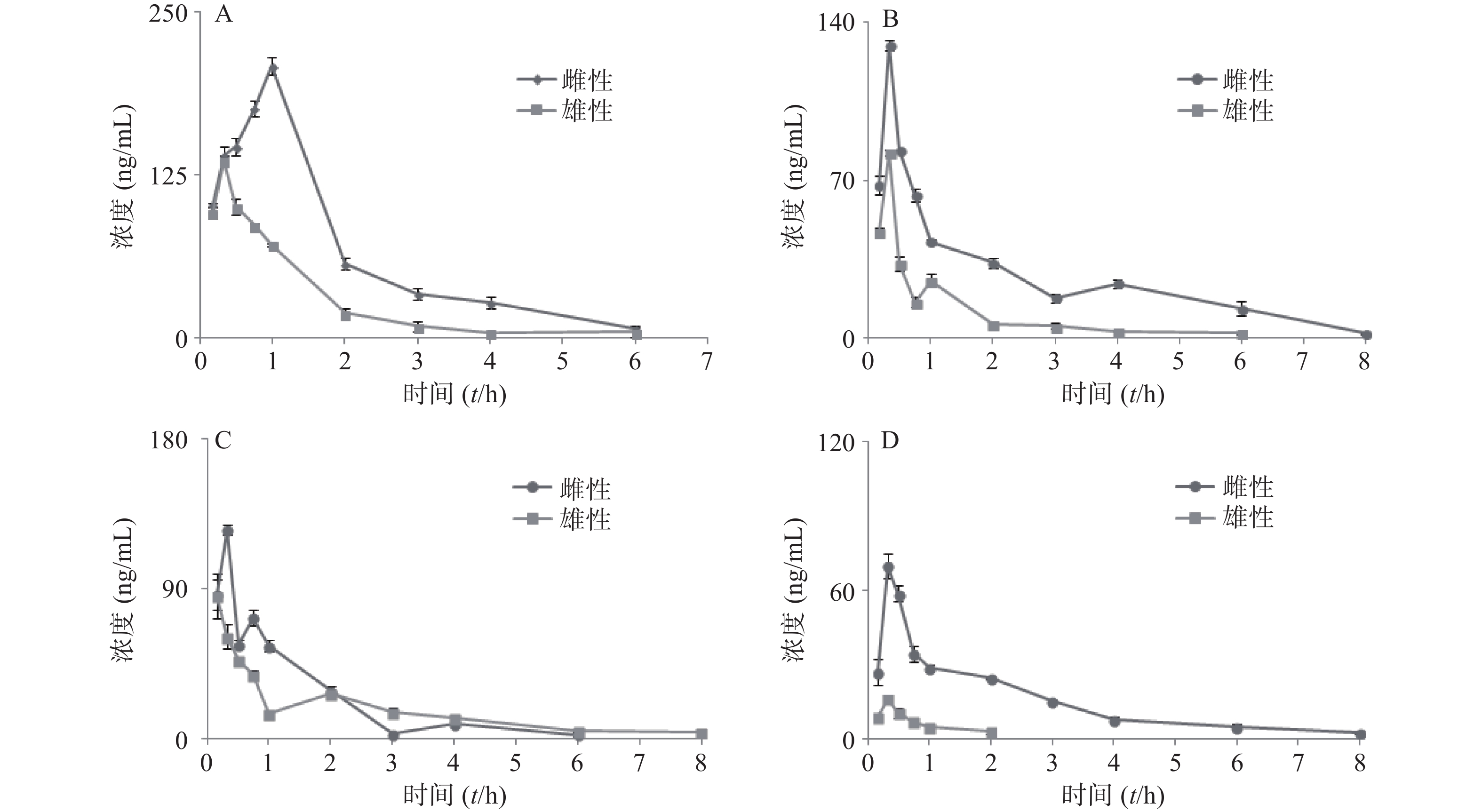
大鼠经口灌胃夏枯草消瘤合剂后,各待测物在大鼠体内的平均血药浓度-时间曲线见图4,采用 DAS2.0 软件的非房室模型计算分析大鼠给药后的药动学参数,详见表7。其中芦丁在大鼠体内血药浓度低于定量下限,白术内酯Ⅱ在生物样品中未被检测出,故未能进行计算。
参数 咖啡酸 迷迭香酸 丁香酸 白术内酯Ⅲ 雌性 雄性 雌性 雄性 雌性 雄性 雌性 雄性 AUC(0-t)(mg·h/L) 384.1±2.946** 156.6±3.658 203.6±9.177** 67.57±2.932 151.5±9.637 115.8±18.63 114.0±2.869** 10.32±3.749 AUC(0-∞)(mg·h/L) 395.5±7.342** 161.8±4.451 230.7±32.08* 73.84±7.545 167.0±11.45 144.8±11.22 125.3±5.275** 12.33±2.454 MRT(0-t)(t/h) 1.596± 0.05 0.997±0.052 2.265±0.136 1.339±0.056 1.219±0.020 1.846±0.616 1.959±0.212 0.573±0.178 MRT(0-∞)(t/h) 1.771± 0.122 1.110±0.05 3.285± 1.298 2.205±0.776 1.634±0.067 3.082±0.605 2.707±0.253 0.890±0.213 t1/2z(t/h) 1.041 ±0.094 0.765± 0.060 2.160±1.005 2.071±1.334 1.199±0.097 2.137±0.402 1.797±0.346* 0.426±0.040 tmax(t/h) 1** 0.33 0.33 0.33 0.33* 0.167 0.33 0.33 CLz/F(L/h) 0.008 ±0.001** 0.018± 0.001 0.013±0.002** 0.041±0.004 0.018±0.001 0.021±0.002 0.024±0.001* 0.249±0.045 cmax(mg/L) 208.2 ±6.721** 136.0 ±3.798 129.9±2.462** 82.46±1.373 125.057±3.101* 85.510±13.597 69.90±5.080** 16.23±1.411 *P<0.05, **P<0.01,与雄性大鼠组比较。 -
本次实验采用单次灌胃给予夏枯草消瘤合剂,给药后不同时间点眼眶取血,利用LC-MS/MS检测合剂中主要药效成分(包括咖啡酸、迷迭香酸、丁香酸、白术内脂Ⅲ等)的血药浓度,并绘制“药-时曲线”,结果呈现出典型的血管外给药特征。其中,咖啡酸和迷迭香酸在大鼠体内代谢的半衰期及达峰时间较两种成分单独代谢均显著延长[21],表明复方配伍可以减缓咖啡酸和迷迭香酸在大鼠体内代谢速度,延长两者在体内发挥作用的时间,起到了增加疗效的作用。其中,咖啡酸为迷迭香酸的代谢产物[22],合剂中咖啡酸半衰期及达峰时间的延长可能与迷迭香酸等酚酸类成分的代谢相关,迷迭香酸的代谢造成了体内咖啡酸的总量增加,间接造成了咖啡酸“在体内拥有更长的滞留时间及进入血液循环更高的药物浓度”的表象。此外,丁香酸的药时曲线出现双峰,而此前未有文献报道此类情况,原因有待进一步研究。白术内酯III在复方中代谢与其单独代谢时相比达峰时间明显缩短[23],可能是复方中的成分影响体内代谢酶活性,进而促进了白术内酯III的代谢。
本研究结果显示,合剂中各成分的代谢均存在显著性别差异。咖啡酸在雌性与雄性大鼠体内暴露的AUC值具有2倍的差异,且雌性相较于雄性到达tmax(峰浓度时间)有30 min延迟,提示雌雄个体间药物进入血液循环的相对量及达峰时间存在明显的差异。同样,白术内酯Ⅲ的性别差异也极为明显,AUC存在约10倍的差异,MRT、t1/2、cmax存在4倍差异,提示白术内酯Ⅲ在雌性体内仍发挥药效的同时在雄性大鼠体内早已被清除。现有研究证实[24-25],大鼠药动学的性别差异主要原因是雌性与雄性大鼠肝中CYP450酶的种类差异。大鼠与人类的CYP450酶系在酶的组成和活性上亦存在明显差异[26]。
在夏枯草消瘤合剂用于临床治疗非小细胞肺癌的合理用药方案制订、与化疗药物等相互作用评价、药物本身体内代谢过程监测及根据患者性别不同的个体差异化给药方案调整等过程中,本次实验的研究结果将为中药临床药动学方面的评估提供科学依据。
In vivo pharmacokinetic study on determination of effective components in Xiakucao Xiaoliu mixture in Normal Rat Plasma By LC-MS/MS
doi: 10.12206/j.issn.2097-2024.202111008
- Received Date: 2021-11-02
- Rev Recd Date: 2022-03-27
- Available Online: 2023-07-14
- Publish Date: 2023-06-25
-
Key words:
- Pharmacokinetics /
- Xiakucao Xiaoliu mixture /
- LC-MS
Abstract:
| Citation: | DAI Yuanyuan, MA Minghua, XU Xiaohong, WANG Xiaohe, ZHANG Ruoxi, XU Zhiru, NIAN Hua. In vivo pharmacokinetic study on determination of effective components in Xiakucao Xiaoliu mixture in Normal Rat Plasma By LC-MS/MS[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(6): 358-365. doi: 10.12206/j.issn.2097-2024.202111008 |








 DownLoad:
DownLoad: