-
胆汁淤积是以胆汁生成障碍和胆汁排泄受阻为特征的病理性疾病,是一种临床常见的综合征[1]。胆汁淤积由胆管阻塞、肝细胞分泌缺陷等多种原因引起,肝脏和体循环中胆汁酸、胆固醇及胆红素等成分过多堆积,从而造成机体及肝细胞的损伤[2]。由于胆汁淤积的病因和损伤机制复杂,严重影响了其相关新药的开发,因此研究胆汁淤积性肝损伤的病因,寻找有效的治疗药至关重要。目前,经FDA批准用于临床治疗胆汁淤积的药物只有熊去氧胆酸(UDCA)和奥贝胆酸(OCA)[3-4]。我国中草药资源丰富,为中医药的开发和研究提供了物质基础。此中药组合物是一种用于治疗肝损伤的中药民间验方,主要由醋鳖甲、佛手、芍药等13味组成。本课题组在四氯化碳诱导的肝纤维化动物模型中证实了此中药组合物具有保肝和治疗肝纤维化等慢性肝损伤的作用[5-7],其作用温和、无毒、无副作用,可以明显降低慢性肝损伤小鼠血清谷丙转氨酶(ALT)、谷草转氨酶(AST)的水平[6-8],并且该作用效果可能与基质金属蛋白酶-2(MMP-2)和CD147的表达增加、基质金属蛋白酶抑制因子-2(TIMP-2)的表达降低有关[8-9],在肝病防治领域应用前景广泛。但到目前为止,尚未见此中药组合物从改善胆汁淤积角度进行肝病防治的研究,更未见其在α-萘异硫氰酸酯(ANIT)诱导的小鼠胆汁淤积型肝损伤模型中的相关报道。为此,本课题组采用ANIT构建小鼠胆汁淤积性肝损伤模型,研究此中药组合物在防治胆汁淤积肝病方面的研究,探索此中药组合物在防治肝病领域的理论与实践,进而为研究其在防治慢性肝病的分子机制及其临床应用方面提供理论基础。
-
8周龄SPF级昆明小鼠[动物伦理号:DWLL202003225,许可证号:SCXK(豫)2021-0015,伦理负责单位:河南中医药大学]60只,雌雄各半,体质量18~22 g,由郑州大学动物实验中心提供,饲养于河南中医药大学实验动物中心;中药组合物(各组分均为颗粒剂,河南中医药大学第一附属医院中成药房);熊去氧胆酸(UDCA)胶囊(商品名:优思弗,批号:G62222,规格:250 mg/粒,河南中医药大学第一附属医院);水合氯醛(北京化工厂);α-ANIT(批号:K2325417,上海阿拉丁生化科技股份有限公司);谷丙转氨酶(ALT)、谷草转氨酶(AST)、碱性磷酸酶(ALP)、总胆汁酸(TBA)、总胆红素(TBIL)、γ-谷氨酰转肽酶(γ-GT)、超氧化物歧化酶(SOD)、丙二醛(MDA)、谷胱甘肽过氧化物酶(GSH-PX)试剂盒(南京建成生物工程研究所);全自动酶标仪[美国伯乐(BIO-RAD)];4℃冷藏冰箱(GENERAL公司);−80℃冰箱[赛默飞世尔科技(中国)有限公司];正置显微镜[奥林巴斯集团公司(BX53)]。
-
取8周龄SPF级昆明小鼠,饲养于通风、安静的环境中,定时喂食,饮水不限,进行一周的适应性饲养后随机分成6组,每组10只,分别为空白对照组(灌胃生理盐水),模型对照组(灌胃生理盐水),中药组合物低(灌胃剂量5.25 g/kg)、中(灌胃剂量11.25 g/kg)、高(灌胃剂量20.25 g/kg)剂量组,阳性对照组(熊去氧胆酸,UDCA,0.1 g/kg)。将中药组合物颗粒与实验动物中心提供的50 ml温热纯水溶解(水温37℃),UDCA与25 ml温热纯水溶解(水温37℃),再使用超声多普勒仪器,使其充分溶解,根据体表面积法换算人与小鼠的给药剂量[10],每日1次,灌胃7 d,第5天,除对照组外其他各组灌胃ANIT(65 mg/kg)造模,对照组灌胃给予等量橄榄油。造模48 h后,各组小鼠末次给药后30 min,收集小鼠血液和肝组织样本,进行各项指标的检测。每日定时观察小鼠进食、体质量、饮水、活动、精神、皮毛、大小便等情况。
-
收集小鼠血液和肝组织样本用于各项指标分析。经腹腔注射10%水合氯醛(0.03 ml/10 g)麻醉后,摘眼球取血,放EP管中,于4℃冰箱内静置2 h左右,
3500 r/min离心20 min,取上清液,按照试剂盒说明书检测ALT、AST、ALP、TBA、γ-GT、TBIL、MDA、SOD、GSH-PX含量。打开腹腔取出肝脏,先用磷酸盐缓冲液(PBS)平衡盐溶液将上面的血液等杂质小心洗干净,用滤纸吸干,然后用刀片切下一部分左叶放入10%中性福尔马林溶液中固定,做常规病理切片,进行苏木精-伊红(HE)染色,观察小鼠肝脏病理变化。剩余肝组织置于冻存管中,然后速放入液氮中,再转−80℃冰箱冻存。 -
①石蜡切片脱蜡至水:依次将切片放入二甲苯Ⅰ、二甲苯Ⅱ各20 min,无水乙醇Ⅰ、无水乙醇Ⅱ、75%酒精各5 min,然后用自来水冲洗。②苏木素染色:切片入苏木素染液3~5 min,然后用自来水冲洗,再用分化液分化,然后用自来水冲洗,再用返蓝液返蓝,再进行流水冲洗。③伊红染色:切片依次入85%、95%的酒精脱水5 min,放入伊红中染色5 min。 ④脱水封片:切片依次放入无水乙醇Ⅰ、无水乙醇Ⅱ 、无水乙醇Ⅲ、二甲苯Ⅰ各5 min,然后再放二甲苯Ⅱ5 min进行透明,最后用中性树胶封片。
-
采用SPSS 22.0软件,计量资料用均数±标准差(
$ \bar x \pm s $ )表示,组内比较采用单因素方差分析,两两比较采用LSD-t检验,以P<0.05,P<0.01为差异有统计学意义。 -
与对照组相比,给予ANIT后,模型组小鼠血清中肝功酶学指标(ALT、AST、ALP)水平显著升高(P<0.01);与模型组相比,中药组合物中、高剂量组和UDCA组小鼠外周血清中ALT、AST、ALP含量明显降低(P<0.01),低剂量组ALT的表达降低(P<0.05),表明中药组合物能够改善肝损伤血清生化指标(表1)。
组别 ALT(U/L) AST(U/L) ALP(金氏单位) 对照组 35.31±3.24 123.58±7.86 3.15±1.14 模型组 69.76±5.33## 186.65±8.25## 12.25±3.46## 中药组合物低剂量组 42.59±2.13* 142.12±10.34** 5.17±2.52** 中药组合物中剂量组 36.22±3.24** 125.73±9.63** 3.25±1.16** 中药组合物高剂量组 40.15±4.33** 136.68±8.45** 4.43±2.45** UDCA组 35.46±3.65** 124.24±7.41** 3.76±1.36** *P<0.05,**P<0.01,与模型组比较;##P<0.01,与对照组比较。 -
与对照组相比,给予ANIT后,模型组黄疸特异性指标(TBA、TBIL和γ-GT)的表达水平明显升高(P<0.01)。与模型组相比,中药组合物中、高剂量组和UDCA组能够显著降低小鼠外周血清中TBA、TBIL和γ-GT含量(P<0.01);中药组合物低剂量组TBA和TBIL表达降低(P<0.05),γ-GT表达降低(P<0.01),表明中药组合物对胆汁淤积肝损伤小鼠具有降低黄疸的作用(表2)。
组别 TBA(μmol/L) TBIL(μmol/L) γ-GT(U/L) 对照组 2.34±1.64 12.06±4.54 2.27±1.18 模型组 10.87±2.32## 35.17±2.64## 11.37±3.54## 中药组合物低剂量组 4.36±2.25* 18.02±4.62* 3.87±2.02** 中药组合物中剂量组 3.13±1.45** 14.67±2.56** 3.27±2.21** 中药组合物高剂量组 4.36±2.16** 15.37±3.45** 3.23±1.25** UDCA组 4.21±2.58** 13.15±3.26** 3.02±1.45** *P<0.05,**P<0.01,与模型组比较;##P<0.01,与对照组比较。 -
与对照组相比,给予ANIT后,模型组肝脏抗氧化因子(SOD、GSH-Px)活性降低明显(P<0.01),而MDA的表达水平上升明显(P<0.01),与模型组相比,中药组合物中剂量组、高剂量组和UDCA组SOD、GSH-Px的表达水平显著升高(P<0.01),同时中药组合物低、中、高剂量组MDA的表达水平显著降低(P<0.01),中药组合物低剂量组SOD、GSH-PX的表达升高(P<0.05),UDCA组MDA的表达降低(P<0.05)。结果提示,中药组合物能够通过清除氧化自由基减少炎症反应进而保护肝脏(表3)。
组别 SOD(U/ml) MDA(nmol/ml) GSH-PX[nmol·
(min·g)−1]对照组 305.65±8.21 7.75±3.24 139.58±8.02 模型组 210.46±8.96## 20.65±2.86## 86.05±9.78## 中药组合物低剂量组 257.32±5.42* 13.31±4.21** 118.87±6.52* 中药组合物中剂量组 297.35±5.64** 8.76±3.46** 130.35±4.15** 中药组合物高剂量组 275.34±6.54** 11.75±5.12** 126.48±6.06** UDCA组 300.02±7.38** 8.52±3.35* 132.34±5.47** *P<0.05,**P<0.01,与模型组比较;##P<0.01,与对照组比较。 -
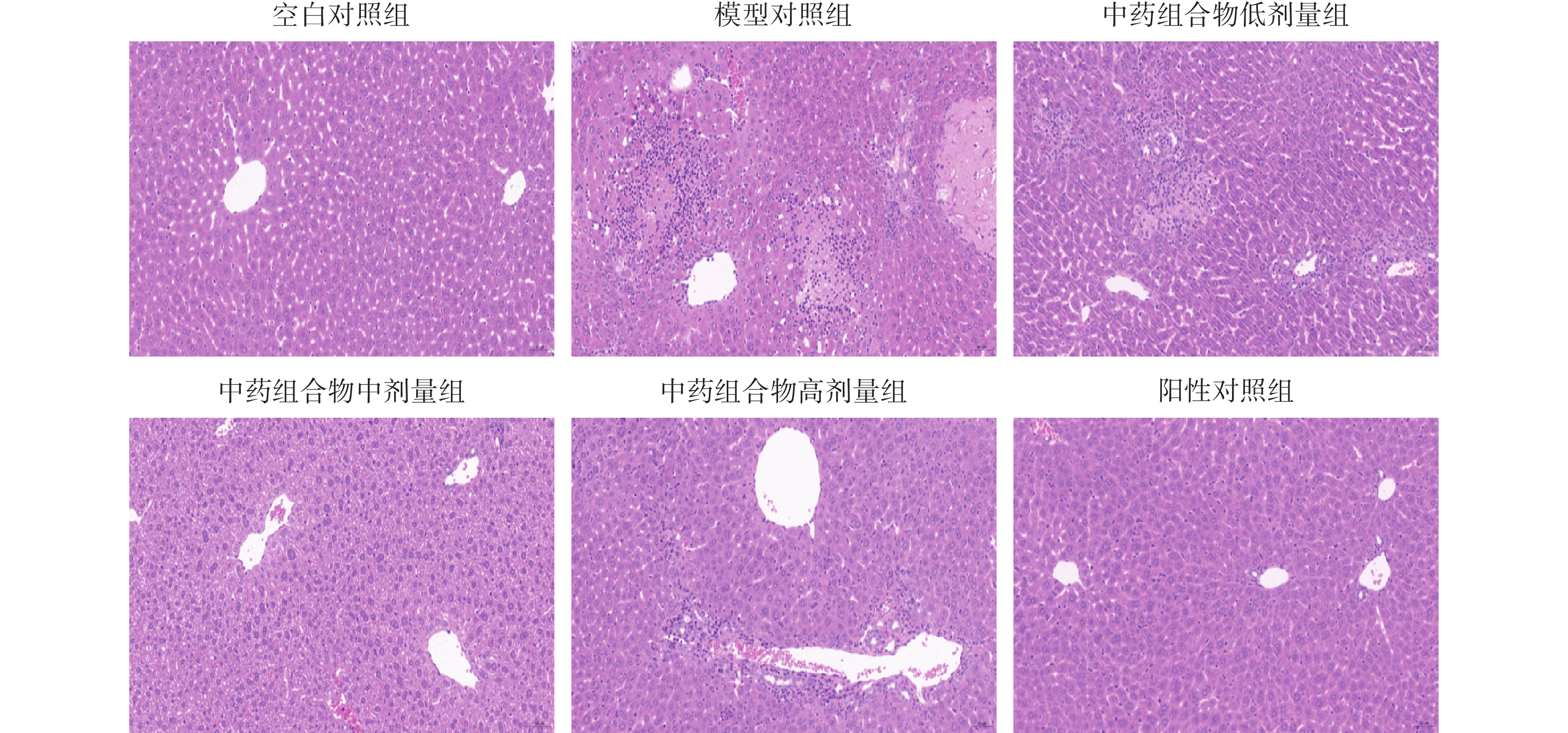
对照组小鼠肝脏病理切片显示肝细胞胞浆丰富,肝细胞圆润饱满,小叶结构完整清晰,无纤维组织,肝细胞围绕中央静脉呈放射状排列,无炎症细胞浸润;与对照组相比,模型组小鼠肝脏病理切片显示肝脏细胞呈现脂肪变性,中央静脉周围汇管区炎症细胞浸润,部分肝细胞排列紊乱,出现点状坏死;与模型组相比,中药组合物低剂量组、高剂量组肝脏细胞炎症浸润稍微减轻,较模型组好转。中药组合物中剂量组肝脏细胞脂肪变性显著减少,肝脏细胞排列紊乱恢复,炎症细胞浸润现象较模型组改善明显,肝小叶结构完整;UDCA组部分肝细胞水肿,中央静脉周围有少量散在炎症细胞浸润,逐渐恢复细胞序列。具体见图1。
-
目前认为胆汁淤积与各种肝脏疾病密切相关[11],如黄疸、胆结石、急性肝炎、原发性硬化性胆管炎(PSC)、原发性胆汁性肝硬化(PBC)等,其中引起成年胆汁淤积的两种最主要的疾病就是PSC和PBC,这些疾病不仅使人们的生活质量下降,也大大增加了家庭和社会负担[12],因此,开发治疗胆汁淤积肝疾病更有效的药物是临床面临的迫切任务,深入阐述胆汁淤积肝损伤的病理机制则有助于寻找更加精准的治疗靶点。
ANIT是急性胆汁淤积肝损伤动物模型常用造模试剂,在肝脏中经细胞色素P450超家族(CYP)与谷胱甘肽(GSH)结合之后被转运至胆管并集中在胆汁内,造成胆管上皮细胞损伤,以致肝小叶间胆管炎症及肝内胆管增生,导致胆管阻塞,使胆汁酸等物质在肝脏中过度堆积,从而产生肝毒性[13-14]。同时胆汁反流及扩散,使周围的肝细胞损伤,引发系统出现炎症,进而导致高胆红素血症。
本研究通过灌胃ANIT构建小鼠胆汁淤积肝病模型,小鼠血清中ALT、AST、ALP、TBIL、TBA、MDA及γ-GT水平显著升高,GSH-PX、SOD表达显著下降,经苏木精-伊红染色肝脏出现病理损伤。
ALT、AST作为肝脏中不可或缺的酶,主要分布在肝细胞胞浆和线粒体内,是临床最常用的检测肝功能的指标,能在一定程度上反映肝损伤程度,随着肝细胞结构和功能的破坏,可被大量释放到外周血,其血清浓度与肝脏损伤程度呈正相关关系[6]。本研究结果显示,此中药组合物能显著降低胆汁淤积模型小鼠清中ALT、AST的表达水平,提示其具有保护肝脏免受ANIT所致损伤的作用。
临床上将血清ALP 升高作为各种原因所致胆汁淤积早期诊断的特征性指标之一[15],当肝脏出现损伤或胆汁排泄不畅时,血清中ALP的浓度就会升高[15]。本研究结果显示,此中药组合物各剂量组能不同程度降低小鼠胆汁淤积模型血清中ALP的浓度。
肝脏是TBIL和TBA主要的代谢场所,当肝细胞发生病变或肝内外出现阻塞时,肝脏正常的代谢和排泄功能发生障碍,TBA和TBIL在血液中积聚,出现黄疸现象[16-17]。γ-GT在肝内主要分布于肝细胞胞浆和肝内胆管上皮。临床上此酶主要用于诊断肝胆疾病,是胆道梗阻和肝炎活动的指标之一。本研究结果显示,此中药组合物能显著降低胆汁淤积模型小鼠血清TBA、TBIL和γ-GT水平,改善黄疸症状。
脏损伤通常伴有氧化应激反应的发生[18-19],因此本研究还检测了抗氧化因子SOD和GSH-PX表达。结果显示,中药组合物能够明显增加胆汁淤积小鼠血清中SOD和GSH-PX的含量,表明中药组合物能通过其抗氧化活性发挥保护肝损伤的作用。UDCA作为临床治疗胆汁淤积的常用药物,在本实验研究中,其改善胆汁淤积肝损伤血清学指标、降低黄疸以及清除氧化自由基效果明显。MDA作为机体自由基与生物膜发生脂质过氧化反应的重要产物[20-21],其表达水平与细胞发生自由基氧化的程度密切相关,本研究结果显示此中药组合物能够显著降低胆汁淤积小鼠MDA的表达水平,提示其能够改善ANIT所致的氧化损伤。
综合肝脏病理学变化和血清生化结果,中药组合物中剂量组(11.25 g/kg)效果较为明显,其发挥改善胆汁淤积型肝病的效果较好,其整体改善作用与阳性药相当。
综上所述,此中药组合物对ANIT诱导的小鼠胆汁淤积性肝病具有明显的保护作用,其改善胆汁淤积的作用可能与改善肝损伤血清学指标、降低黄疸以及清除氧化自由基、减轻炎症反应等有关。本研究进一步丰富了此中药组合物防治肝病的研究资料,为此中药组合物防治肝病提供了参考依据和理论基础。
The protective effect of a traditional chinese medicine composition on ANIT induced liver injury in mice with cholestasis
doi: 10.12206/j.issn.2097-2024.202305008
- Received Date: 2023-05-06
- Rev Recd Date: 2024-01-19
- Available Online: 2024-12-20
- Publish Date: 2024-12-25
-
Key words:
- Traditional chinese medicine composition /
- Cholestasis /
- Biochemical indexes /
- Pathological changes
Abstract:
| Citation: | YANG Nian, ZHANG Bole, ZHANG Junxia, ZHANG Zhenqiang. The protective effect of a traditional chinese medicine composition on ANIT induced liver injury in mice with cholestasis[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(12): 508-511, 519. doi: 10.12206/j.issn.2097-2024.202305008 |









 DownLoad:
DownLoad: