-
糖尿病脑血管并发症是其致死致残的常见原因。鉴于内皮祖细胞(EPCs)在血管形成中的重要作用,近年来在心脑血管方面的研究越来越受到重视。多项研究证实EPCs移植治疗对脑缺血性疾病有改善作用[1-2]。然而糖尿病可影响EPCs功能[3],单纯EPCs移植治疗的效果并不理想。前期研究证实雌激素体外孵育可改善糖尿病大鼠的EPCs的功能。因此,本研究通过分离培养糖尿病大鼠EPCs,经雌激素孵育后对糖尿病缺血性脑卒中大鼠进行移植治疗并评价治疗的效果,为临床治疗糖尿病脑血管并发症提供新的思路。
-
雄性Wistar大鼠,体重(180±10)g(上海斯莱克实验动物有限公司)。动物房保持室温在22 ℃左右,相对湿度70%左右。所有实验动物均符合实验动物伦理学要求。
-
雌激素 (Abcam公司);链脲佐菌素(Sigma aldrich公司); EGM-2 培养基(LON-ZA 公司);2,3,5-氯化三苯基四氮唑(TTC, Sigma Aldrich);水合氯醛、甲醛溶液(国药集团化学试剂有限公司)。
-
Wistar雄性大鼠,10~12周,适应性地喂养1周后,连续7 d空腹腹腔注射新鲜配制的链脲佐菌素(streptozotocin, STZ)55 mg/(kg·d),对照组大鼠腹腔注射等体积枸橼酸钠缓冲液。7 d后测空腹血糖(禁食12 h),将血糖值为(13.5~25) mmol/L的大鼠作为糖尿病大鼠进行实验。
-
分离收集大鼠骨髓,采用密度梯度离心法获取骨髓单核细胞。将单核细胞重悬于培养基EGM-2并调整细胞浓度至1×106个/ml并接种于预先包被好纤维连接蛋白的细胞培养皿,置于细胞培养箱37 ℃和5%CO2条件下培养。培养3 d后洗去未贴壁细胞,继续培养至7 d。PBS洗去未贴壁细胞,贴壁细胞供实验用。
-
将消化下来的细胞悬液置于无菌离心管中,加入M199培养基离心后弃掉上清液。加入1 ml稀释液C重悬细胞。PKH26染料用稀释液C稀释。将细胞悬液加入到PKH26稀释液中,立即用吸管混匀样本,在25 ℃孵育2~5 min,并定时轻轻的颠倒离心管保证充分混匀。加入等量血清孵育1 min终止染色反应。细胞清洗3次后用PBS重悬备用。
-
1.5%异氟烷在氮气/氧气(70/30)吸入式麻醉后,大鼠手术期间置于保温垫上维持体温在(37±0.5) ℃,在体视显微镜下分离出左侧颈总、颈内和颈外动脉,结扎颈外及颈总动脉,从一侧颈总动脉插入线栓经颈内动脉至大脑中动脉并阻断其血流,手术期间应用激光散斑仪实时监测小鼠大脑皮层血流量的变化情况。
-
实验分3组:①对照组和②糖尿病组:M199培养基;③雌激素组:含雌激素10 nmol/L的M199培养基。在37 ℃、5% CO2培养箱中孵育24 h 后进行实验。
-
实验动物分4组:①正常雄性Wistar大鼠脑缺血组(CI);②糖尿病大鼠脑缺血组(DM+CI);③接受糖尿病大鼠EPCs移植治疗的糖尿病大鼠组(DM+CI+EPCs);④接受经雌激素体外干预糖尿病EPCs移植治疗的糖尿病大鼠(DM+CI+EPCs+estrogen)。所有大鼠行脑缺血术后24 h,③和④组大鼠经尾静脉注入相应的经PKH26标记的EPCs悬液(1×106个细胞,悬于PBS中),①和②组经尾静脉注入等体积PBS。术后3 d进行实验。
-
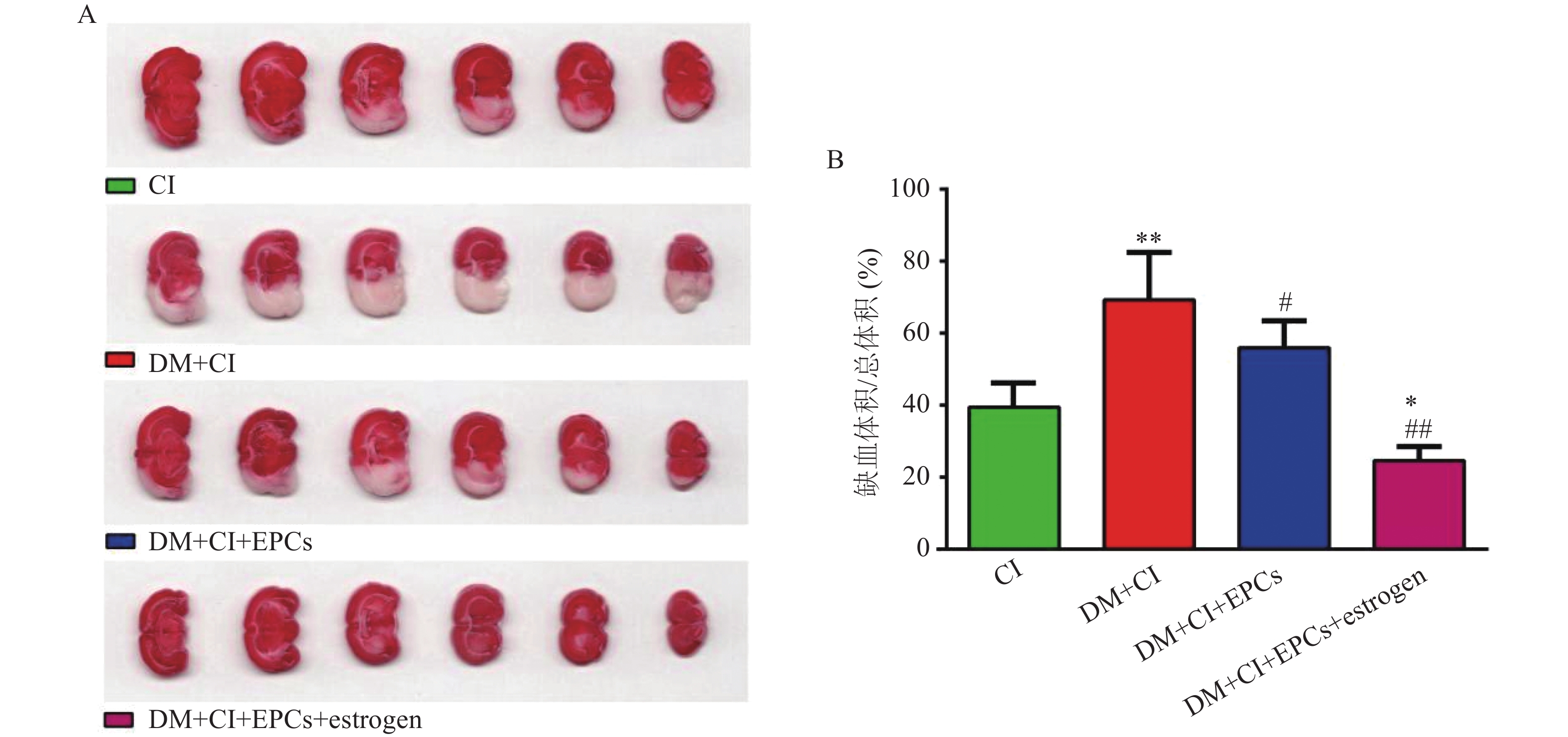
移植后3 d,大鼠麻醉处死取脑,将各组大鼠脑组织切为厚约3 mm的切片并浸入1% TTC中,37 ℃染色30 min。正常脑组织TTC染色为鲜红色,而缺血区脑组织为白色,拍照并计算脑缺血体积。
-
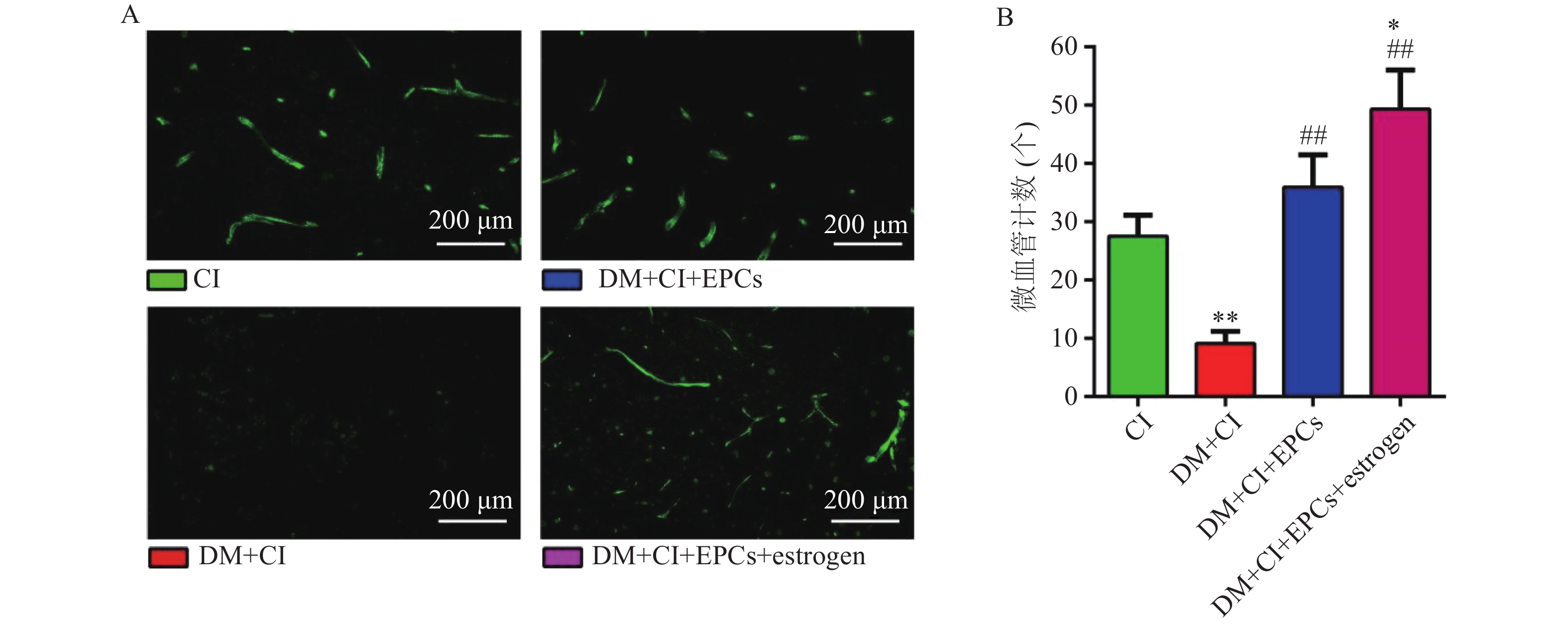
移植后3 d,取各组大鼠脑组织做冰冻切片,160倍荧光显微镜下观察并获取图像,计数PKH26标记的细胞数并统计分析。同时进行VEGFR2免疫组化,每张切片取5个不同视野在160倍荧光显微镜下观察计数新生小管数。
-
与CI组比较,DM+CI组脑梗死体积明显增加(P<0.01);经EPCs移植治疗后糖尿病大鼠的脑缺血体积明显减小且经雌激素体外干预后的EPCs移植能进一步减少脑缺血梗死体积,说明雌激素干预可能加强了EPCs对脑卒中大鼠脑部缺血损伤的治疗作用(P<0.05, P<0.01,与 DM+CI组比较; P<0.05, 与 CI组比较),见图1。
-
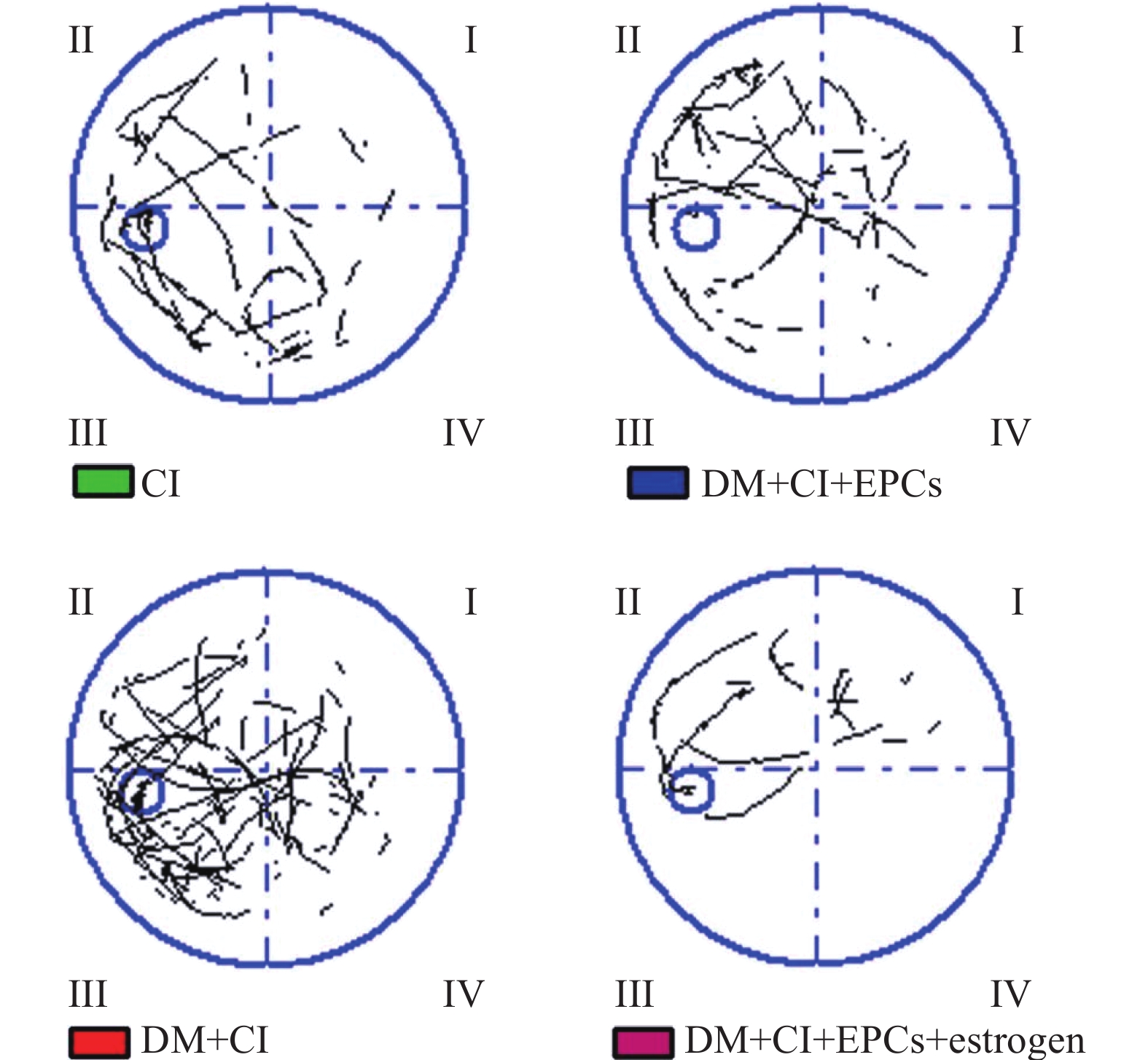
水迷宫实验结果显示, DM+CI组大鼠游泳路程和总时间相较于CI组明显增加(P<0.05),平均速度和潜伏时间则无明显变化(P>0.05);与DM+CI组比较,DM+CI+EPCs组以及DM+CI+EPCs+ estrogen组游泳总时间明显缩短(P<0.05),平均速度均明显提高(P<0.05),游泳总路程则无明显变化(P>0.05);但只有DM+CI+EPCs+ estrogen组潜伏期明显降低(P<0.01),见图2和表1。
组别(n=8) 总时间(s) 总路程(mm) 平均速度(mm/s) 潜伏期(s) CI 30.71±18.28 6721.56±4838.88 198.36±78.74 26.34±21.20 DM+CI 35.15±15.29* 7855.55±4262.89* 197.49±59.9 27.93±20.36 DM+CI+EPCs 29.49±13.46# 7459.41±4829.74 236.85±56.49# 25.14±16.04 DM+CI+EPCs+estrogen 28.80±13.75## 8477.04±5226.29 269.68±94.16## 22.41±14.16## *P<0.05,与CI组比较;#P<0.01,##P<0.01,与DM+CI组比较。 -
免疫荧光结果显示,与CI组比较,DM+CI组大鼠脑梗死区域新生血管数量明显降低(P<0.01);而DM+CI+EPCs组以及DM+CI+EPCs+ estrogen组大鼠脑梗死区域新生血管数量明显增加(P<0.01),且DM+CI+EPCs+ estrogen新生血管数量高于CI组(P<0.05),见图3。
-
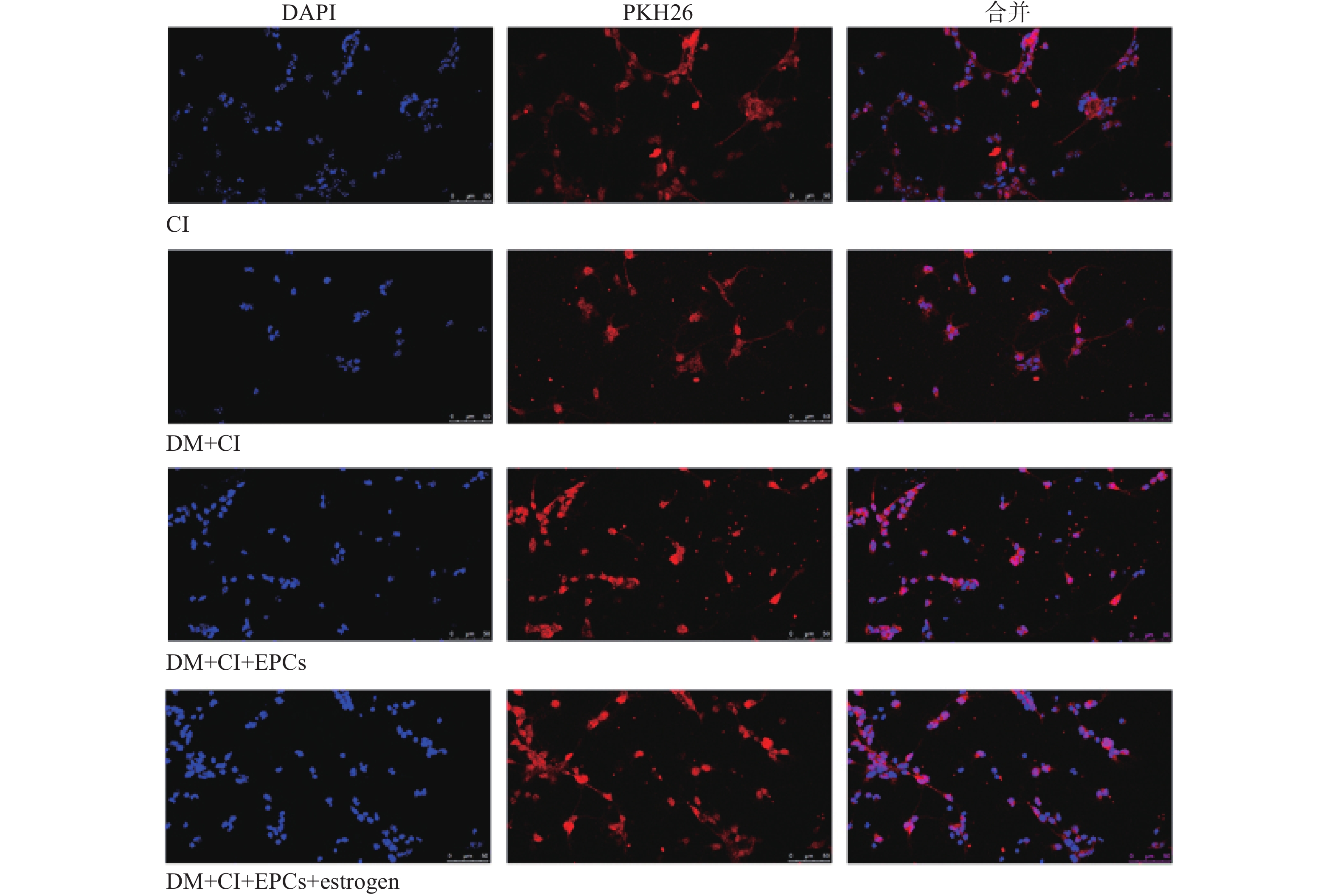
EPCs移植后第3天,取大鼠脑组织,切片后观察EPCs归巢情况。与CI组比较,DM+CI组大鼠脑组织缺血区域EPCs数量明显降低;而EPCs移植组大鼠脑组织梗死区域EPCs数量均明显增加,其中经过雌激素干预的EPCs移植组显示出梗死区域有更多数量的EPCs(图4)。
-
糖尿病是脑卒中的独立风险因素,且糖尿病脑缺血具有高致死及致残率的特点。但受到目前治疗条件的限制,急需新的治疗手段。内皮祖细胞(EPCs)为一种来源于骨髓并能分化为内皮细胞的前体细胞。在适当条件下(如缺血、缺氧)能从骨髓动员、迁移并归巢到受损部位,分化为内皮细胞,参与受损血管内皮的再内皮化及受损部位的血管新生[11]。研究表明,通过外源性的补充内皮祖细胞,外源的内皮祖细胞可以归巢于损伤部位,促进受损部位的血管新生及组织的修复[12]。Wang[13]等通过制造小鼠颈动脉球囊损伤模型并采用EPCs移植治疗,结果显示EPCs移植后附着在受伤的动脉内膜上能促进受伤部位的再内皮化和抑制新内膜增生。Huang[14]等的研究显示,EPCs移植通过增加循环中的EPCs数量和脑微血管密度可以恢复全脑放疗对血脑屏障和脑部毛细血管造成的损害。Garbuzova-Davis[8]等将人骨髓内皮祖细胞(hBMEPCs)系统移植至G93A小鼠,改善肌受损的萎缩性脊髓炎的血脊髓屏障的同时明显改善疾病结局。此外, EPCs移植治疗可有效改善心肌梗死、冠心病、糖尿病肾病及脑缺血等缺血性疾病[2, 15-17]。因此,鉴于EPCs移植在心脑血管疾病中的良好治疗作用,EPC移植有可能成为治疗糖尿病脑血管并发症的重要措施。然而高龄及疾病可导致自身内皮祖细胞数量减少及功能降低[3],单纯EPCs移植治疗的效果将大打折扣。若通过药物改善糖尿病EPCs功能,将使EPCs移植治疗达到事半功倍的效果。
雌激素在激发女性第二性征的出现及维持中发挥不可替代的作用,并参与机体脂肪、水盐及肌肉蛋白质的合成与代谢。雌激素对心脑血管具有保护作用,可能与其增强EPCs的功能相关[18-19]。本课题组前期研究结果显示雌激素体外孵育能改善糖尿病大鼠的EPCs的功能。Ying等研究表明雌激素通过改善糖尿病小鼠的EPCs功能进而促进其伤口处血管新生和伤口愈合[20]。但雌激素干预后的EPCs移植治疗糖尿病脑卒中的研究较少。
本研究采用EPCs对糖尿病脑卒中大鼠进行移植治疗,并采用TTC染色法对各组大鼠的脑组织染色以比较不同EPCs移植后大鼠脑梗死体积的变化。结果显示,糖尿病大鼠脑缺血梗死体积较正常大鼠相比明显增加,EPCs移植能明显减小脑梗死体积。与单独移植EPCs相比,雌激素干预的EPCs移植能进一步降低大鼠的脑梗死体积。同时水迷宫实验结果显示,雌激素干预EPCs移植明显提高脑卒中大鼠平均游泳速度、游泳总时间和明显缩短潜伏期,说明雌激素干预的EPCs移植对糖尿病脑卒中大鼠的脑缺血损伤有治疗作用。
研究表明EPCs通过促进缺血部位的血管新生在改善缺血性疾病预后中发挥重要作用[21-23]。为探讨EPCs移植治疗降低脑缺血体积是否与促进缺血部位血管新生相关,本实验采用免疫荧光法观察脑缺血部位的血管新生情况。结果发现与对照组大鼠相比,糖尿病组大鼠脑梗死区域新生血管数量明显降低,而EPCs移植组以及雌激素干预的EPCs移植组大鼠脑梗死区域新生血管数量明显增加,且雌激素干预的EPCs移植组新生血管数量最多并高于对照组,说明雌激素干预的EPCs移植通过促进缺血部位血管新生改善糖尿病脑卒中大鼠的脑缺血损伤。
研究发现移植的EPCs可以归巢于损伤部位促进血管新生[24-25]。为了解移植的EPCs是否归巢于脑缺血部位,我们利用荧光染料PKH26标记EPCs并将标记的EPCs移植入脑卒中大鼠体内,快速冰冻切片后置于荧光显微镜下观察。结果显示,糖尿病组大鼠脑组织缺血区域EPCs归巢数量较少;而EPCs移植组大鼠脑组织梗死区域EPCs归巢数量明显增加,且经雌激素干预的EPCs移植组显示出梗死区域有最多数量的EPCs归巢,说明雌激素能促进EPCs的归巢。
综上所述,雌激素体外孵育的糖尿病EPCs移植对糖尿病大鼠缺血性脑卒中有治疗作用,作用机制可能与雌激素促进EPCs归巢于缺血部位并促进缺血部位血管新生相关。该研究结果为糖尿病缺血性脑卒中提供了潜在的治疗手段。
Therapeutic effects of estrogen-intervened EPCs transplantation on diabetic ischemic stroke rats
doi: 10.12206/j.issn.2097-2024.202111111
- Received Date: 2021-11-30
- Rev Recd Date: 2022-04-19
- Available Online: 2023-07-14
- Publish Date: 2023-01-25
-
Key words:
- estrogen /
- EPCs transplantation /
- diabetic ischemic stroke /
- neovascularization /
- homing
Abstract:
| Citation: | DONG Yafen, WANG Jian, CHEN Ye, LI Shushu, LIU Helong, QIU Yan. Therapeutic effects of estrogen-intervened EPCs transplantation on diabetic ischemic stroke rats[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(1): 40-44, 49. doi: 10.12206/j.issn.2097-2024.202111111 |








 DownLoad:
DownLoad: