-
肿瘤恶病质是肿瘤患者较为常见且复杂的并发症,主要表现为厌食、严重的体力和体重下降、虚弱、贫血、水肿等症状。患者机体出现糖、脂、蛋白质代谢的异常反应变化,进行性的骨骼肌量减少,从而引起功能性障碍,对肿瘤患者的心理健康、社会功能产生极大的负面影响,严重影响肿瘤患者的生活质量[1-2]。研究表明50%~80%的肿瘤患者会发生不同程度的恶病质,影响患者放化疗的进行和生活质量,甚至超过20%患者死于恶病质[3]。目前,针对肿瘤恶病质的标准治疗方案主要是在营养支持基础上加用孕激素类药物,如醋酸甲羟孕酮。由于恶病质期单纯营养支持疗效欠佳[4],且孕激素类药物存在一些药物不良反应,因此,仍需更多治疗手段解决肿瘤恶病质带来的一系列临床医学问题。
相关研究显示,中药由于多成分、多靶点的作用,能对肿瘤恶病质进行整体干预,起到较好的疗效。中医药在改善恶病质患者体重、体力情况、食欲等方面可能有一定疗效[5],其中以参苓白术散为代表的益气健脾法在临床实践中取得了较好的疗效,得到专家共同的推荐,被用于减少肿瘤患者化疗和术后的不良反应,恢复胃肠道功能,提高生活质量[6],但其具体作用机制仍不明确[7]。参苓白术散首载于《太平惠民和剂局方》,由人参、茯苓、白术、白扁豆、山药、甘草、莲子、砂仁、薏苡仁、桔梗10味中药组成,方中以人参、白术、茯苓为君药,人参补益脏器,白术健脾燥湿,茯苓既善健脾补虚,又兼利水渗湿,三者共达益气健脾之效;另加山药补脾养胃,薏苡仁健脾渗湿,白扁豆补脾化湿,莲子补脾止泻,共为臣药,加强健脾益气、渗湿止泻之功。砂仁为佐,醒脾和胃,行气化滞,桔梗和甘草为佐使,桔梗以宣肺利气,通调水道,引气上升,甘草则健脾和中,调和诸药。诸药合用,共奏健脾益气、和胃渗湿之效。脾胃健运,湿滞得化,水谷精微生化恢复,使患者衰弱的机体可以逐步好转。因此,本研究借助网络药理学对参苓白术散治疗肿瘤恶病质的有效成分及靶点进行预测,探讨其作用机制。
-
检索中药系统药理学数据库和分析平台[8](TCMSP;https://old.tcmsp-e.com/tcmsp.php)获取人参、茯苓、白术、山药、白扁豆、薏苡仁、砂仁、桔梗、甘草9味中药的化学成分,根据口服利用度(OB)≥30%且类药性(DL)≥ 0.18进行活性成分初步筛选,以获得活性化合物及其作用的蛋白质靶点。检索BATMAN-TCM数据库(http://bionet.ncpsb.org.cn/batman-tcm/)[9],设置Score cutoff ≥20,P≤0.05,获取莲子的化学成分和靶点信息。筛选结束后,统一在Uniprot蛋白质数据库(https://www.uniprot.org)将化合物作用的蛋白质靶点进行规范。
-
以“Cancer cachexia”为关键词,在OMIM(https://omim.org/)、Genecards(https://www.genecards.org/)、Disgenet(https://www.disgenet.org/)三个基因数据库寻找肿瘤恶病质的潜在靶点(检索时间为2021年8月27日),在DRUGBANK数据库(https://go.drugbank.com/)中检索改善肿瘤恶病质临床一线用药作用靶点进行补充[10]。合并检索的4个数据库靶点后,删除重复值,并使用Uniprot蛋白质数据库进行规范。然后将肿瘤恶病质相关靶点与参苓白术散处方活性成分潜在作用靶点导入Venny 2.1.0,筛选出共同靶点,生成韦恩图。
-
通过Cytoscape3.7.2软件来构建“药物-活性成分-靶点”网络,其中用节点表示成分或靶点,用边表示两者之间的关系;采用Cytoscape3.7.2软件内置的Network Analyzer分析工具分析网络特征参数,包括连接度、介度及紧密度等,研究参苓白术散中较为重要的成分和靶点及它们之间的关系。
-
为进一步研究参苓白术散治疗肿瘤恶病质靶点蛋白在系统水平的作用,将筛选出来的共同靶点导入STRINGv11数据库[11](https://string-db.org),选择物种为“Homo sapiens”,使用默认设置,以获得PPI网络关系,通过CytoScape3.7.2软件制作PPI网络图,并使用Network Analyzer分析工具进行网络拓扑属性分析。
-
采用整合多个权威数据库的Metascape平台(http://metascape.org/gp/index.html)[12],将筛选后的靶点导入Metascape 平台进行GO及KEGG分析,保存结果并通过R软件进行可视化。
-
通过pubchem(https://pubchem.ncbi.nlm.nih.gov/)数据库下载“药物-活性成分-治疗靶点”网络图中度值排前10的化学成分结构(.sdf),PDB(http://www.rcsb.org/)数据库下载PPI中度值排前10的靶点的结构。用Discovery Studio软件分别对靶点结构进行去除结晶水、加氢等处理,将其进行对接,以libdockscore值作为对接程度的评判标准。
-
从TCMSP数据库初步获取参苓白术散中人参、茯苓、白术、山药、白扁豆、薏苡仁、砂仁、桔梗、甘草成分949个,通过ADME参数筛选后获得179种活性成分,从BATMAN-TCM数据库筛选获取莲子17种活性成分。将靶点预测结果合并重复值,共获得544个靶点。
-
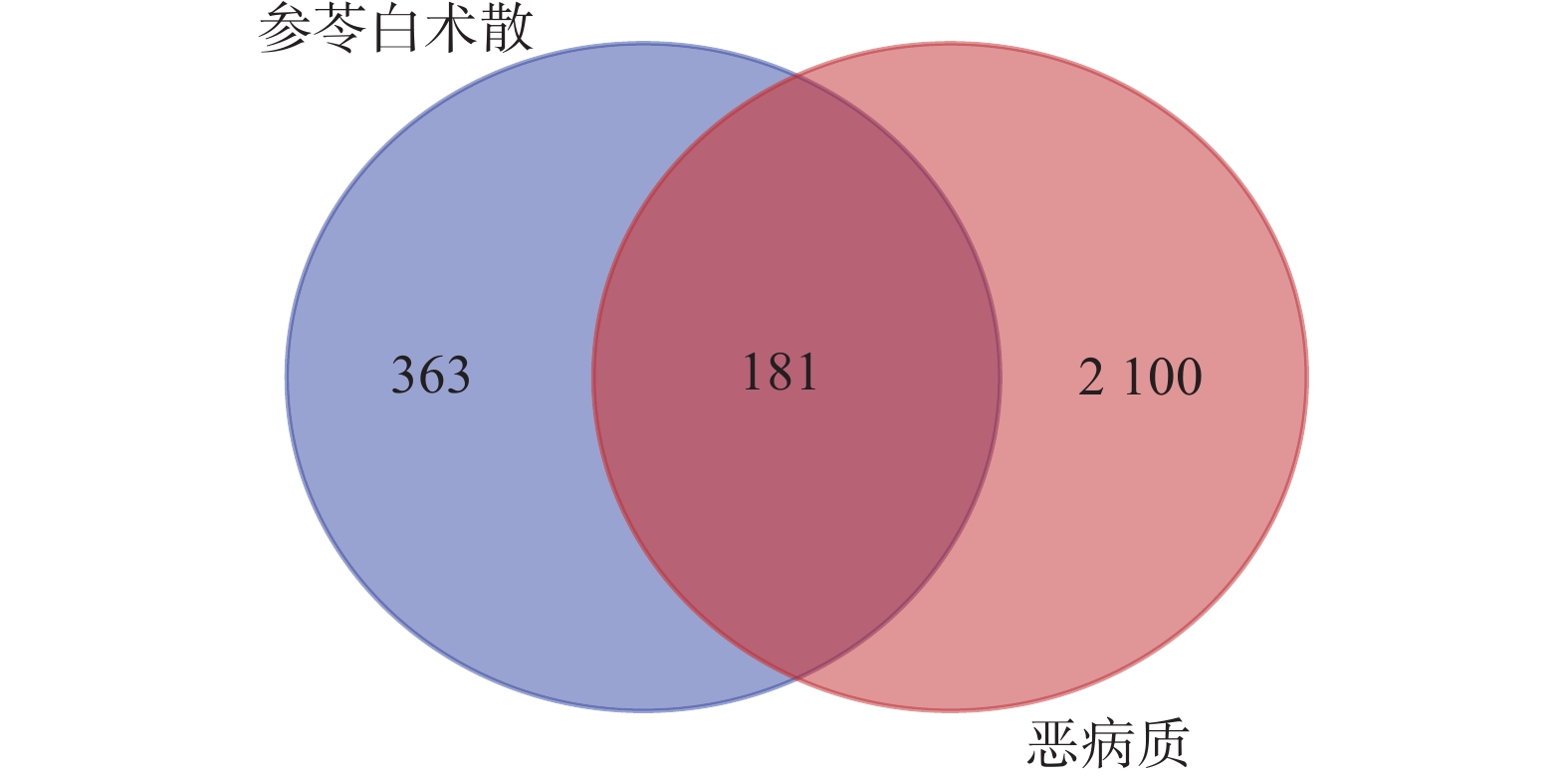
检索Genecards、OMIM、Disgenet、DRUGBANK数据库,获得肿瘤恶病质相关靶点个数分别为1 792、499、110、9,筛选并去重后得到2 281个靶点。药物与疾病靶点交集得到181个共有靶点(图1),作为参苓白术散治疗肿瘤恶病质的潜在靶点。
-
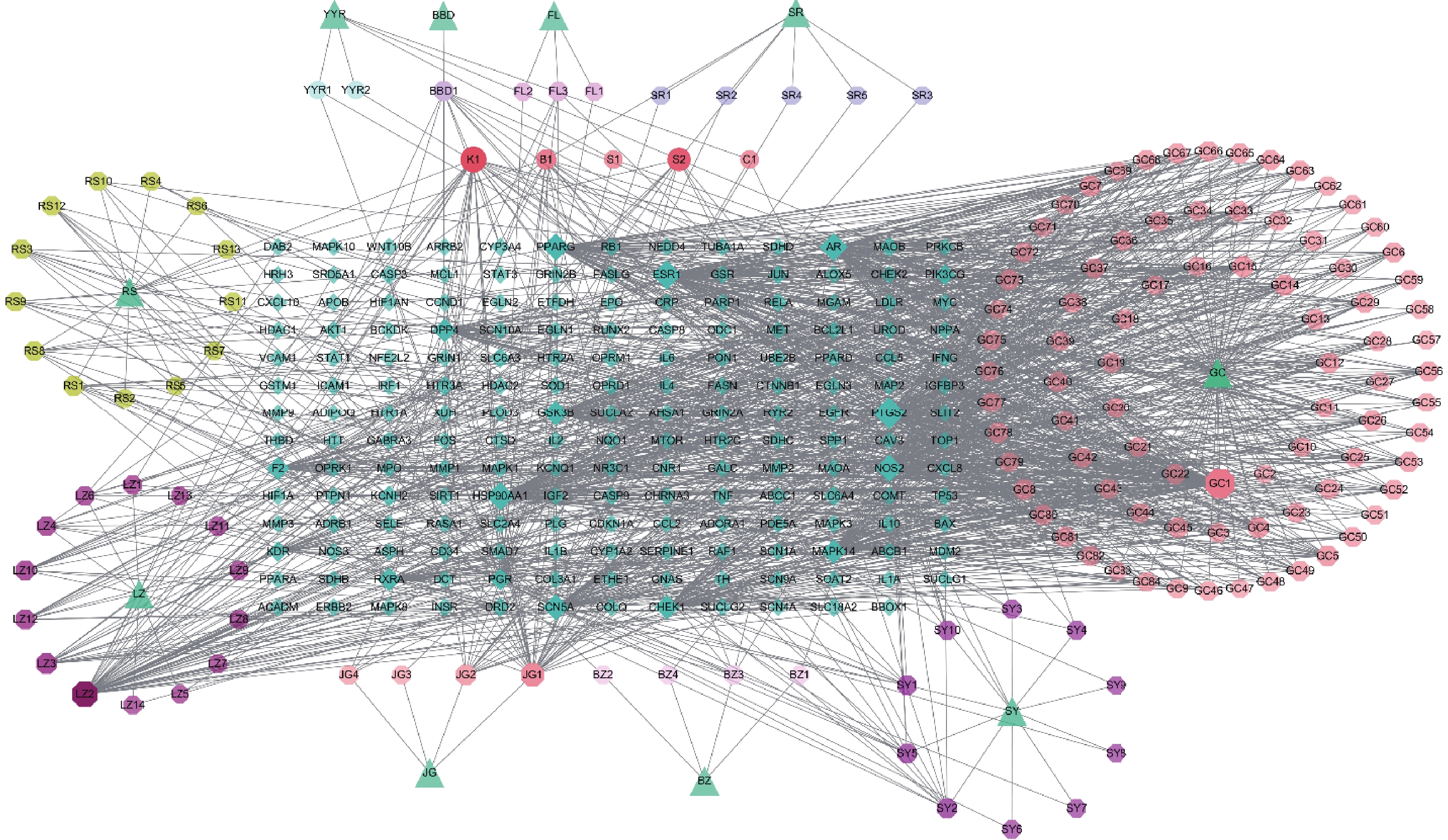
运用CytoScape 3.7.2构建参苓白术散的“药物-活性成分-靶点”网络图,见图2。图中共有336个节点(10个中药节点,145个活性成分节点,181个潜在靶点节点),1 385条边。通过Cytoscape 3.7.2内置的Network Analyzer功能分析参苓白术散治疗肿瘤恶病质的网络拓扑学参数,筛选网络中关键节点,排前10的活性成分节点拓扑参数见表1,排前10的靶点节点拓扑参数见表2。
编号 Mol ID 活性成分 连接度 介度 紧密度 来源 GC1 MOL000098 quercetin 81 0.271 114 0.436 767 甘草 K1 MOL000422 kaempferol 58 0.053 66 0.389 082 人参、甘草 LZ2 MOL000663 pyrolignous acid 55 0.274 946 0.394 582 莲子 S2 MOL000449 stigmasterol 40 0.031 101 0.381 115 人参、砂仁、山药、薏苡仁 JG1 MOL000006 luteolin 37 0.058 771 0.380 25 桔梗 B1 MOL000358 beta-sitosterol 34 0.039 925 0.391 813 人参、砂仁 GC5 MOL000392 formononetin 19 0.009 376 0.374 302 甘草 GC14 MOL003896 7-methoxy-2-methyl isoflavone 19 0.010 022 0.378 531 甘草 GC7 MOL000497 licochalcone a 17 0.013 409 0.375 14 甘草 JG2 MOL001689 acacetin 16 0.015 687 0.359 828 桔梗 靶点 连接度 介度 紧密度 PTGS2 111 0.155 87 0.448 461 ESR1 82 0.063 534 0.355 249 HSP90AA1 76 0.054 254 0.406 061 AR 74 0.133 102 0.453 315 PPARG 69 0.028 901 0.389 988 NOS2 69 0.014 526 0.350 785 SCN5A 61 0.140 382 0.442 536 GSK3B 57 0.006 328 0.319 962 CHEK1 47 0.004 578 0.311 628 MAPK14 47 0.003 912 0.309 898 -
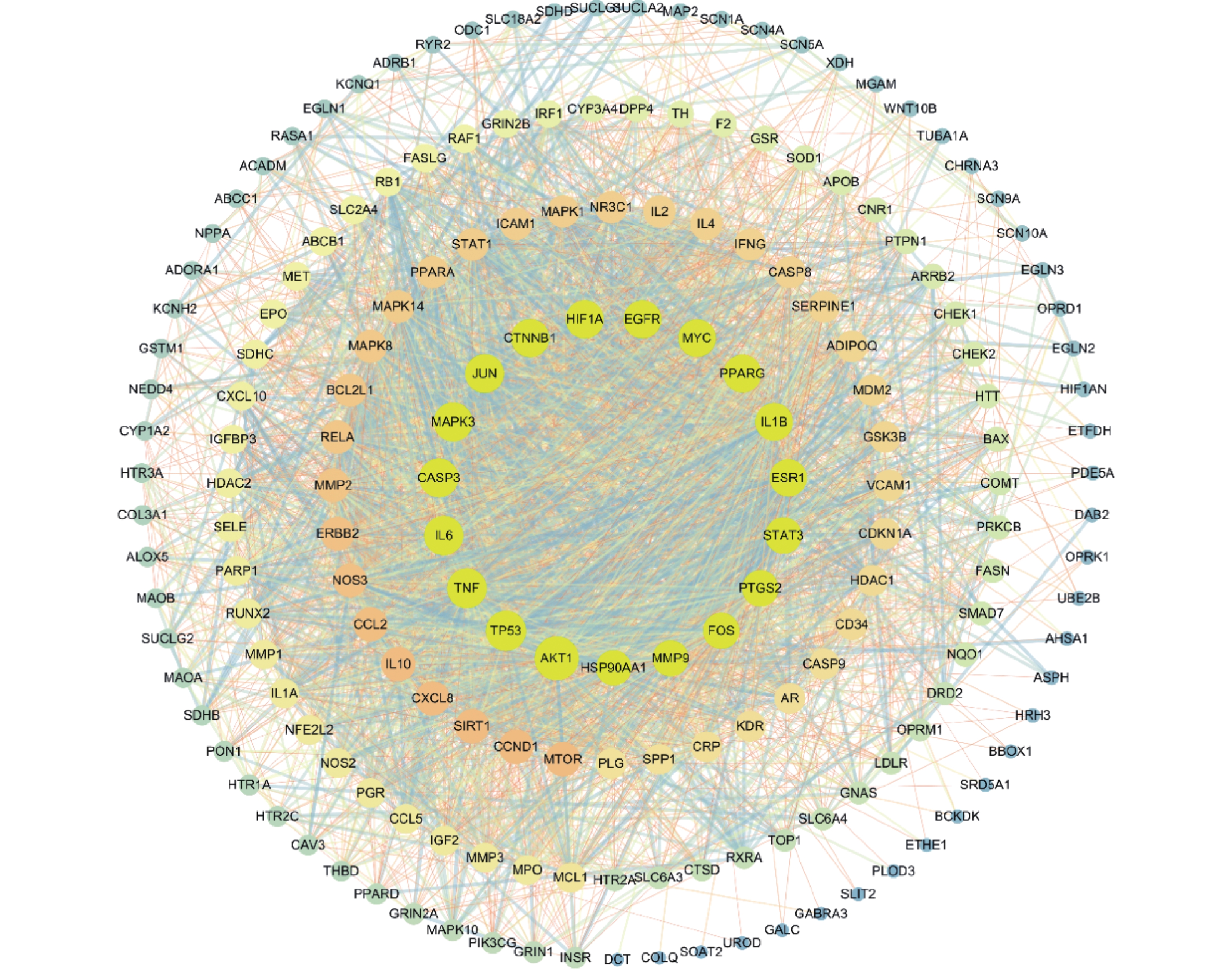
将在STRING构建的蛋白互作网络导入Cytoscape 3.7.2进行可视化,见图3。图中42个治疗靶点存在362条相互作用。对网络中的节点进行拓扑分析得出节点的平均度中心性(degree centrality,DC)为36.143 7,平均中介中心性(betweenness centrality,BC)为0.000 57,平均紧密中心性(closeness centrality,CC)为0.507 1,其中以上3个参数都大于平均值的节点有30个,其排名前10的参数详见表3。
靶点 连接度 介度 紧密度 AKT1 128 0.086 792 0.765 957 TP53 105 0.035 549 0.674 157 TNF 102 0.022 326 0.671 642 IL-6 100 0.020 958 0.669 145 MAPK3 98 0.017 936 0.664 207 CASP3 98 0.024 522 0.661 765 JUN 96 0.017 324 0.654 545 CTNNB1 93 0.033 266 0.654 545 HIF1A 93 0.031 81 0.640 569 EGFR 92 0.019 349 0.652 174 -
应用Metascape数据平台对参苓白术散治疗肿瘤恶病质的相关靶点进行信号通路分析,选择富集计数和-lgP 排名前20的与肿瘤恶病质相关结果,借助R语言将其进行可视化,结果见图4A。共同靶点主要参与的生物学过程,包括对有机环状化合物的细胞反应、对脂多糖的反应、对无机物质的反应、对生长因子的反应、凋亡信号通路、蛋白质磷酸化的正调控等;细胞的组成主要包括轴突、膜筏细胞质核周区;分子功能方面涉及蛋白质结构域特异性结合、RNA特异性聚合酶IIDNA-结合转录因子结合、氧化还原酶活性、激酶结合、磷酸酶结合、蛋白激酶活性等。
KEGG富集显示,参与的相关通路主要有癌症的途径、糖尿病并发症中的AGE-RAGE信号通路、癌症中的蛋白多糖、HIF-1信号通路、多巴胺能突触、5-羟色胺能突触、癌症中的转录失调、雌激素信号通路等,结果见图4B。
-
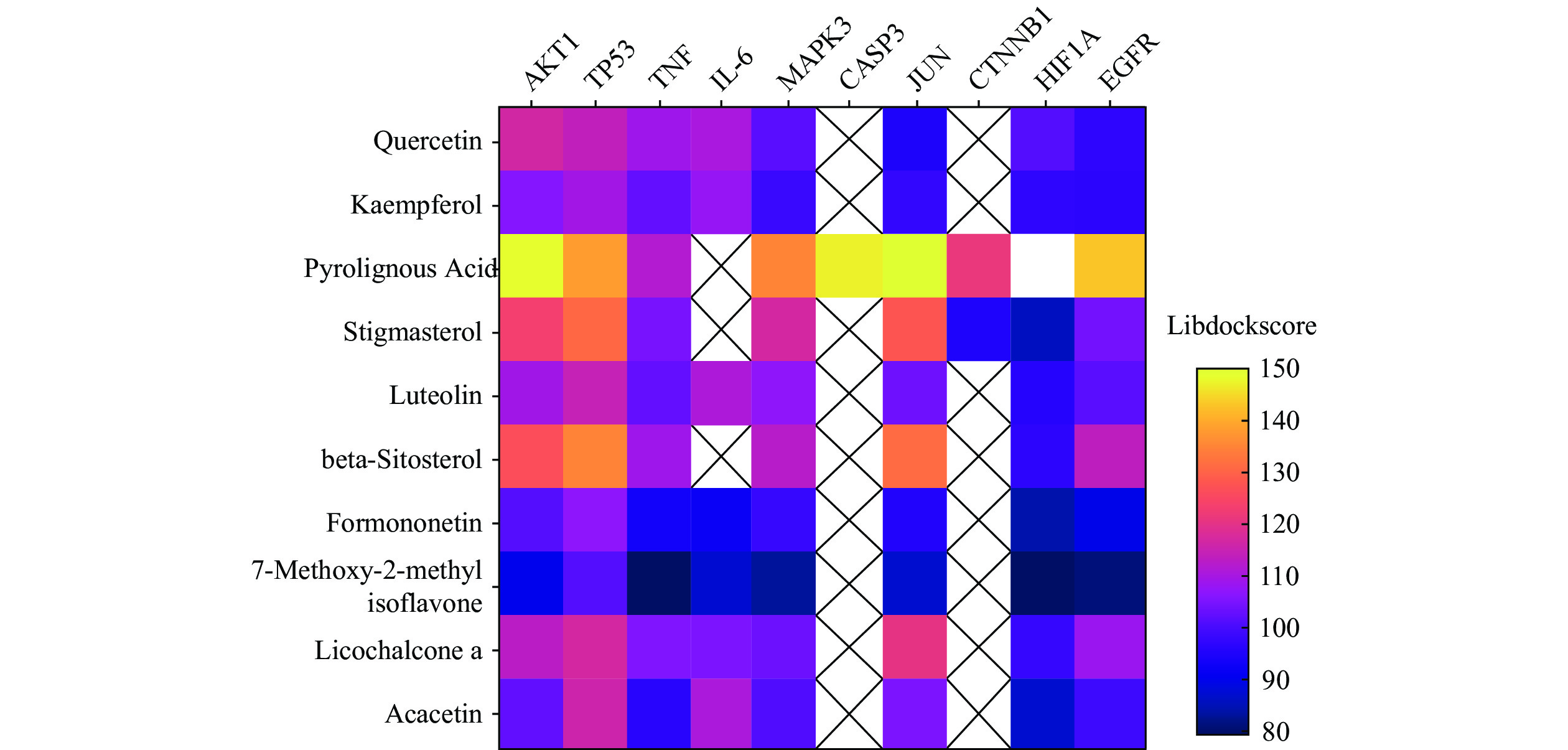
运用Discovery Studio软件对靶点和化学成分进行分子对接,进一步说明靶点与化合物之间的结合性,其结合度越高,得分越高,对接结果见图5,图中横坐标代表靶蛋白,纵轴为参苓白术散活性成分,图中颜色越紫代表结合能越低,颜色越黄代表结合能越高,每个靶点均有活性成分与其对接,最低libdockscore>79。木蜡酸、豆甾醇、β谷甾醇与靶点对接值较高,ATK1、TP53、MAPK3、CASP3、JUN、EGFR等靶点与活性成分均有较高的对接值,选择部分活性成分绘制对接模式图见图6,可见活性成分与靶点之间形成氢键,结合稳定。
-
肿瘤相关恶病质会使患者虚弱,难以接受进一步的治疗,且几乎没有有效的治疗方法[13]。恶病质不仅发生在癌症晚期,许多早期癌症也可能发生恶病质。恶病质降低了患者机体对药物、射线等抗肿瘤治疗反应的敏感性与耐受性,直接影响肿瘤治疗效果,且会增加并发症,缩短癌症患者的生存期[14]。恶病质的特征是全身炎症和多器官消耗紊乱,伴随着体重减轻,特别是脂肪组织和骨骼肌的质量减少[15-16]。目前尚不清楚恶病质的发病机制,多认为是由于厌食症、全身系统性炎症、能量代谢异常和信号通路等相互作用造成的[17-19]。中医药辅助治疗不仅可以针对肿瘤进行治疗,同时可改善晚期恶性肿瘤患者体倦乏力、食少纳呆等脾虚现象,以及放、化疗引起的胃肠道不适等不良反应。郭东霖等[20]对中药汤剂治疗肿瘤恶病质疗效进行meta分析,结果显示,中药汤剂对肿瘤恶病质的治疗有一定的疗效,且不良反应较小。
以益气健脾为治法的参苓白术散联合放、化疗治疗肺癌、胃癌、结直肠癌,不仅能提高对肿瘤的治疗效果,还能增强机体免疫力,改善化疗后的毒副作用,提高患者生活质量[21-24]。本研究通过网络药理学初步筛选出参苓白术散治疗肿瘤恶病质的主要活性成分为槲皮素、山奈酚、木蜡酸、豆甾醇、木犀草素、β谷甾醇等。相关研究表明,槲皮素可能通过影响脂肪的消化吸收、甘油磷脂代谢、SCAP-SREBP2-LDLr信号通路等改善脂质代谢[25],同时可以直接或间接作用于脂多糖,抑制脂多糖诱导产生TNF-α,进而抑制机体炎症[26]。山柰酚可增强脂肪分解并减少高级脂肪酸及受损葡萄糖的摄取、糖原合成、AMPK活性以及葡萄糖转运蛋白4的表达[27],可下调炎症通路蛋白的表达[28]。豆甾醇通过下调NF-κB P65的表达来抑制促炎介质(TNF-α,IL-6,IL-1β等)的表达,增加抗炎细胞因子(IL-10)的表达[29]。木犀草素能借助AMP活化蛋白激酶1信号传导,降低THP-1细胞衍生的巨噬细胞中总胆固醇水平,同时抑制巨噬细胞趋化因子和炎性细胞因子表达[30]。研究表明,槲皮素、山奈酚、豆甾醇三种成分作为抗癌候选药,可通过调节多种生物途径,以诱导和促进肿瘤细胞凋亡、抑制肿瘤血管新生、防止肿瘤细胞增殖的方式抑制肿瘤的发展[31-34]。
KEGG通路富集分析发现,参苓白术散治疗肿瘤恶病质作用的通路可分为3个方面。一是抑制炎症,改善食欲。肿瘤生长增加了血浆色氨酸浓度,大脑中色氨酸浓度增加可引起下丘脑腹内侧核5-羟色胺能神经元活性增强,从而增强下丘脑厌食神经元的活性[35]。IL-1β、TNF-α、IL-6等细胞因子在厌食症发病过程中起到重要作用,促进了厌食症的发生和发展。参苓白术散可能通过作用于血清学突触、多巴胺能突触、HIF-1信号通路、雌激素信号等通路,抑制炎症因子产生,降低5-羟色胺能神经元活性,从而促进食欲。二是作用于代谢相关通路,纠正肿瘤恶病质机体能量恶性消耗、代谢紊乱的状态。如糖尿病并发症中的AGE-RAGE信号通路、脂肪细胞脂解调节、PPAR信号通路等与脂肪代谢有关的通路。糖化应激通过AGEs与RAGE结合,诱导炎症和氧化应激,研究表明,AGE-RAGE可介导高胆固醇血症引起的动脉粥样硬化等多种病变,并且sRAGE水平与HDL呈正相关[36]。PPAR通路中,PPAR家族调控参与细胞增殖、分化以及炎症和免疫相关蛋白的表达,同时也是参与调控脂肪细胞分化和葡萄糖稳态的关键细胞因子[37]。推测参苓白术散通过这些通路修复肿瘤恶病质所致的高甘油三酯和低胆固醇症等。HIF-1 信号通路是治疗肿瘤具有潜在价值的通路,抑制PIK3/Akt-HIF-1α途径能够显著减少多种细胞的糖酵解[38],而糖酵解正是恶性肿瘤细胞获取能量的主要方式,也是造成糖代谢紊乱的原因。HIF-1α蛋白升高诱导瘦素信号通路,从而增强白色脂肪组织储存的脂肪分解[39]。三是直接作用于肿瘤相关的通路(癌症中的通路、癌症中的蛋白多糖、Wnt信号通路),抑制肿瘤活性。刘羽茜等[40]通过网络药理学研究发现,参苓白术散作用涉及到肺癌的多种信号通路,其通过调节相关信号通路从而抑制炎症因子的活化,缓解机体过激的免疫反应,发挥对肺癌的治疗作用。田婷婷等[41]运用网络药理学探索参苓白术散治疗结直肠癌作用机制,发现其可能通过调节炎性反应、优化肠道菌群结构发挥治疗作用。参苓白术散基础方四君子汤治疗恶病质的作用[42]与本研究中参苓白术散调节代谢和抑制肿瘤的作用基本一致,参苓白术散在四君子汤基础上添加的药物,增加了抗击炎症和增强食欲的作用。
研究结果表明,参苓白术散同一活性成分可调控不同靶点,而同一靶点可干预不同的生物学过程及信号通路。分子对接结果显示,排名前10的活性成分和靶点之间存在较好的结合活性,具有良好的相互作用,体现了参苓白术散多通路、多靶点联合作用的特点,为参苓白术散临床运用提供了科学依据,也为挖掘参苓白术散潜在作用机制提供新方向。本研究主要是以生物信息学与海量数据计算的结果为基础,后续将在此基础上进一步进行动物或细胞实验验证,进而明晰参苓白术散的主要调控靶点。
Molecular mechanism of Shenling Baizhu powder in treatment of cancer cachexia based on network pharmacology
doi: 10.12206/j.issn.2097-2024.202208115
- Received Date: 2022-08-31
- Rev Recd Date: 2023-09-22
- Available Online: 2024-02-26
-
Key words:
- Shenling Baizhu powder /
- network pharmacology /
- cancer cachexia /
- molecular docking
Abstract:
| Citation: | KE Gang, DONG Qingke, XIAO Shirong, GONG Qian, LI Rong, WANG Daijie. Molecular mechanism of Shenling Baizhu powder in treatment of cancer cachexia based on network pharmacology[J]. Journal of Pharmaceutical Practice and Service. doi: 10.12206/j.issn.2097-2024.202208115 |










 DownLoad:
DownLoad: