-
糖尿病是一种由遗传和环境因素共同引起的糖代谢紊乱综合征,根据文献报告,全世界大约有4.35亿人被诊断患有糖尿病,预计到2040年这一数字将超过6.42亿[1]。糖尿病在我国患病率逐渐增高,居世界首位,严重影响人民生活健康,且与炎症密切相关。目前以肠组织为靶器官的研究相对较少,尤其是肠道免疫系统和肠道微生物的研究[2-4]。中药材黄精,是中国传统的大宗药材[5],在民间又是一种使用面非常广的药食同源植物,《中国药典》2015年版记载为滇黄精P. kingianum Coll. et Hemsl.、黄精P. sibiricum Red.或多花黄精P. cyrtonema Hua的干燥根茎[6];黄精在抗氧化和抗衰老、降血糖、调节免疫力、调血脂、抑制老年痴呆和改善记忆力、抗肿瘤、扩张血管、抗菌和抗病毒等方面显示出潜在的药用价值[7-8]。本研究以高脂高糖饲料喂养和一次性注射链脲菌素复制糖尿病模型,比较高、中、低剂量黄精多糖对小鼠降血糖以及对肠道组织结构的改善作用,旨在为中药治疗糖尿病提供理论依据,为黄精药材的开发应用奠定基础。
-
ICR小鼠,雄性,体重18~22 g,购于重庆腾鑫生物技术有限责任公司,合格证号:SCXK(京)2019-0010,伦理编号:伦审〔2021〕2-077号,饲养于遵义医科大学动物中心。饲养环境为光照,通风良好,温度(23±2) ℃。试验开始前分笼饲养,适应环境7 d,整个试验过程自由进食和饮水。普通饲料(碳水化合物占65.08%,脂肪占11.85%,蛋白质占23.07%),高脂饲料的重量组成以 67%普通饲料为基料,添加10%猪油、20%蔗糖、2.5%胆固醇、0.5%胆酸钠,购自北京科奥协力饲料有限公司。
黄精多糖(批号:TZR200714,陕西天之润生物科技有限公司);链脲佐菌素(Sigma公司);甲醛(批号:2018080201,成都科隆化学品有限公司);柠檬酸(批号:2020052090)、柠檬酸钠(批号:2019072051)购自天津市致远化学试剂有限公司。
-
ME104E/02 电子天平[梅特勒-托利多仪器(上海)有限公司];DW-86L386 立式超低温保存箱(青岛海尔特种电器有限公司);GA-3型血糖仪及配套试纸(三诺生物传感股份有限公司);Multifuge X1R 冷冻离心机[赛默飞世尔科技(中国)有限公司];DM400B 显微镜、PELORIS型脱水机、YB-9LF型包埋机、RM 2235切片机(德国莱卡公司);202-1AB型电热恒温干燥箱、DK-98-IIA 电热恒温水浴锅(天津市泰斯特仪器有限公司)。
-
分别称取0.625、1.25、2.5 g黄精多糖溶于50ml 蒸馏水,配置成12.5、25、50 mg/ml的低、中和高剂量黄精多糖溶液,备用。
-
称取柠檬酸2.1g加入双蒸水至100 ml中配成柠檬酸溶液,称取柠檬酸钠2.94 g加入100 ml双蒸水配成柠檬酸钠溶液,将柠檬酸溶液和柠檬酸钠溶液按1∶1.1的比例混合,用柠檬酸钠溶液调节pH至4.4,配制浓度1%的链脲佐菌素,现配现用。
-
将小鼠饲养于小鼠笼中,温度(23±2) ℃,昼夜12 h条件下饲养。适应性喂养7 d,整个试验过程自由进食和饮水,之后开始建立2型糖尿病小鼠模型。除正常对照组外,其余小鼠均高糖高脂饲料喂养6周,一次性给予40 mg/kg 链脲佐菌素腹腔注射,继续喂养高糖高脂饲料;尾静脉取血检测小鼠血糖值,血糖值≥11.1mmol/L的小鼠则造模成功。将小鼠分为5组,每组10只,正常对照组:正常饲养;模型组:给予高脂高糖饲料并灌胃20ml/kg生理盐水;黄精多糖低、中、高剂量组:给予高脂高糖饲料并分别灌胃125、250、500 mg/kg[9]黄精多糖;每天早上九点灌胃,连续4周,每周测一次小鼠体重和血糖值,实验结束使用浓度为2%的戊巴比妥钠,以50 mg/kg腹腔注射麻醉后颈椎脱位法处死小鼠。
-
小鼠禁食不禁水,每周称取体重,观察小鼠生长情况。
-
每周进行小鼠尾静脉取血测血糖。
-
实验结束前收集各组小鼠的粪便于冻存管,立即存放至液氮中,委托诺禾致源生物科技有限公司进行16S rRNA 基因高通量测序工作。
-
依据文献所述方法[10-11],末次给药1 h后结束实验,解剖小鼠,取5 cm小肠用生理盐水轻轻冲洗,固定于10%甲醛溶液中,进行梯度酒精脱水、石蜡包埋、组织切片制备、HE染色及中性树胶封片,至光学显微镜下观察小肠组织各部分结构。
-
数据分析采用SPSS 22.0 统计软件,计量资料以均数±标准差(
$ \bar x \pm s $ )表示,多组比较用单因素方差分析,两两比较用 LSD-t 检验,P<0.05为差异有统计学意义,P<0.01为差异极显著。 -
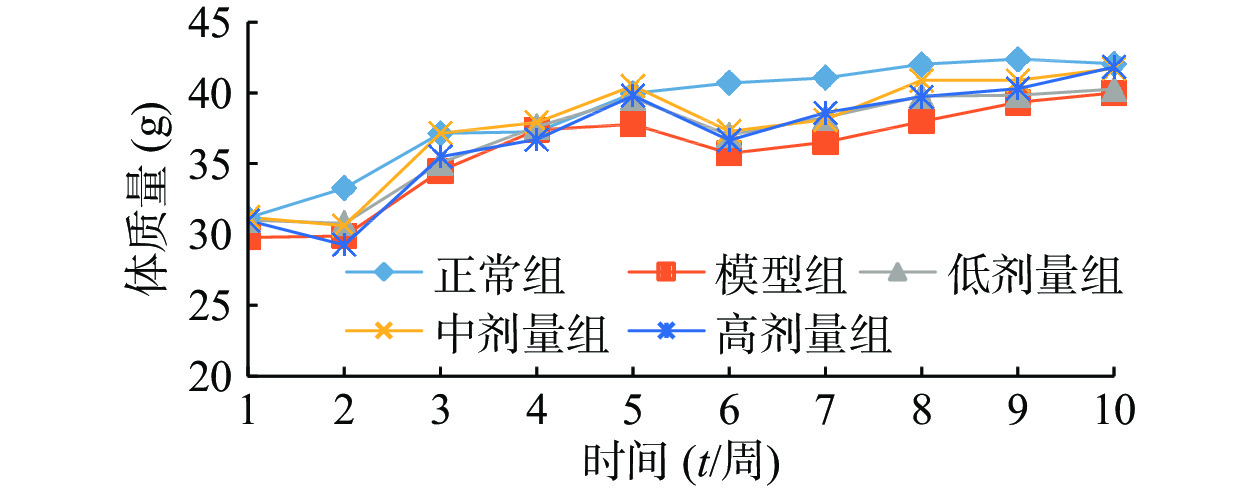
由图1可知,正常组小鼠体重整个实验中一直呈增长趋势,但随着饲养时间增长,增长幅度变小;其余组小鼠在给予高脂高糖饲料一周后体质量下降,随后呈上升趋势分别增长26%、29%、32%和36%,而正常组增长率约为20%,第6周一次性注射STZ后,小鼠体重均急速下降,模型组小鼠体重下降5.31%,黄精多糖高、中、低剂量组分别下降7.85%、7.91%和6.18%,如图2;模型组给予生理盐水灌胃,继续添加高脂高糖饲料饲养,体重呈缓慢增长趋势,给药组分别以高、中、低剂量黄精多糖给予灌胃,继续喂养高脂高糖饲料,各组小鼠体重分别增加14.24%、11.97%、8.78%,如图3。由此可见,糖尿病影响小鼠体重变化,灌胃黄精多糖溶液后,各组小鼠体重均有恢复,且高剂量效果明显。
-
实验过程中,给予充足的饮水和饲料,正常对照组小鼠活动正常,精神状态良好,色泽光亮,粪便呈浅墨绿色;模型组小鼠给予高脂高糖饲料第一周食欲下降,体重减轻,注射STZ后体重下降,表现不活泼、精神状态不好,竖毛,尿液呈深黄色,粪便呈黄棕色。黄精多糖组小鼠灌胃相应剂量后精神、饮食活动改善,尿液颜色变淡等,说明黄精多糖可以改善糖尿病小鼠的不良症状。结果表明,可能是STZ与高脂高糖饮食联合,破坏胰腺组织β细胞,并致胰岛素分泌发生不足,进而使体内糖代谢絮乱,发生胰岛素抵抗 [11-12]。
-
如表1所示,建模前血糖无显著差异(P>0.05),建模成功后的7 d和14 d时,与正常对照组相比,模型组小鼠血糖极显著升高(P<0.01);与模型组相比,黄精多糖各剂量组均无显著差异(P>0.05)。在21 d时,与正常对照组相比,模型组差异极显著(P<0.01) ;与模型组相比,黄精多糖低剂量和中剂量组无显著差异(P>0.05),高剂量组具有极显著性差异(P<0.01)。在28 d时,与正常对照组相比,模型组差异极显著(P<0.01);与模型组相比,黄精多糖各剂量组小鼠血糖值均降低,差异极显著(P<0.01)。
组别 血糖含量 建模前 建模后7 d 建模后14 d 建模后21 d 建模后28 d 正常组 5.67±0.33 5.95±0.24 5.95±0.21 5.97±0.22 5.99±0.24 模型组 5.6±0.43 15.85±0.28** 15.79±0.15** 15.59±0.16** 15.25±0.13** 黄精多糖低剂量组 5.76±0.36 15.88±0.40 15.45±0.18 14.75±0.11 13.77±0.11## 黄精多糖中剂量组 5.44±0.25 15.86±0.23 14.7±0.11 13.69±0.16 12.32±0.12## 黄精多糖高剂量组 5.57±0.29 15.85±0.21 13.81±0.10 12.3±0.16## 11.63±0.36## *P<0.05、**P<0.01,与正常对照组比较;#P<0.05、##P<0.01,与模型对照组比较 -
小鼠小肠病理组织切片HE染色结果显示,正常组小鼠小肠绒毛较长,结构完整,排列整齐;模型组小鼠小肠绒毛明显缩短,小肠绒毛断裂不完整,排列不紧密,与正常组比较差异显著;黄精多糖各剂量组小鼠小肠呈明显恢复状态,绒毛增长,排列较整齐,效果随黄精多糖剂量升高而表现明显,见图4。
-
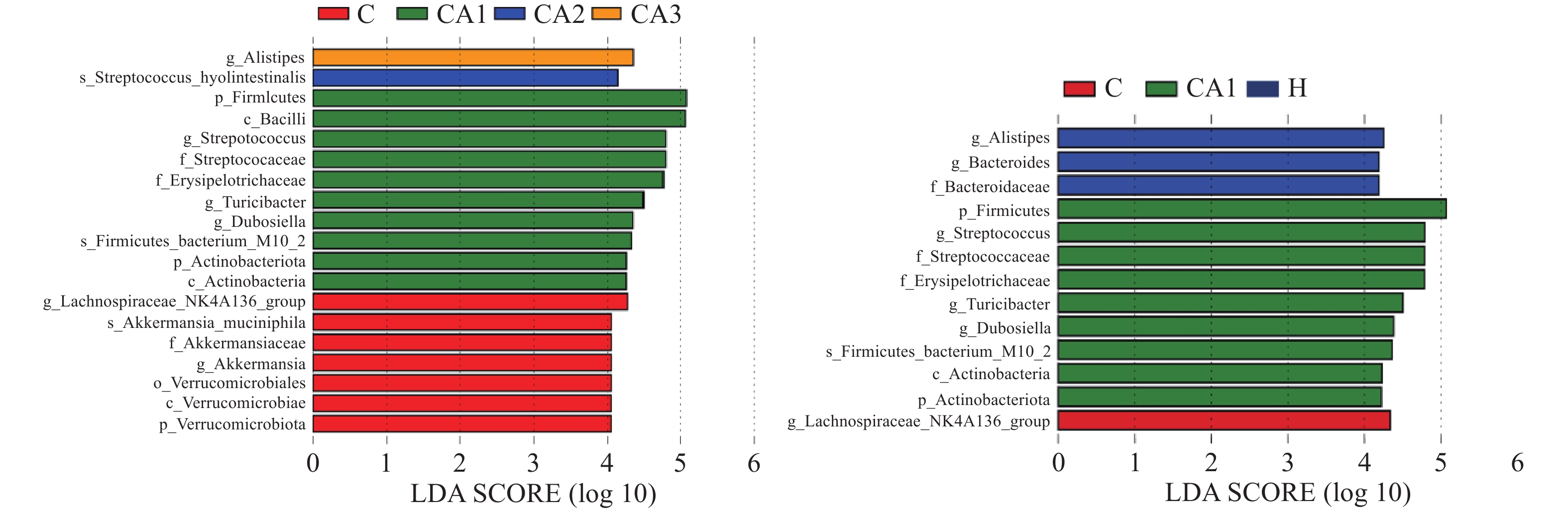
生物信息学分析结果表明,肠道微生物组成丰富,如图5,健康组和黄精多糖灌胃组中厚壁菌门和拟杆菌门丰富,而这两种菌是人类肠道内的优势有益菌,对人体健康起着重要作用,而糖尿病模型组中疣微菌门增多,这种菌可能会损害子代的消化和代谢能力,糖尿病造成了肠道菌群的紊乱。由此可推测,黄精多糖可以调节小鼠肠道微生物组成,增加有益菌丰富度,从而保护肠道。
-
糖尿病动物模型可通过高脂高糖饲料饲养和注射链脲佐菌素等多种方法制备,复制成功率高。本实验中发现,小鼠对高脂高糖饲料有一段过渡期,一开始饲喂高脂高糖饲料会影响小鼠食欲,导致体重下降,后体重迅速增长,小鼠肥胖,肥胖高脂的小鼠更容易患糖尿病。黄精多糖是中药材黄精的主要活性成分,已被证实具有治疗糖尿病的作用。黄精多糖可通过多种保护机制对糖尿病动物的各器官进行保护[13],但目前以肠组织为靶点的研究很少,且随着黄精属药用植物的广泛应用以及价格不断上涨,出现混用、滥用现象,黄精的临床规范应用受到一定程度的限制。黄精多糖可显著改善老龄鼠肠组织病理状态[14],本实验结果证实,黄精多糖可降低糖尿病小鼠血糖,恢复糖尿病小鼠代谢能力,改善其小肠绒毛排序结构,对肠道黏膜免疫功能可能存在一定作用,但目前未作进一步研究。肠道结构是肠道屏障功能的一部分,肠道微生物是影响代谢性疾病的关键因素,改变肠道微生物的群落结构,会引起代谢功能紊乱,肠道菌群与糖尿病存在密切关系,调节肠道菌群可以在一定程度上缓解患者症状,减轻黏膜炎症,有实验证明通过服用蜂胶、蒲公英、党参等,可调节小鼠肠道微生物,从而改善其肠道屏障功能[15-16]。肠道微生物区系组成的调节方法可能有助于糖尿病的治疗[17],本研究结果提示,糖尿病模型组中疣微菌门增多,可能会损害子代的消化和代谢能力,而健康组和黄精多糖灌胃组中厚壁菌门和拟杆菌门丰富,而这两种菌是人类肠道内的优势有益菌,且黄精多糖可调节肥胖小鼠的肠道菌群[18-19],这为进一步将肠道微生物和肠道免疫调控作为预防和治疗糖尿病的靶点提供理论基础,对临床治疗具有重要指导意义,为中药材黄精在糖尿病患者饮食辅助的治疗应用中提供重要的参考价值。
Study on hypoglycemic effect and intestinal effect of Polygonatum sibiricum polysaccharides in diabetic mice
doi: 10.12206/j.issn.2097-2024.202206057
- Received Date: 2022-06-14
- Rev Recd Date: 2022-09-22
- Available Online: 2022-11-28
- Publish Date: 2022-11-25
-
Key words:
- Polygonatum sibiricum polysaccharides /
- diabetes mellitus /
- hypoglycemia /
- mice /
- intestinal microorganisms
Abstract:
| Citation: | REN Qunli, ZHANG Xinqun, WANG Miao, LI Xiaolan, YAO Yanzi, RAN Yinghui, WANG Qian. Study on hypoglycemic effect and intestinal effect of Polygonatum sibiricum polysaccharides in diabetic mice[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(6): 510-514. doi: 10.12206/j.issn.2097-2024.202206057 |












 DownLoad:
DownLoad: