-
蛋白降解靶向嵌合体(proteolysis targeting chimera, PROTAC)是近年来最受期待的蛋白降解技术[1]。PROTAC是一种异双功能分子,一端与靶蛋白结合,另一端结合E3连接酶形成靶蛋白-PROTAC-E3三元复合物,E3连接酶诱导靶蛋白泛素化,随后被蛋白酶体识别并降解。PROTAC相较传统药物具有诸多优势,如使难成药靶点实现可成药性、大幅增加可用靶点数量、克服耐药性、提高选择性和活性、降低毒副作用等。但是,PROTAC依赖E3连接酶和蛋白酶体,也存在一些固有缺陷,如主要针对细胞质中可溶性蛋白进行降解,不能降解蛋白聚集物和大的蛋白质,对非蛋白物质的降解无能为力[2]。
自噬-溶酶体途径(ALP)是广泛存在于真核细胞中的蛋白降解系统,它是指在自噬关键蛋白LC3参与下,细胞内膜结构形成自噬小体并将底物包裹,随后转运至溶酶体实现底物降解的过程[3]。该机制涉及的底物范围十分广泛,包括蛋白质聚集体、衰老或受损的细胞器、入侵的病原微生物等。ALP与泛素-蛋白酶体途径互为补充,在细胞中发挥重要的生理功能[4-5]。
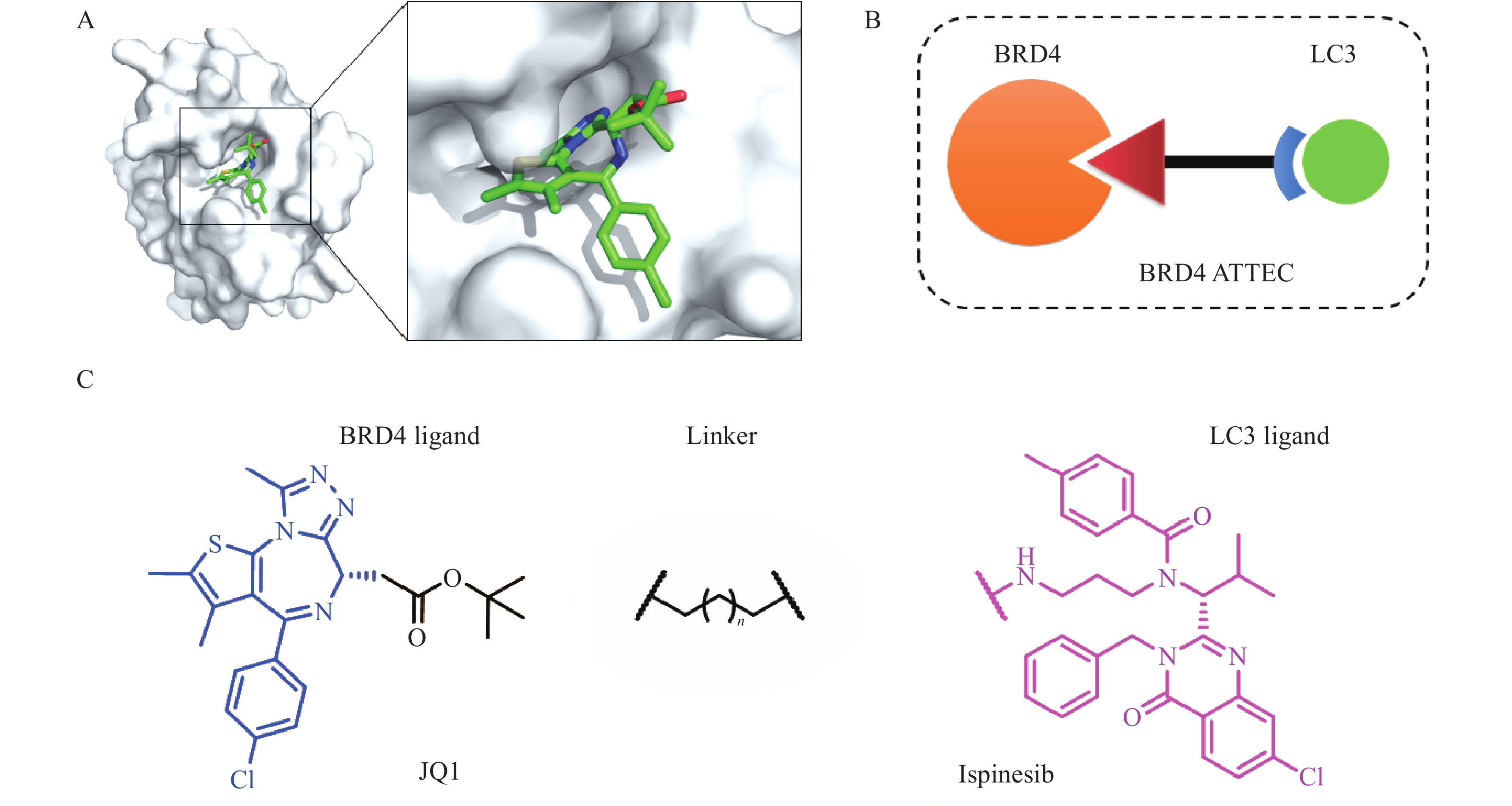
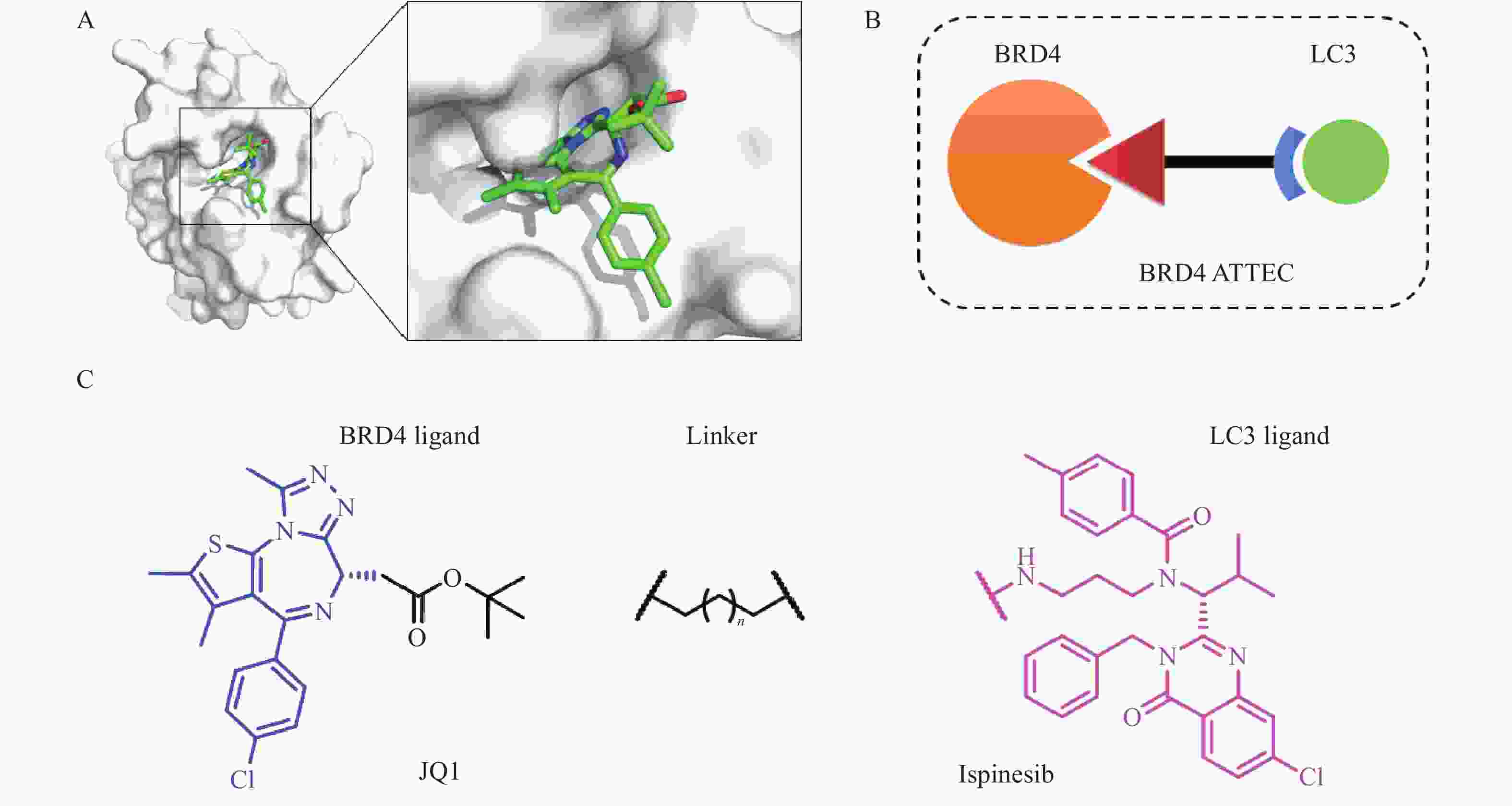
2019年,复旦大学鲁伯埙团队基于自噬-溶酶体途径首次提出自噬小体绑定化合物(ATTEC)概念。ATTEC是一种双功能分子,同时结合LC3与靶蛋白,将靶蛋白包裹进自噬小体,并在溶酶体中实现降解。基于这一思想,研究人员将小分子库固定在芯片上,筛选以“分子胶”方式将突变型亨廷顿蛋白(mHTT)和LC3蛋白“黏合”的分子,成功获得4个小分子化合物(10O5、ispinesib、AN1-2, 图1)。这些化合物能有效降解mHTT蛋白,并减弱亨廷顿病相关表型[6]。ATTEC分子通过直接连接自噬蛋白LC3,绕过泛素化过程,是一种利用自噬降解靶标最为直接的策略,对于降解不同类型的靶标具有很大的潜力。但是,ATTEC技术尚处于概念验证阶段,急需拓展靶标应用范围,推动技术的不断成熟。
BRD4是BET溴结构域蛋白家族中最重要的成员之一,在人体中广泛分布,对细胞正常生长及细胞周期的调控具有重要意义,与肿瘤发生密切相关,是肿瘤治疗的热门靶点[7],基于PROTAC的BRD4降解剂相继报道[8-9]。BRD4已成为靶向蛋白降解研究的经典体系,为了验证ATTEC降解策略的普适性和可行性,我们以BRD4靶点为研究对象,开展BRD4-ATTEC分子设计、合成和蛋白降解活性评价研究。
-
BRD4-ATTEC分子由三部分组成:BRD4抑制剂、连接子Linker和LC3配体。我们选择经典BRD4抑制剂JQ1作为靶蛋白配体,JQ1与BRD4共晶结构如图2A所示。JQ1的酯基部分暴露于溶剂中,适合作为Linker连接位点,不影响BRD4蛋白结合活性[10];Ispinesib作为LC3配体,由于鲁伯埙团队在进行高通量筛选时,化合物ispinesib的氨基端连接于分子芯片,故选择氨基端作为Linker连接另一位点,不会影响其与LC3蛋白的结合;随后,使用不同长度的烷烃链将两个配体相连设计得到相应目标化合物(图2B、C)。目标化合物通过同时结合BRD4与LC3蛋白,将BRD4靶向至自噬小体中,从而被溶酶体吞噬完成降解。
-
化学原料均为市售分析纯;免抗BRD4抗体(Abcam,ab128874);免抗GAPDH抗体(Abcam,ab181602);山羊抗免IgG H&L (Alexa Fluor® 680) (Abcam,ab175773);Bruker AVANCE600(Bruker Company, Germany)核磁共振仪,TMS作为内标,化学位移与偶合常数分别用ppm和Hz表示;Agilent 6538 UHD Accurate-Mass Q-TOF LC/MS高分辨质谱(HRMS)仪;上海申光WRR目视熔点仪;Bioteck Synergy2多功能酶标仪;Biorad ChemiDoc成像仪。
-
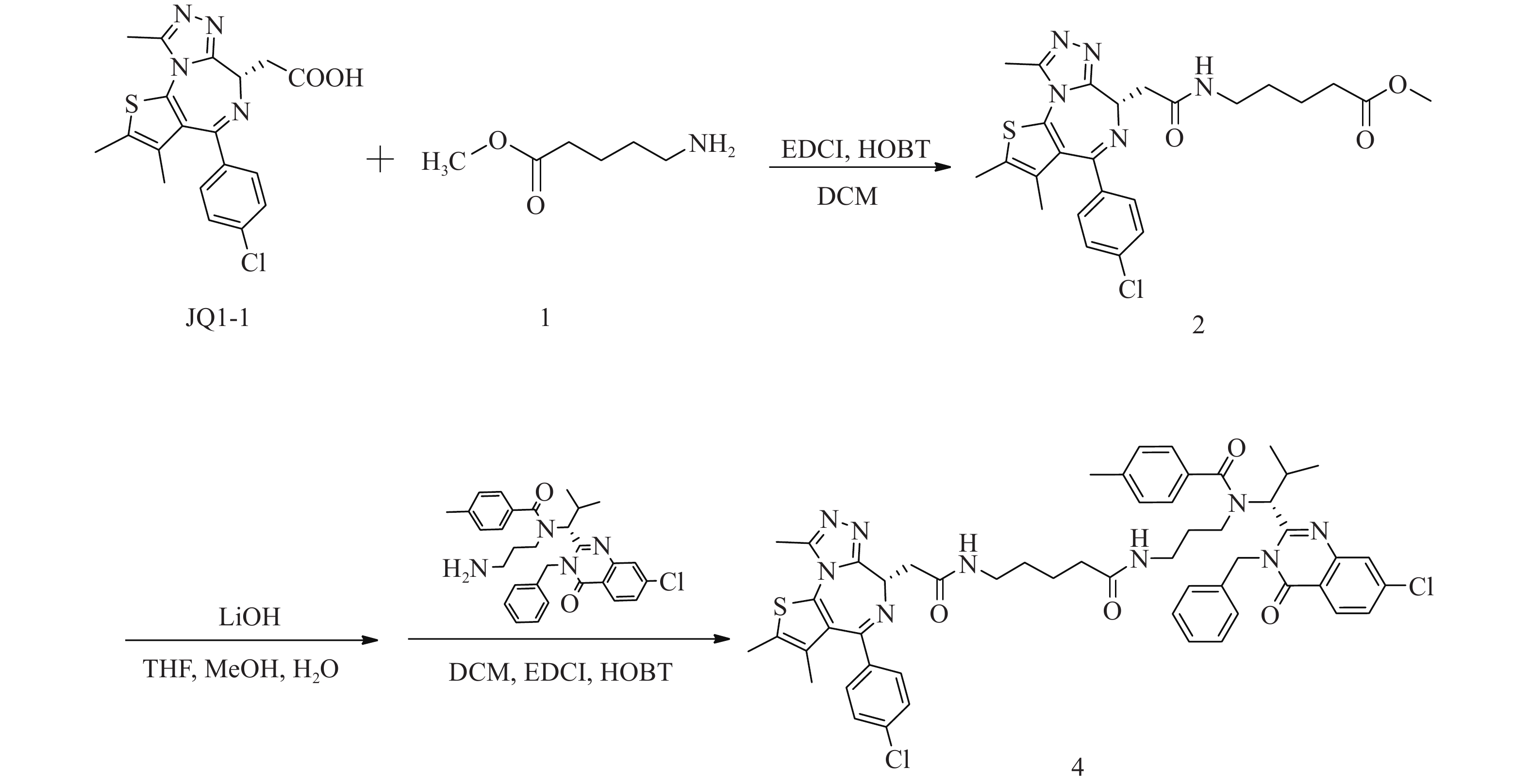
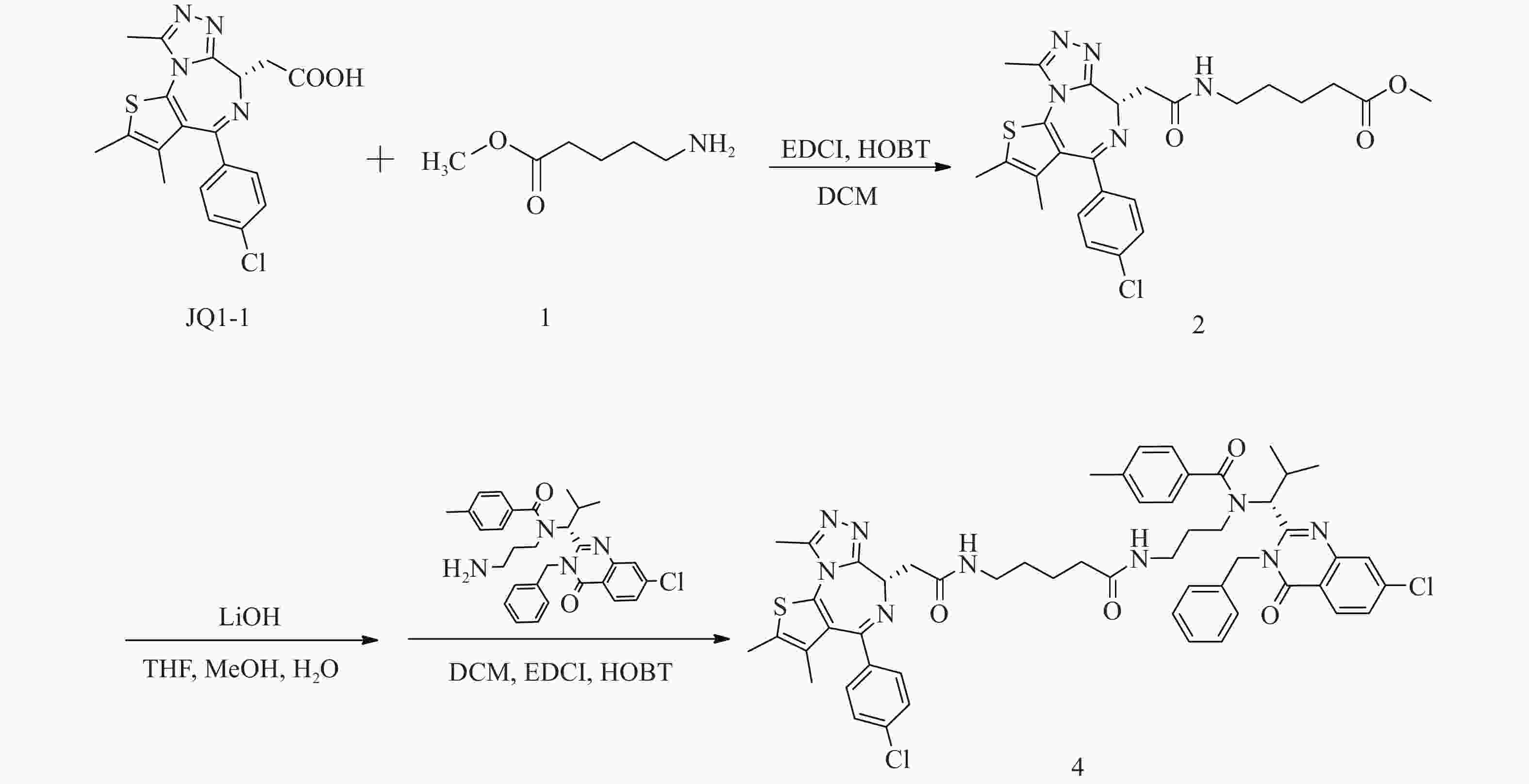
化合物4的合成路线见图3。
(S)-5-(2-(4-(4-氯苯基)-2, 3, 9-三甲基-6H-噻吩并[3, 2-f][1, 2, 4]三唑并[4, 3-a][1, 4]二氮杂卓-6-基)乙酰氨基)戊酸甲酯(2)的制备:
将JQ1-1(100 mg,0.25 mmol)溶解于二氯甲烷(DCM, 10 ml)中,加入5-氨基戊酸甲酯(1)(39 mg,0.30 mmol)、EDCI(73 mg,0.38 mmol)和HOBT(51 mg,0.38 mmol),室温下反应8 h。反应完后,加水(200 ml)稀释,并用DCM(50 ml×3)萃取,收集有机层,使用无水硫酸钠干燥,蒸干溶剂,硅胶柱色谱分离(DCM∶MeOH = 98∶2),得淡黄色油状液体(2)91 mg,产率71%;1H NMR (600 MHz, DMSO−d6) δ: 8.20 (t, J=5.7 Hz, 1 H), 7.48 (d, J=8.8 Hz, 2 H), 7.42 (d, J=8.6 Hz, 2 H), 4.50 (dd, J=8.4, 5.7 Hz, 1 H), 3.57 (s, 3 H), 3.28−3.22 (m, 1 H), 3.19−3.12 (m, 2 H), 3.10−3.03 (m, 1 H), 2.59 (s, 3 H), 2.41 (d, J=0.6 Hz, 3 H), 2.33 (t, J=7.4 Hz, 2 H), 1.62 (s, 3 H), 1.59−1.53 (m, 2 H), 1.48−1.42 (m, 2 H)。
N-((R)-1-(3-苄基-7-氯-4-氧代-3, 4-二氢喹唑啉-2-基)-2-甲基丙基)-N-(3-(5-(2-((S)-4-(4-氯苯基)-2, 3, 9-三甲基-6H-噻吩并[3, 2-f][1, 2, 4]三唑并[4, 3-a][1, 4]二氮杂卓-6-基)乙酰胺基)戊酰胺基丙基)-4-甲基苯甲酰胺(4)的制备:
将化合物2(91 mg,0.18 mmol)溶于THF-MeOH-H2O(3∶2∶1)混合溶剂(6 ml),加入LiOH(17 mg,0.72 mmol),室温反应5 h后蒸干溶剂,使用1 mol/L稀盐酸调pH至6,过滤,收集固体并干燥,得白色固体49 mg(0.10 mmol);将所得白色固体(49 mg,0.10 mmol)溶于DCM(15 ml),加入EDCI(29 mg,0.15 mmol)、HOBT(20 mg,0.15 mmol)和ispinesib(0.10 mmol,52 mg),室温反应8 h后,加水(300 ml)稀释,用DCM(100 ml×3)萃取,收集有机层,使用无水硫酸钠干燥,蒸干溶剂,C18反相柱色谱分离(MeOH∶H2O=63∶37),得白色固体(4)59 mg(0.06 mmol),两步收率33%;1H NMR (600 MHz, DMSO−d6) δ: 8.24 (dd, J=8.6, 3.9 Hz, 1 H), 8.17 (t, J=5.6 Hz, 1 H), 7.81 (t, J=1.7 Hz, 1 H), 7.69−7.65 (m, 1 H), 7.50−7.47 (m, 2 H), 7.42 (d, J=8.3 Hz, 2 H), 7.40−7.35 (m, 3 H), 7.34−7.30 (m, 1 H), 7.29−7.20 (m, 6 H), 5.89 (d, J=16.0 Hz, 1 H), 5.55 (d, J=10.6 Hz, 1 H), 5.06 (d, J=16.3 Hz, 1 H), 4.51 (dd, J=8.1, 5.9 Hz, 1 H), 3.30−3.23 (m, 3 H), 3.20−3.15 (m, 1 H), 3.15−3.09 (m, 1 H), 3.05−2.97 (m, 1 H), 2.79−2.71 (m, 1 H), 2.61 (s, 3 H), 2.58−2.54 (m, 1 H), 2.49 (d, J=7.0 Hz, 1 H), 2.42 (s, 3 H), 2.34 (s, 3 H), 1.87−1.76 (m, 2 H), 1.64−1.60 (m, 3 H), 1.40−1.31 (m, 5 H), 0.91 (d, J=6.4 Hz, 3 H), 0.82−0.87 (m, 1 H), 0.49 (d, J=6.2 Hz, 3 H);13C NMR (151 MHz, DMSO−d6) δ: 172.44, 171.88, 169.76, 163.43, 161.57, 155.70, 155.59, 150.23, 147.64, 139.97, 139.11, 137.22, 137.16, 135.64, 134.25, 132.72, 131.14, 130.57, 130.27, 130.02, 129.34, 129.11, 128.91, 128.48, 127.88, 127.11, 126.89, 126.33, 119.55, 59.44, 54.35, 45.61, 42.90, 38.74, 38.11, 36.17, 35.36, 30.71, 29.33, 28.81, 23.06, 21.36, 19.95, 18.61, 14.48, 13.13, 11.75;HRMS(ESI) m/z calcd for C54H56Cl2N9O4S (M-H)− 996.3559, found 996.3542;熔程:143.1~146.3 ℃。
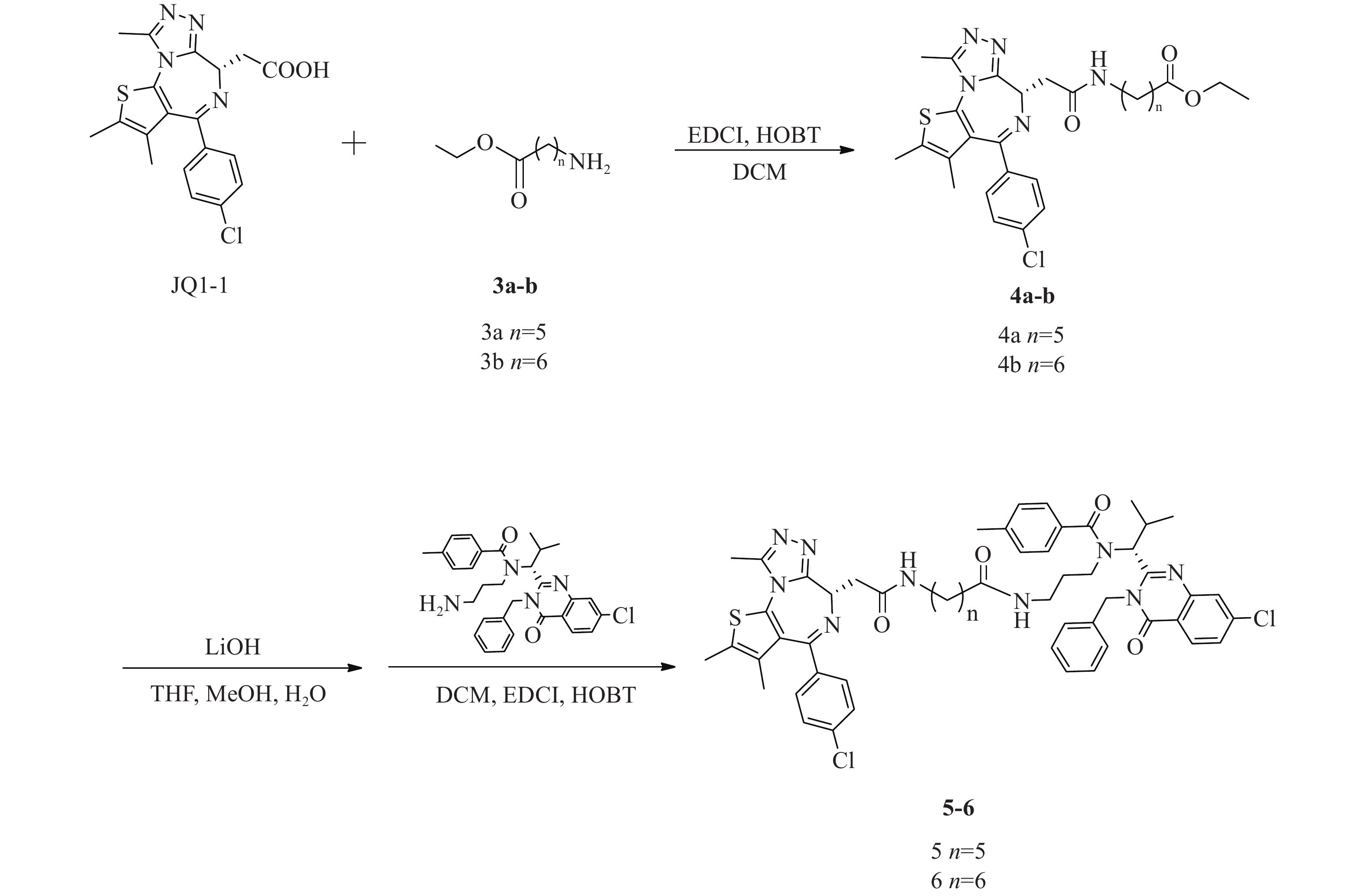
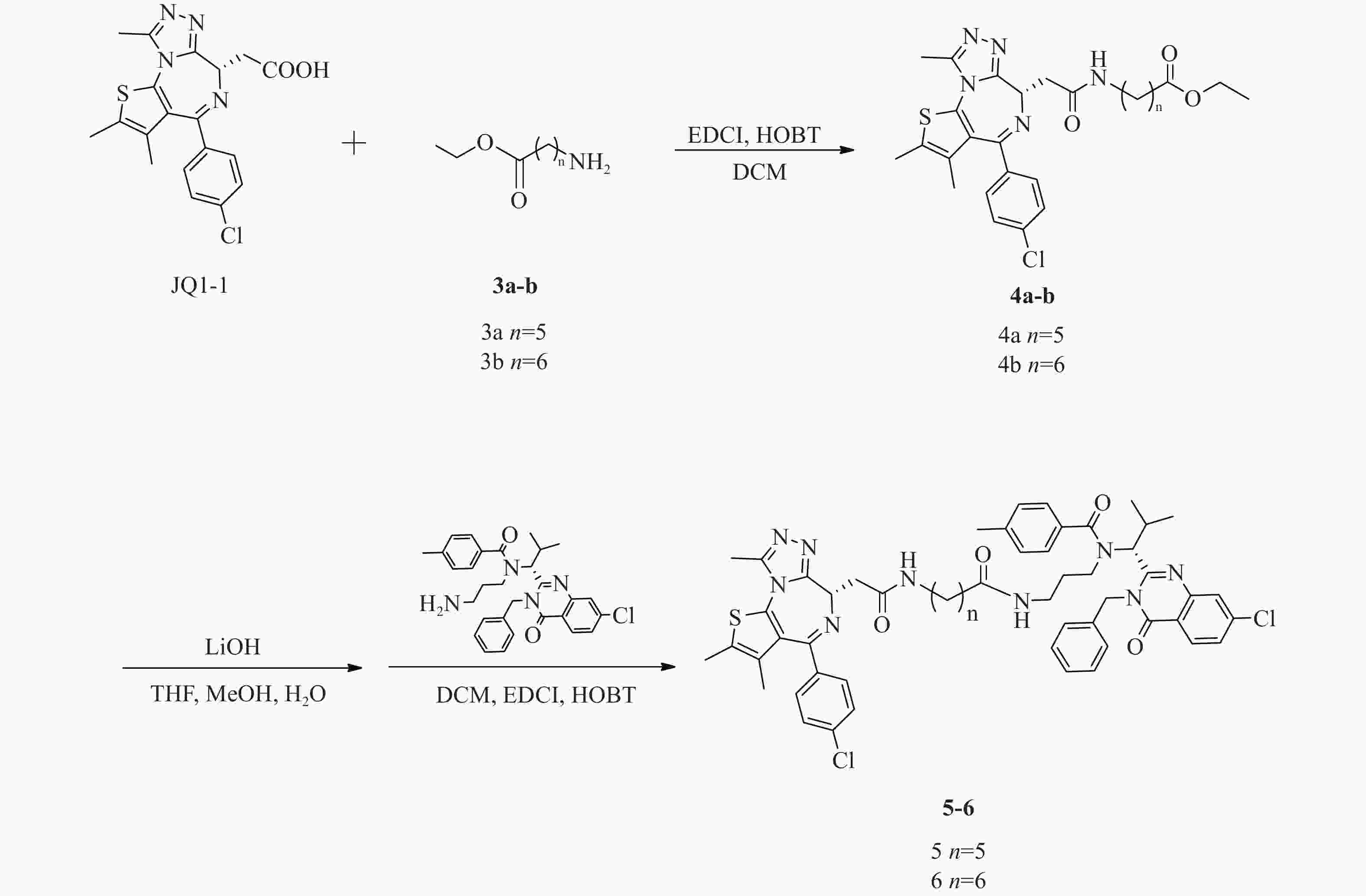
化合物5、6合成路线如图4所示。
(S)-6-(2-(4-(4-氯苯基)-2, 3, 9-三甲基-6H-噻吩并[3, 2-f][1, 2, 4]三唑并[4, 3-a][1, 4]二氮杂卓-6-基)乙酰氨基)己酸乙酯(4a)的制备:
将JQ1-1(100 mg,0.25 mmol)溶解于DCM(10 ml),加入化合物6-氨基己酸甲酯(3a,44 mg,0.30 mmol)、EDCI(73 mg,0.38 mmol)和HOBT(51 mg,0.38 mmol),室温反应8 h。反应完后,加水(200 ml)稀释,并用DCM(50 ml×3)萃取,收集有机层,使用无水硫酸钠干燥,蒸干溶剂,硅胶柱色谱分离(DCM∶MeOH=98∶2),得淡黄色油状液体(4a)80 mg,产率59%;1H NMR (600 MHz, DMSO - d6) δ∶8.17 (t, J=5.7 Hz, 1 H), 7.48 (d, J=8.8 Hz, 2 H), 7.42 (d, J=8.6 Hz, 2 H), 4.50 (dd, J=8.3, 5.8 Hz, 1 H), 4.04 (q, J=7.2 Hz, 2 H), 3.28−3.22 (m, 1 H), 3.20−3.10 (m, 2 H), 3.08−3.02 (m, 1 H), 2.59 (s, 3 H), 2.41 (s, 3 H), 2.26 (t, J=7.5 Hz, 2 H), 1.62 (s, 3 H), 1.56−1.50 (m, 2 H), 1.47−1.41 (m, 2 H), 1.34−1.27 (m, 2 H), 1.17 (t, J=7.1 Hz, 3 H)。
(S)-7-(2-(4-(4-氯苯基)-2, 3, 9-三甲基-6H-噻吩并[3, 2-f][1, 2, 4]三唑并[4, 3-a][1, 4]二氮杂卓-6-基)乙酰氨基)庚酸乙酯(4b)的制备:
中间体4b的合成步骤参照中间体4a合成,得淡黄色油状液体(4b)99 mg,产率76%;1H NMR (600 MHz, DMSO−d6) δ: 8.16 (t, J=5.7 Hz, 1 H), 7.48 (d, J=8.8 Hz, 2 H), 7.42 (d, J=8.4 Hz, 2 H), 4.50 (dd, J=8.4, 5.9 Hz, 1 H), 4.04 (q, J=7.1 Hz, 2 H), 3.28−3.22 (m, 1 H), 3.20−3.10 (m, 2 H), 3.08−3.02 (m, 1 H), 2.59 (s, 3 H), 2.41 (s, 3 H), 2.26 (t, J=7.4 Hz, 2 H), 1.62 (s, 3 H), 1.54−1.47 (m, 2 H), 1.46−1.39 (m, 2 H), 1.31−1.26 (m, 4 H), 1.16 (t, J=7.1 Hz, 3 H)。
N-((R)-1-(3-苄基-7-氯-4-氧代-3, 4-二氢喹唑啉-2-基)-2-甲基丙基)-N-(3-(6-(2-((S)-4-(4-氯苯基)-2, 3, 9-三甲基-6H-噻吩并[3, 2-f][1, 2, 4]三唑并[4, 3-a][1, 4]二氮杂卓-6-基)乙酰胺基)己酰胺基)丙基)-4-甲基苯甲酰胺(5)的制备:
将化合物4a(80 mg,0.15 mmol)溶于THF-MeOH-H2O(3∶2∶1)混合溶剂(6 ml),加入LiOH(14 mg, 0.6 mmol),室温反应5 h后蒸干溶剂,使用1 mol/L稀盐酸调pH至6,过滤,收集固体并干燥,得白色固体61 mg(0.12 mmol);将所得白色固体(61 mg,0.12 mmol)溶于 DCM(15 ml)中,加入EDCI(35 mg,0.18 mmol)、HOBT(24 mg,0.18 mmol)和化合物ispinesib(0.12 mmol,62 mg),室温下反应8 h后,加水(300 ml)稀释,并用DCM(100 ml × 3)萃取,收集有机层,使用无水硫酸钠干燥,蒸干溶剂,C18反相柱色谱分离(MeOH∶H2O=63∶37),得白色固体(5)(64 mg,0.06 mmol),两步收率42%;1H NMR (600 MHz, DMSO−d6) δ: 8.23 (d, J=8.6 Hz, 1 H), 8.17 (t, J=5.6 Hz, 1 H), 7.79 (d, J=1.8 Hz, 1 H), 7.68−7.65 (m, 1 H), 7.49−7.45 (m, 2 H), 7.42 (d, J=7.9 Hz, 2 H), 7.39−7.34 (m, 3 H), 7.33−7.28 (m, 1 H), 7.28−7.19 (m, 6 H), 5.88 (d, J=16.1 Hz, 1 H), 5.54 (d, J=10.3 Hz, 1 H), 5.05 (d, J=16.5 Hz, 1 H), 4.51 (dd, J=8.2, 6.1 Hz, 1 H), 3.29−3.21 (m, 3 H), 3.21−3.15 (m, 1 H), 3.14−3.07 (m, 1 H), 3.07−3.00 (m, 1 H), 2.77−2.69 (m, 1 H), 2.60 (d, J=1.3 Hz, 3 H), 2.55−2.52 (m, 1 H), 2.50−2.47 (m, 1 H), 2.41 (s, 3 H), 2.33 (s, 3 H), 1.83−1.71 (m, 2 H), 1.62 (s, 3 H), 1.43−1.37 (m, 2 H), 1.36−1.27 (m, 3 H), 1.22−1.15 (m, 2 H), 0.90 (d, J=6.6 Hz, 3 H), 0.87−0.80 (m, 1 H), 0.48 (d, J=5.9 Hz, 3 H); 13C NMR (151 MHz, DMSO−d6) δ: 172.45, 171.96, 169.74, 163.43, 161.57, 155.70, 155.60, 150.24, 147.64, 139.96, 139.11, 137.22, 137.16, 135.67, 134.25, 132.71, 131.16, 130.55, 130.27, 130.03, 129.34, 129.11, 128.90, 128.48, 127.88, 127.13, 126.88, 126.33, 119.55, 59.44, 54.36, 45.62, 42.90, 38.89, 38.08, 36.18, 35.70, 30.69, 29.48, 28.81, 26.61, 25.35, 21.36, 19.95, 18.61, 14.49, 13.13, 11.74;HRMS(ESI) m/z calcd for C55H59Cl3N9O4S (M+Cl)− 1046.3482, found 1046.3443;熔程:143.0~145.2 ℃。
N-((R)-1-(3-苄基-7-氯-4-氧代-3, 4-二氢喹唑啉-2-基)-2-甲基丙基)-N-(3-(7-(2-((S)-4-(4-氯苯基)-2, 3, 9-三甲基-6H-噻吩并[3, 2-f][1, 2, 4]三唑并[4, 3-a][1, 4]二氮杂卓-6-基)乙酰胺基)庚酰胺基)丙基)-4-甲基苯甲酰胺(6)的制备:
化合物6的合成步骤参照化合物5合成,得白色固体(6)(71 mg,0.07 mmol),两步收率36%;1H NMR (600 MHz, DMSO−d6) δ: 8.23 (d, J=8.6 Hz, 1 H), 8.17 (t, J=5.6 Hz, 1 H), 7.79 (d, J=2.0 Hz, 1 H), 7.66 (dd, J=8.6, 2.0 Hz, 1 H), 7.50−7.46 (m, 2 H), 7.44−7.40 (m, 2 H), 7.40−7.34 (m, 3 H), 7.33−7.28 (m, 1 H), 7.27−7.19 (m, 6 H), 5.88 (d, J=16.1 Hz, 1 H), 5.54 (d, J=10.5 Hz, 1 H), 5.05 (d, J=16.3 Hz, 1 H), 4.51 (dd, J=8.3, 6.1 Hz, 1 H), 3.29−3.21 (m, 3 H), 3.21−3.17 (m, 1 H), 3.16−3.09 (m, 1 H), 3.08−3.01 (m, 1 H), 2.77−2.69 (m, 1 H), 2.59 (s, 3 H), 2.57−2.53 (m, 1 H), 2.50−2.46 (m, 1 H), 2.41 (s, 3 H), 2.33 (s, 3 H), 1.84−1.70 (m, 2 H), 1.61 (s, 3 H), 1.44−1.38 (m, 2 H), 1.36−1.28 (m, 3 H), 1.27−1.25 (m, 1 H), 1.23−1.21 (m, 1 H), 1.19−1.12 (m, 2 H), 0.89 (d, J=6.6 Hz, 3 H), 0.87−0.81 (m, 1 H), 0.47 (d, J=6.2 Hz, 3 H);13C NMR (151 MHz, DMSO−d6) δ: 172.45, 172.00, 169.74, 163.44, 161.56, 155.70, 155.60, 150.24, 147.64, 139.95, 139.09, 137.21, 137.16, 135.69, 134.25, 132.71, 131.15, 130.54, 130.26, 130.04, 129.33, 129.11, 128.90, 128.47, 127.88, 127.12, 126.87, 126.32, 119.55, 59.44, 54.37, 45.62, 42.90, 38.90, 38.11, 36.16, 35.67, 30.71, 29.61, 28.89, 28.81, 26.61, 25.53, 21.36, 19.95, 18.60, 14.48, 13.13, 11.74;HRMS(ESI) m/z calcd for C56H61Cl2N9O4SNa (M+Na)+ 1048.3836, found 1048.3892;熔程:142.0~144.7 ℃。
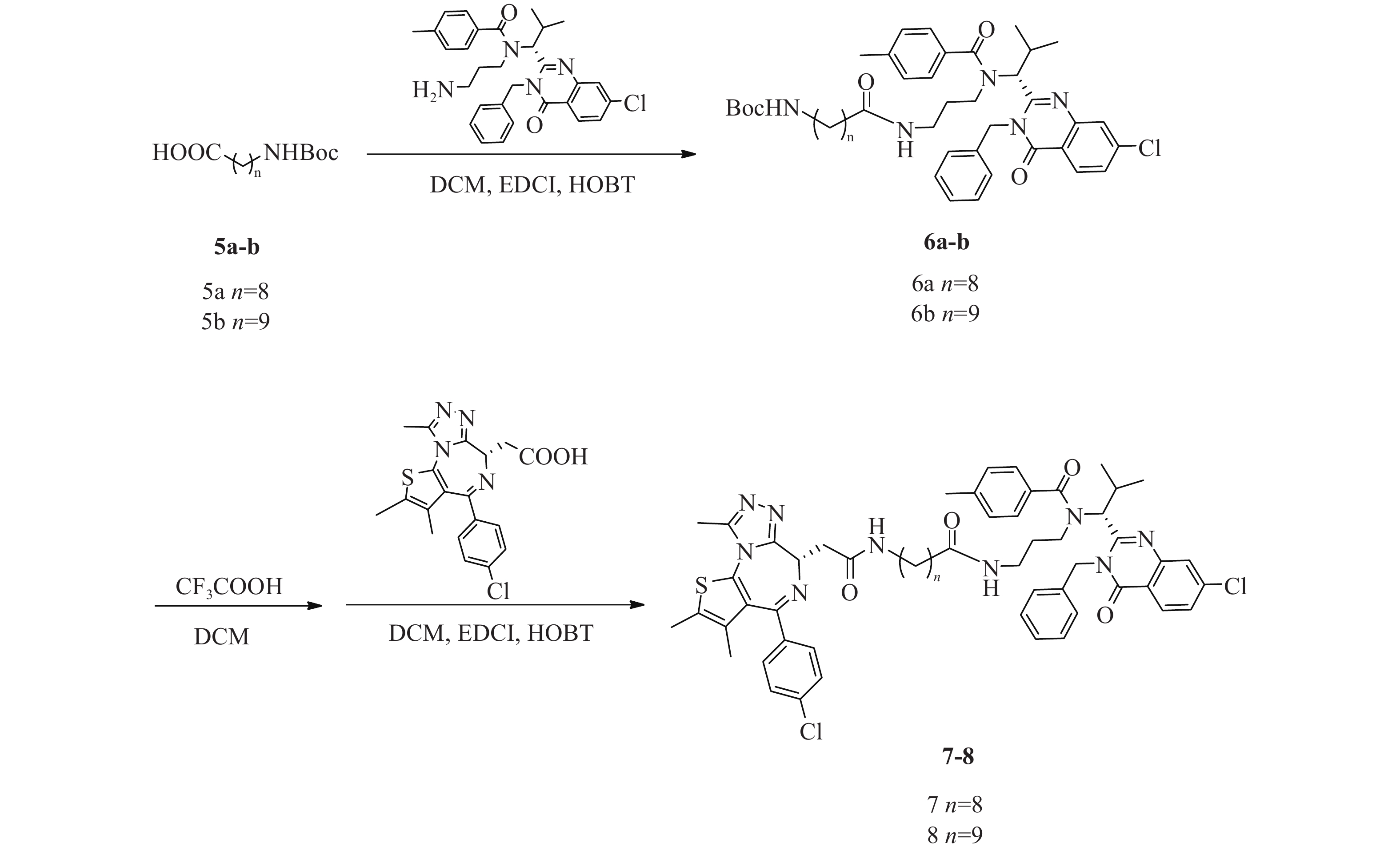
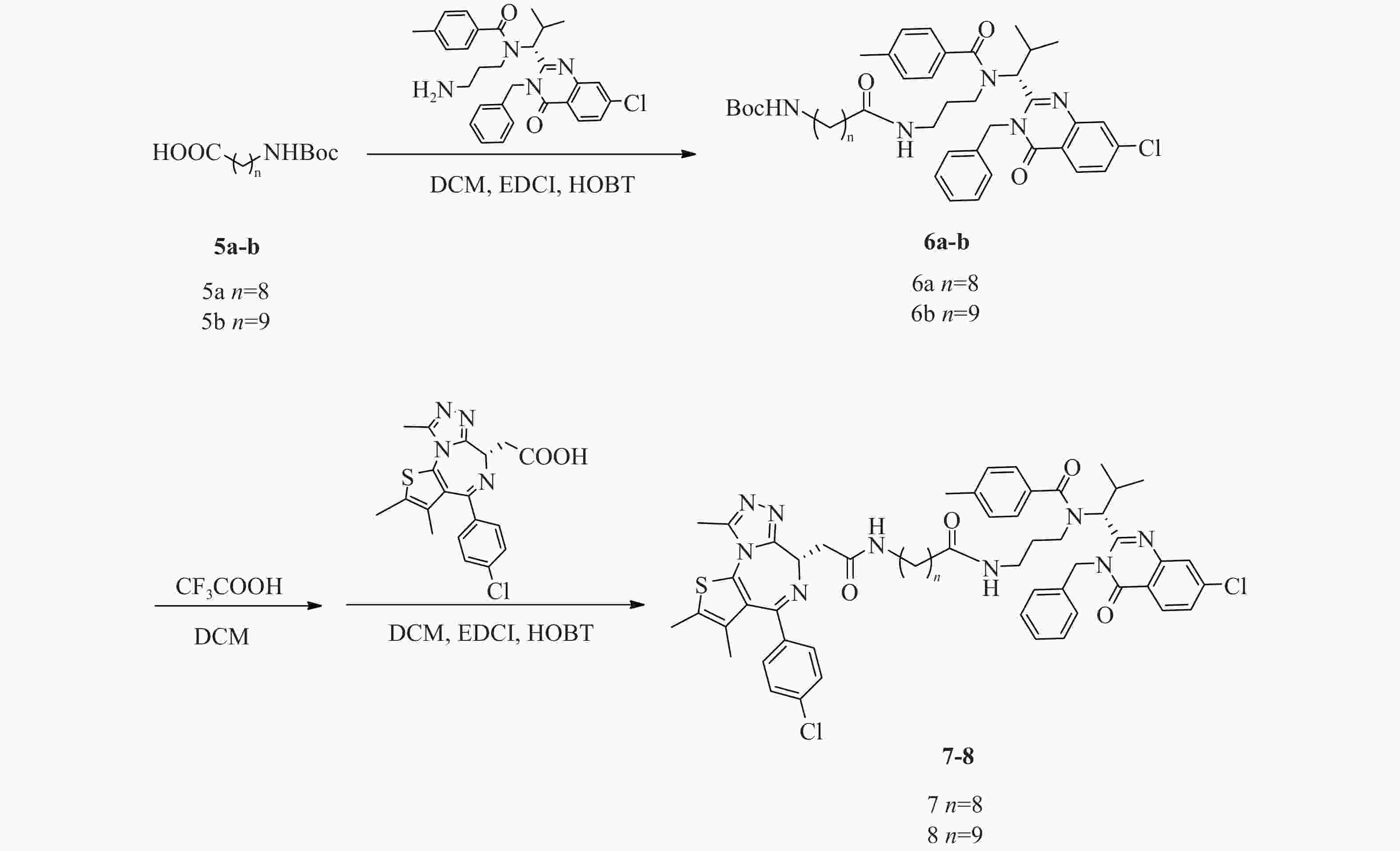
化合物7、8合成路线如图5所示。
(R)-(9-((3-(N-(1-(3-苄基-7-氯-4-氧代 -3, 4-二氢喹唑啉 -2-基)-2-甲基丙基)-4-甲基苯甲酰胺基)丙基)氨基)-9-氧代壬基)氨基甲酸叔丁酯(6a)的制备:
将ispinesib(100 mg,0.2 mmol)溶解于DCM(10 ml)中,加入化合物9-((叔丁氧基羰基)氨基)壬酸(5a)(66 mg,0.24 mmol),EDCI(58 mg,0.30 mmol)和HOBT(41 mg,0.30 mmol),室温下反应8 h。反应完后,加水(200 ml)稀释,并用DCM(50 ml×3)萃取,收集有机层,使用无水硫酸钠干燥,蒸干溶剂,硅胶柱色谱分离(DCM∶MeOH=98∶2),得淡黄色油状液体(6a)93 mg,产率60%;1H NMR (600 MHz, DMSO−d6) δ: 8.22 (d, J=8.4 Hz, 1 H), 7.77 (d, J=2.0 Hz, 1 H), 7.65 (dd, J=8.5, 2.1 Hz, 1 H), 7.38−7.31 (m, 3 H), 7.31−7.27 (m, 1 H), 7.26−7.19 (m, 6 H), 6.74 (t, J=5.5 Hz, 1 H), 5.88 (d, J=16.1 Hz, 1 H), 5.53 (d, J=10.5 Hz, 1 H), 5.05 (d, J=16.3 Hz, 1 H), 3.26−3.20 (m, 2 H), 2.87 (q, J=6.7 Hz, 2 H), 2.76−2.68 (m, 1 H), 2.55−2.51 (m, 1 H), 2.49−2.45 (m, 1 H), 2.33 (s, 3 H), 1.81−1.69 (m, 2 H), 1.36 (s, 9 H), 1.33−1.27 (m, 4 H), 1.23−1.15 (m, 7 H), 1.14−1.08 (m, 2 H), 0.89 (d, J=6.8 Hz, 3 H), 0.86−0.80 (m, 1 H), 0.47 (d, J=6.2 Hz, 3 H)。
(R)-(10-((3-(N-(1-(3-苄基-7-氯-4-氧代 -3, 4-二氢喹唑啉 -2-基)-2-甲基丙基)-4-甲基苯甲酰胺基)丙基)氨基)-9-氧代壬基)氨基甲酸叔丁酯(6b)的制备:
中间体6b的合成步骤参照中间体6a合成,得淡黄色油状液体(6b)82 mg,产率52%;1H NMR (600 MHz, DMSO−d6) δ: 8.22 (d, J=8.6 Hz, 1 H), 7.78 (d, J=2.0 Hz, 1 H), 7.65 (dd, J=8.6, 2.0 Hz, 1 H), 7.38−7.28 (m, 4 H), 7.26−7.18 (m, 6 H), 6.75 (t, J=5.5 Hz, 1 H), 5.87 (d, J=16.7 Hz, 1 H), 5.53 (d, J=10.5 Hz, 1 H), 5.04 (d, J=16.7 Hz, 1 H), 3.25−3.19 (m, 2 H), 2.87 (q, J=6.4 Hz, 2 H), 2.75−2.68 (m, 1 H), 2.54−2.51 (m, 1 H), 2.49−2.46 (m, 1 H), 2.33 (s, 3 H), 1.81−1.68 (m, 2 H), 1.36 (s, 9 H), 1.34−1.09 (m, 15 H), 0.89 (d, J=6.8 Hz, 3 H), 0.86−0.80 (m, 1 H), 0.47 (d, J=6.2 Hz, 3 H)。
N-((R)-1-(3-苄基-7-氯-4-氧代-3, 4-二氢喹唑啉-2-基)-2-甲基丙基)-N-(3-(9-(2-((S)-4-(4-氯苯基)-2, 3, 9-三甲基-6H-噻吩并[3, 2-f][1, 2, 4]三唑并[4, 3-a][1, 4]二氮杂卓-6-基)乙酰胺基)壬酰胺基)丙基)-4-甲基苯甲酰胺(7)的制备:
将化合物6a(93 mg,0.12 mmol)溶于DCM(3 ml)中,加入CF3COOH(1 ml),室温反应4 h后蒸干溶剂,得无色油状液体73 mg(0.11 mmol);将所得无色油状液体(73 mg,0.11 mmol)溶于DCM(15 ml)中,加入EDCI(31 mg,0.16 mmol)、HOBT(22 mg,0.16 mmol)和化合物JQ1-1(0.11 mmol,44 mg),室温下反应8 h,加水(300 ml)稀释,并用DCM(100 ml×3)萃取,收集有机层,使用无水硫酸钠干燥,蒸干溶剂,C18反相柱色谱分离(MeOH∶H2O=68∶32),得白色固体(7)(53 mg,0.05 mmol),两步收率46%;1H NMR (600 MHz, DMSO−d6) δ: 8.24 (d, J=8.6 Hz, 1 H), 8.19 (t, J=5.5 Hz, 1 H), 7.80 (d, J=1.8 Hz, 1 H), 7.67 (dd, J=8.6, 2.0 Hz, 1 H), 7.50−7.47 (m, 2 H), 7.46−7.42 (m, 2 H), 7.40−7.34 (m, 3 H), 7.34−7.29 (m, 1 H), 7.29−7.21 (m, 6 H), 5.89 (d, J=16.0 Hz, 1 H), 5.55 (d, J=10.6 Hz, 1 H), 5.06 (d, J=16.3 Hz, 1 H), 4.52 (dd, J=8.3, 6.1 Hz, 1 H), 3.30−3.22 (m, 3 H), 3.22−3.17 (m, 1 H), 3.16−3.05 (m, 2 H), 2.77−2.70 (m, 1 H), 2.61 (s, 3 H), 2.58−2.53 (m, 1 H), 2.51−2.47 (m, 1 H), 2.42 (s, 3 H), 2.34 (s, 3 H), 1.84−1.71 (m, 2 H), 1.63 (s, 3 H), 1.48−1.41 (m, 2 H), 1.35−1.25 (m, 7 H), 1.23−1.17 (m, 2 H), 1.17−1.11 (m, 2 H), 0.91 (d, J=6.8 Hz, 3 H), 0.88−0.82 (m, 1 H), 0.49 (d, J=6.2 Hz, 3 H);13C NMR (151 MHz, DMSO−d6) δ: 172.45, 172.02, 169.76, 163.41, 161.56, 155.70, 155.59, 150.23, 147.64, 139.94, 139.10, 137.20, 137.15, 135.68, 134.25, 132.71, 131.16, 130.56, 130.25, 130.03, 129.33, 129.11, 128.87, 128.46, 127.87, 127.12, 126.87, 126.33, 119.55, 59.44, 54.38, 45.63, 42.91, 38.91, 38.13, 36.17, 35.73, 30.69, 29.71, 29.23, 29.14, 28.81, 26.85, 25.57, 21.36, 19.96, 18.61, 14.48, 13.12, 11.74;HRMS(ESI) m/z calcd for C58H66Cl2N9O4S (M+H)+ 1054.433, found 1054.4367;熔程:134.7~139.1 ℃。
N-((R)-1-(3-苄基-7-氯-4-氧代-3, 4-二氢喹唑啉-2-基)-2-甲基丙基)-N-(3-(10-(2-((S)-4-(4-氯苯基)-2, 3, 9-三甲基-6H-噻吩并[3, 2-f][1, 2, 4]三唑并[4, 3-a][1, 4]二氮杂卓-6-基)乙酰胺基)癸酰胺基)丙基)-4-甲基苯甲酰胺(8)的制备:
化合物8的合成步骤参照化合物7合成,得白色固体47 mg,两步收率49%;1H NMR (600 MHz, DMSO−d6)δ: 8.24 (d, J=8.6 Hz, 1 H), 8.18 (t, J=5.6 Hz, 1 H), 7.80 (d, J=1.8 Hz, 1 H), 7.67 (dd, J=8.6, 2.0 Hz, 1 H), 7.50−7.47 (m, 2 H), 7.46−7.42 (m, 2 H), 7.40−7.34 (m, 3 H), 7.34−7.30 (m, 1 H), 7.29−7.21 (m, 6 H), 5.89 (d, J=16.0 Hz, 1 H), 5.55 (d, J=10.6 Hz, 1 H), 5.06 (d, J=16.5 Hz, 1 H), 4.52 (dd, J=8.3, 5.9 Hz, 1 H), 3.30−3.22 (m, 3 H), 3.21−3.16 (m, 1 H), 3.16−3.11 (m, 1 H), 3.11−3.04 (m, 1 H), 2.77−2.70 (m, 1 H), 2.61 (s, 3 H), 2.58−2.53 (m, 1 H), 2.51−2.47 (m, 1 H), 2.42 (s, 3 H), 2.34 (s, 3 H), 1.83−1.71 (m, 2 H), 1.63 (s, 3 H), 1.48−1.41 (m, 2 H), 1.35−1.25 (m, 8 H), 1.22−1.19 (m, 3 H), 1.16−1.09 (m, 2 H), 0.91 (d, J=6.8 Hz, 3 H), 0.89−0.82 (m, 1 H), 0.49 (d, J=6.2 Hz, 3 H);13C NMR (151 MHz, DMSO−d6) δ: 172.45, 172.02, 169.76, 163.40, 161.56, 155.71, 155.60, 150.23, 147.64, 139.93, 139.10, 137.19, 137.15, 135.69, 134.26, 132.72, 131.16, 130.56, 130.25, 130.04, 129.33, 129.11, 128.87, 128.45, 127.87, 127.12, 126.87, 126.34, 119.55, 59.45, 54.39, 45.63, 42.91, 38.93, 38.15, 36.17, 35.74, 30.69, 29.73, 29.40, 29.27, 29.22, 29.15, 28.81, 26.87, 25.57, 21.36, 19.95, 18.62, 14.48, 13.12, 11.74;HRMS(ESI) m/z calcd for C59H68Cl2N9O4S (M+H)+ 1068.4487, found 1068.4488;熔程:132.9~138.5 ℃。
-
将细胞以4×105个/孔的密度接种于6孔板中,培养24 h;根据实验需要,选取相应浓度的PAGE凝胶快速制备试剂盒,取40 μg总蛋白及5 μl蛋白marker上样,恒压120 V电泳90 min;然后恒流300 mA 转膜180 min转至PVDF膜上;转膜结束后,根据marker剪下目的条带,配制无蛋白快速封闭液(5×)封闭30 min;用TBST清洗残留封闭液,用5% BSA稀释相应一抗,4 ℃摇床孵育过夜;用TBST 洗膜3次,每次10min;使用荧光兔二抗,室温孵育1 h; 用TBST 洗膜3次,每次5 min;最后在Biorad ChemiDoc成像仪下拍照。
-
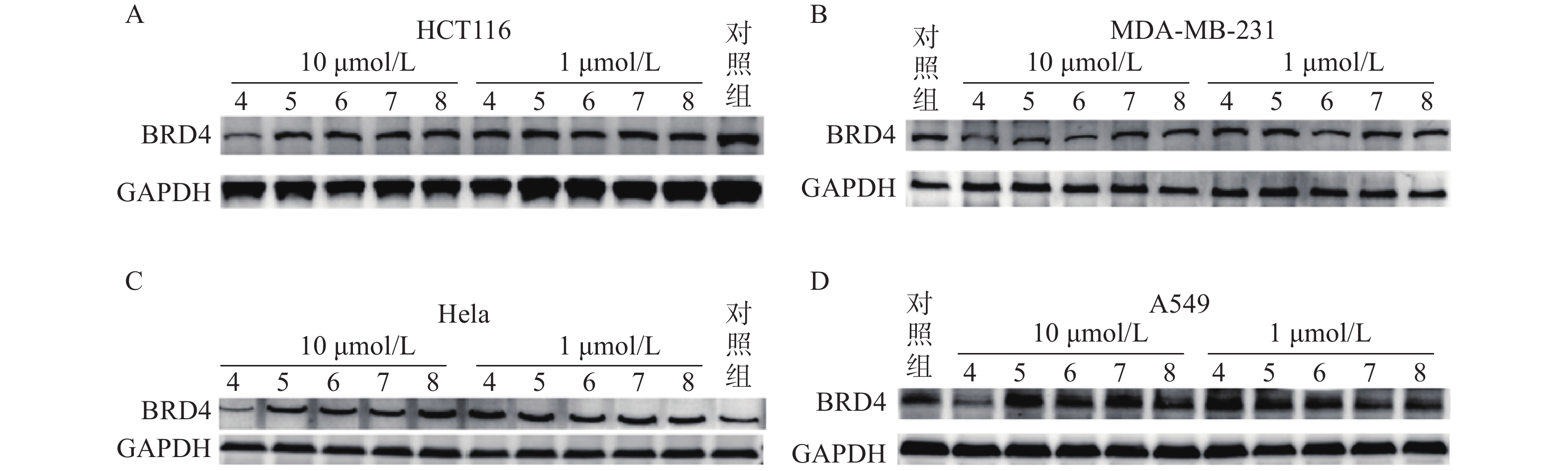
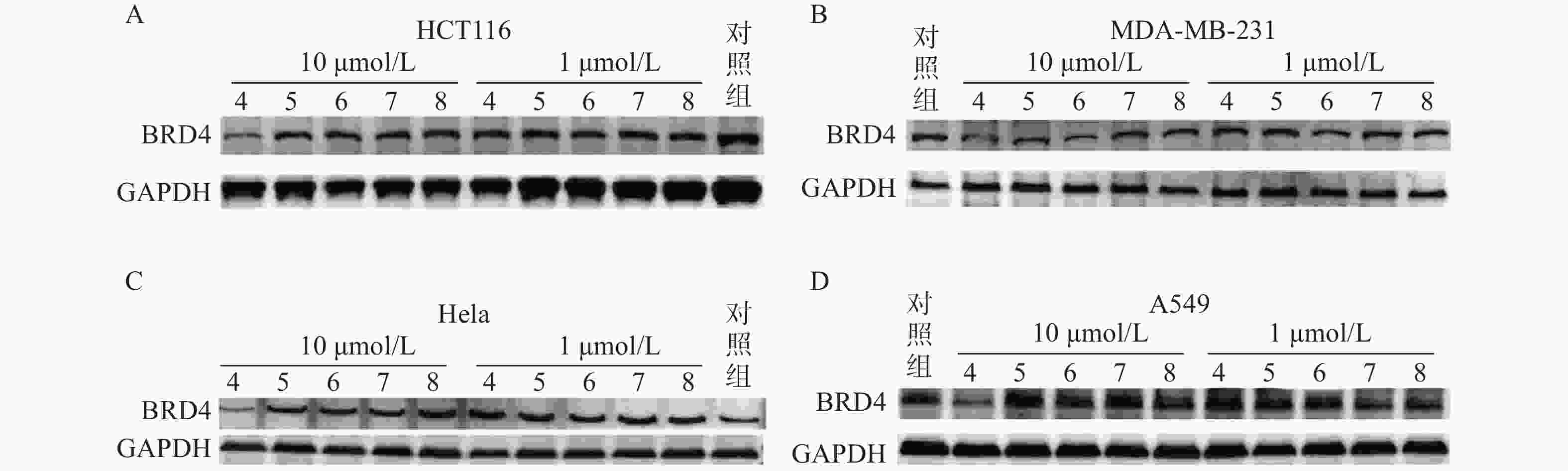
蛋白印迹法结果表明,Linker长度与化合物降解活性密切相关,Linker长度为4个碳原子长度时(化合物4),BRD4降解活性最强。化合物4在HCT116、MDA-MB-231、Hela和A549四种肿瘤细胞中均表现出下调BRD4蛋白水平的活性,而其他化合物在10 μmol/L与1 μmol/L浓度下对胞内BRD4蛋白水平均无明显影响(图6)。
-
基于ATTEC策略,我们设计并成功合成5个BRD4-ATTEC分子,所有目标化合物均经过了核磁和质谱结构确证,纯度均高于95%。在蛋白降解活性测试中,我们发现化合物4在10 μmol/L浓度下对4种肿瘤细胞均显示出诱导BRD4蛋白降解活性,表现出广谱有效的特征,为后续BRD4自噬降解的设计和结构优化提供了有效的先导化合物。本研究验证了ATTEC策略能有效诱导BRD4蛋白降解,并获得BRD4自噬降解剂先导化合物,拓展了靶向自噬降解的适用范围,后续的结构优化研究尚在进展之中。
Design, synthesis and degradation activity of BRD4-targeting ATTECs
doi: 10.12206/j.issn.2097-2024.202206050
- Received Date: 2022-06-13
- Rev Recd Date: 2022-10-10
- Available Online: 2023-07-14
- Publish Date: 2023-01-25
-
Key words:
- autophagosome-tethering compounds /
- BRD4 /
- LC3 /
- Ispinesib
Abstract:
| Citation: | ZHOU Luozhu, SHENG Chunquan. Design, synthesis and degradation activity of BRD4-targeting ATTECs[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(1): 18-25. doi: 10.12206/j.issn.2097-2024.202206050 |







 DownLoad:
DownLoad: