-
衰老是导致老年性骨质疏松症(SOP)的主要致病因素,其通过促进机体活性氧(ROS)的释放诱导氧化应激,从而降低成骨细胞活性,减少其骨形成[1-2]。D-半乳糖(D-gal)是一种还原糖,当其浓度达到一定水平时,就会诱导机体发生代谢紊乱,释放大量ROS,最终促进衰老相关疾病的发生[3]。同时,过量的D-gal还可诱导骨质变化,类似于SOP和衰老过程中的骨质丢失[4],严重危害了我国中老年人的身体健康。
巴戟天丸(Bajitianwan,BJTW)首载于《古今医统大全》,原方用于治疗老年性记忆减退。本课题组前期研究同样表明,巴戟天丸可显著改善D-gal致衰老大鼠的骨微结构,防治骨丢失,证明其具有明确的抗骨质疏松作用;同时还发现巴戟天丸可改善D-gal大鼠的记忆损伤,提示其具有抗老年性骨质疏松潜力[5],然而其在成骨细胞水平上防治D-gal引发骨丢失的作用及机制尚不明确。因此本研究拟采用D-gal损伤成骨细胞,通过成骨细胞活性、氧化应激水平及关键调控通路检测等方法,探究巴戟天丸体外抗老年性骨质疏松症的作用及机制。
-
成骨细胞:新生24 hWistar大鼠(上海西普尔-必凯实验动物有限公司);胎牛血清(美国Gibco);MTT(上海博光);BCIP/NBT碱性磷酸酯酶显色试剂盒、ALP试剂盒(南京建成);ROS检测试剂盒(上海碧云天);α-MEM培养基(天津灏洋);AKT、p-AKT抗体(美国CST)、Nrf2、HO-1、NQO1抗体(英国Abcam)。
-
采用二次消化法从新生24 hWistar大鼠颅盖骨中分离提取成骨细胞,将原代成骨细胞培养于α-MEM培养基中(10%胎牛血清),置于37 ℃、5% CO2的恒温培养箱中培养,取第3~5代成骨细胞进行后续指标检测。
-
将细胞以5×103个/孔接种于无菌96孔板中,孵育24 h。空白组和模型组更换新的完全培养基,给药组分别加入含不同浓度的BJTW水提物的完全培养液(0.001、0.01、0.1、1、10 μg/ml),4 h后模型组和给药组给予D-gal母液进行干预,使完全培养基中D-gal浓度达到100 mol/L。培养48 h后,采用MTT法测定细胞增殖水平。
-
将细胞以2×104个/孔接种于无菌24孔板中,孵育24 h细胞完全贴壁后,空白组和模型组更换新的完全培养基,给药组分别加入含不同浓度的BJTW完全培养液(0.1、1、10 μg/ml),4 h后模型组和给药组给予D-gal母液进行干预,使培养基中D-gal浓度达到100 mmol/L。培养48 h进行染色,于室温进行避光孵育3 d,洗去工作液后拍照。
ALP活性检测:按照“1.3”项的方法进行给药,细胞培养48 h后,取上清液,根据说明书测定ALP活性。
-
将细胞以2×105个/孔接种于6孔板中,孵育24 h细胞完全贴壁后,根据分组加入含不同浓度的BJTW完全培养液(0.1、1、10 μg/ml),4 h后模型组和给药组加入D-gal母液,使其浓度达到100 mmol/L。培养48 h后,吸弃上清液,参照ROS检测试剂盒说明书,装载稀释后的DCFH-DA探针,37 ℃孵育20 min,用0.25%的胰蛋白酶消化细胞并转移至离心管,空白培养基洗涤3次,用300 ml PBS重新混悬细胞后送往流式细胞仪进行检测。
-
将细胞以2×105个/孔接种于6孔板中,孵育24 h细胞完全贴壁后,根据分组分别加入含不同浓度的BJTW完全培养液(1、10 μg/ml),4 h后模型组和给药组加入D-gal母液,使完全培养基中D-gal浓度达到100 mmol/L。孵育48 h后,吸去上清液,PBS洗涤2次。于冰上对细胞进行裂解提取总蛋白,采用BCA试剂盒对蛋白定量。蛋白变性后,经SDS-PAGE凝胶电泳后转移至PVDF膜进行转膜,室温封闭后,加入相应的一抗(4 ℃环境过夜),1×TBST洗涤3次,加二抗于室温孵育1 h。1×TBST洗涤3次,采用ECL化学发光试剂盒检测。
-
将细胞以1×105个/孔的密度接种于激光共聚焦皿中,根据分组分别加入含不同浓度的BJTW完全培养液(1、10 μg/ml),4 h后模型组和给药组给予D-gal母液进行干预,使培养基中D-gal浓度达到100 mmol/L。于皿中培养48 h后,弃上清液,PBS洗涤2次后,组织固定液固定细胞,30 min后用PBS清洗2次,加入0.5%Triton X-100通透20 min。PBS清洗2次,皿中加入5%BSA工作液,室温封闭1 h。PBS清洗后加入一抗(4 ℃冰箱过夜),PBS洗涤皿底,加入荧光二抗,于室温环境孵育45 min,PBS清洗皿底,加入DAPI染液,避光环境放置20 min,清洗去除多余的染液,激光共聚焦显微镜拍照。
-
实验结果用(
$ \bar{\mathrm{x}} $ ±s)表示。采用SPSS 22.0软件、单因素方差分析(One-Way ANOVA)方法对实验数据进行分析。 -
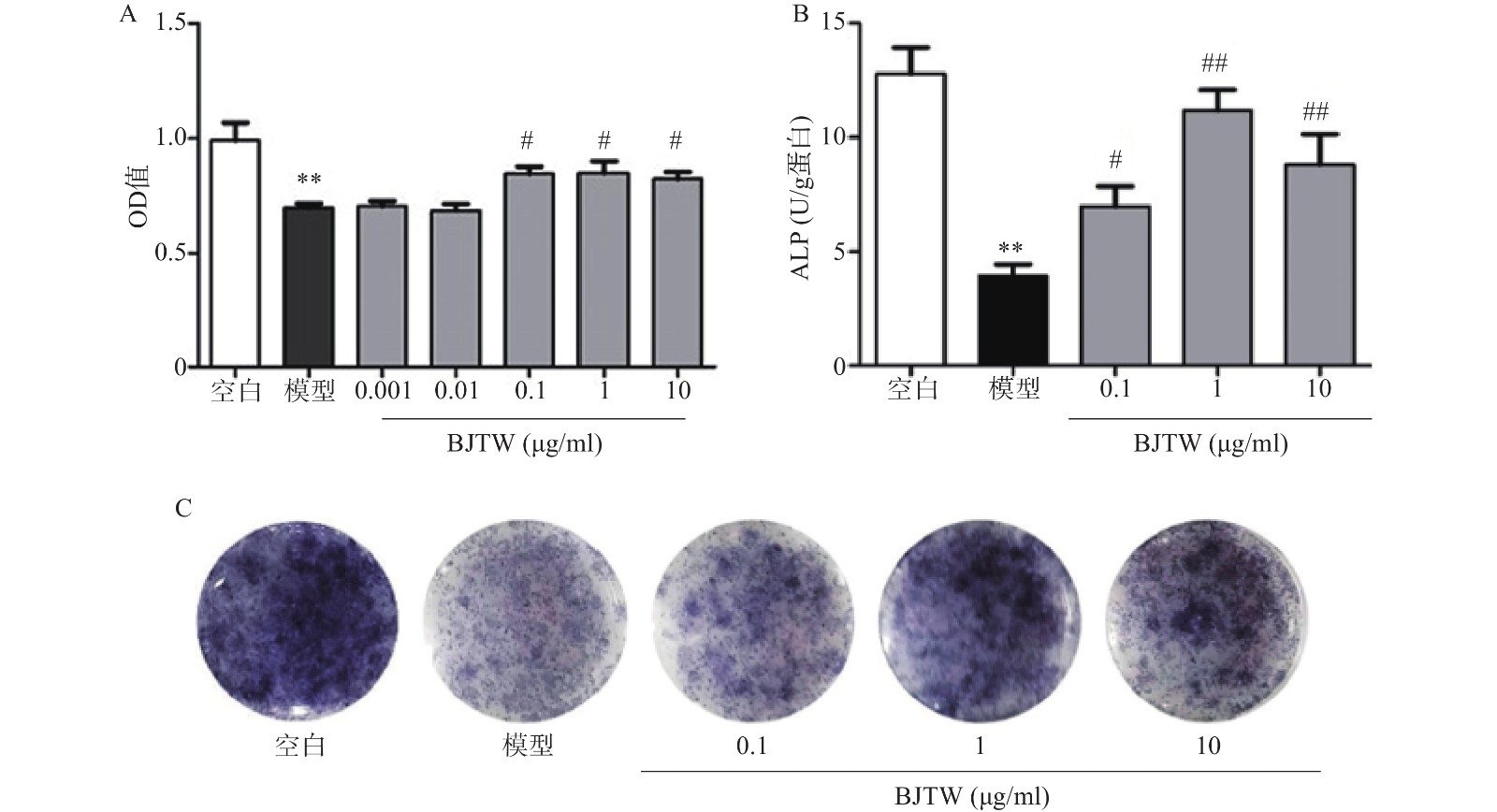
D-gal损伤可显著抑制成骨细胞的增殖;药物干预后,BJTW在0.1、1、10 μg/ml浓度下均能够显著提高成骨细胞的MTT水平,增强D-gal损伤成骨细胞的增殖(图1A)。
-
ALP源自成骨细胞的分泌,能够反映骨形成的能力。对给予D-gal干预的成骨细胞所分泌的ALP的活性进行测定。D-gal损伤成骨细胞后,其ALP活性显著降低;药物干预后,BJTW在0.1、1、10 μg/ml浓度下均可提高D-gal损伤成骨细胞的ALP活性(图1B)。
与此同时,在ALP的催化下,甲苯胺蓝(BCIP)的水解产物会和氯化硝基四氮唑兰(NBT)反应产生不溶的深蓝色至蓝紫色甲瓒结晶。BJTW在0.1、1、10 μg/ml的浓度下均可显著增加培养皿底的甲瓒结晶的颜色深度(图1C),证明其可显著提高D-gal损伤细胞的ALP活性,促进成骨分化。
-
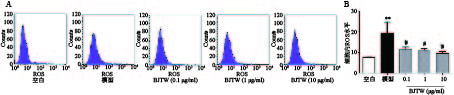
如图2所示,D-gal损伤成骨细胞后,胞内ROS活性显著升高。药物干预后,BJTW在0.1、1、10 μg/ml浓度下均可显著降低D-gal损伤成骨细胞内ROS水平,缓解氧化应激,且该作用呈剂量依赖。
-
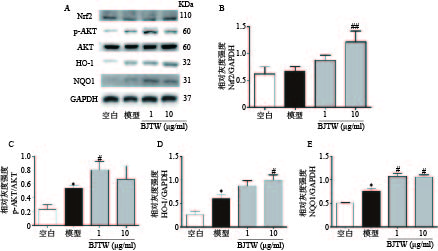
外源性氧化应激会激活细胞内的PI3K/AKT信号通路,使AKT磷酸化水平上升[6]。如图3所示,D-gal损伤成骨细胞后,p-AKT/AKT水平较空白组显著升高;药物干预后,BJTW在1、10 μg/ml浓度下对p-AKT/AKT的水平的促进作用相较模型组表现更为显著。此外,BJTW还可显著促进其下游蛋白Nrf2、HO-1和NQO1的表达,进而激活氧化应激相关PI3K/AKT通路,缓解氧化损伤。
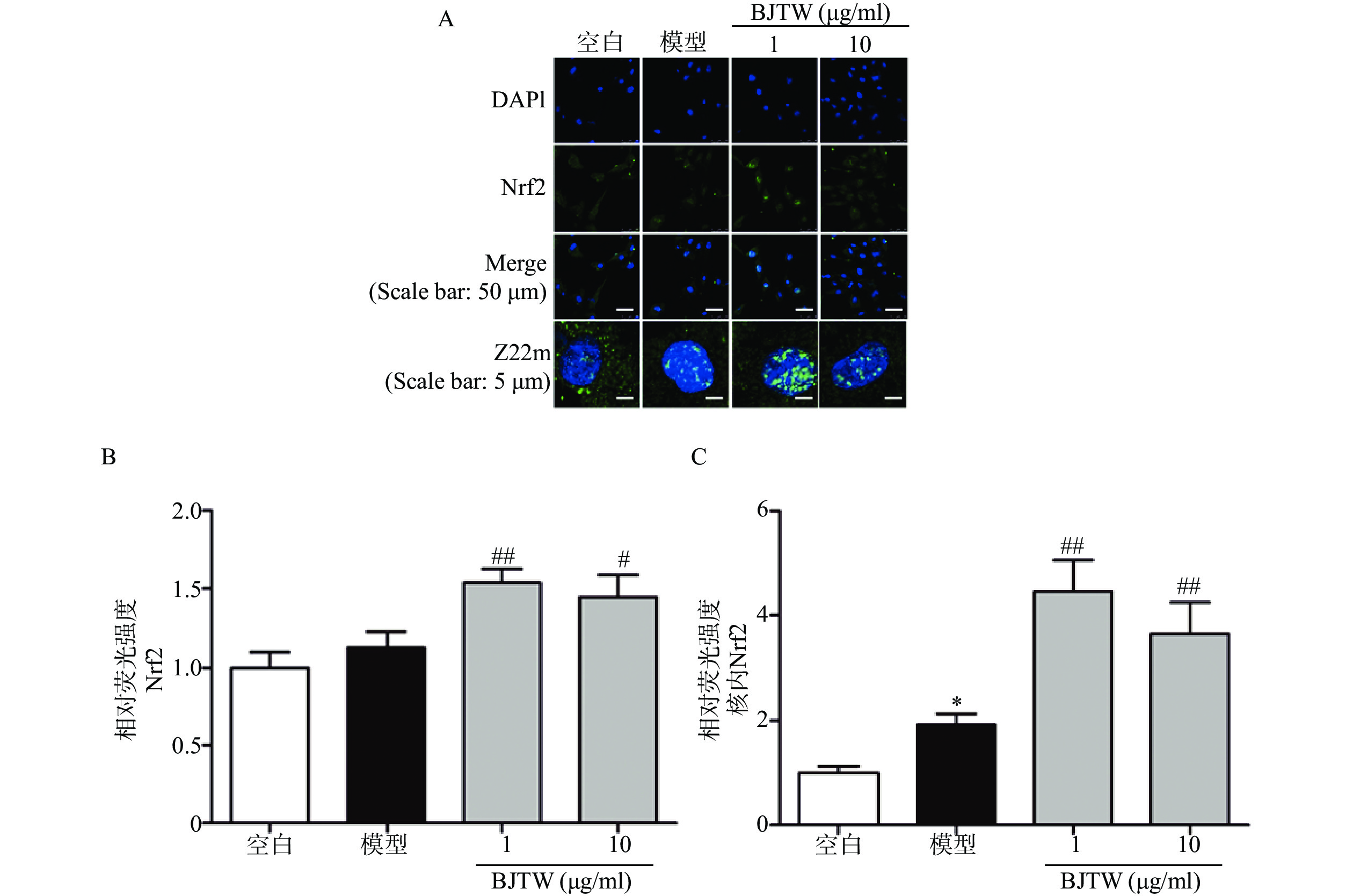
Nrf2通路同样是抗氧化的关键通路,在ROS清除方面发挥重要作用。如图4所示,BJTW可显著促进Nrf2的入核表达(图4),提示BJTW抗氧化、促进骨形成作用与激活Nrf2/AKT通路、促进Nrf2入核有关。
-
成骨细胞主要由骨髓间充质干细胞分化而来,在维持骨稳态和骨重建过程中起着至关重要的作用[7]。与此同时,成骨细胞还是骨形成的主要功能细胞,在骨形成过程中经历增殖、分化、矿化和凋亡四个阶段[8-9]。成骨细胞的增殖水平体现骨形成的强弱,其分泌的ALP是成骨细胞分化阶段的关键酶,可介导骨组织矿化[9-10]。ROS是细胞信号传递和其他生理功能所必需的,然而过多的ROS会导致细胞内的氧化还原反应失衡,扰乱正常的生物功能,导致氧化应激[11-12]。本研究中,D-gal损伤成骨细胞后,细胞的增殖及骨形成指标ALP水平明显降低,细胞内活性氧水平显著上升,而巴戟天丸能够显著促进D-gal损伤成骨细胞的增殖能力和ALP活性,并能显著降低细胞内活性氧水平,表明巴戟天丸可通过抑制胞内ROS释放缓解成骨细胞氧化应激,从而增强成骨细胞的活性,以促进骨的形成。
作为主要的氧化应激调控因子,在骨代谢调控中,Nrf2同样起着重要的作用[13-14]。研究发现,敲除Nrf2基因的小鼠,其股骨的骨密度和骨量显著降低,骨小梁面积减少[15];同时缺失Nrf2会抑制成骨细胞抗氧化酶的表达,提高胞内ROS水平,从而抑制成骨细胞的分化[16]。此外,PI3K/AKT通路作为Nrf2的上游信号通路,在氧化应激调控方面同样起到关键作用[17]。本研究中,药物干预后,巴戟天丸可促进成骨细胞内AKT磷酸化增加,促进Nrf2入核,同时激活下游的HO-1和NQO1的表达,表明巴戟天丸可能通过激活PI3K/AKT和Nrf2信号通路,进而促进通路中相关的蛋白表达,进而增加成骨细胞的抗氧化应激水平,促进骨形成。本研究可为老年性骨质疏松症的临床用药提供一定的实验依据。
Effect and mechanism of Bajitianwan on preventing D-galactose-induced osteoblast bone loss
doi: 10.12206/j.issn.2097-2024.202202030
- Received Date: 2022-02-14
- Rev Recd Date: 2022-10-04
- Available Online: 2023-07-14
- Publish Date: 2023-03-25
-
Key words:
- Bajitianwan /
- D-galactose /
- osteoblast /
- reactive oxygen species /
- Nrf2
Abstract:
| Citation: | XU Weifan, XU Wumu, DING Luying, JIANG Yiping, XIA Tianshuang, XIN Hailiang. Effect and mechanism of Bajitianwan on preventing D-galactose-induced osteoblast bone loss[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(3): 155-159. doi: 10.12206/j.issn.2097-2024.202202030 |









 DownLoad:
DownLoad: