-
急性髓系白血病(AML)是原始和幼稚细胞在骨髓或外周血中恶性克隆、异常增殖的一种恶性血液系统肿瘤疾病,其高复发率、高病死率成为临床治疗的关键与难点[1-3]。在AML的成人患者中,约30%发生了FMS 样酪氨酸激酶(FLT3)突变,使得FLT3成为治疗AML的重要靶点之一[4-5]。吉瑞替尼作为第二代FLT3抑制剂,可同时靶向FLT3的基因内部串联重复(ITD)和酪氨酸激酶结构域(TKD)两个位点,抗肿瘤活性更广,不良反应发生概率更低,是国内外指南中治疗FLT3突变的复发、难治性AML患者的推荐用药[6-7]。吉瑞替尼常见的不良反应有:丙氨酸氨基转移酶升高(25.4%)、天冬氨酸氨基转氨酶升高(24.5%)、贫血(20.1%);其他具有临床意义的严重不良反应包括:QTc间期延长(0.9%)、可逆性后脑部病综合征(0.3%) [8-9]。尽管不良反应发生概率相对较低,但严重不良反应仍使吉瑞替尼在临床应用上受限。该研究报道了临床药师参与1例吉瑞替尼在治疗过程中导致获得性QTc间期延长的药学监护,探讨临床药师在肿瘤药物治疗过程中的重要作用,同时为吉瑞替尼的临床应用提供参考。
-
患者,女,76岁,身高155 cm,体质量44 kg,体表面积1.36 m2。2022年12月,感染新型冠状病毒后痊愈,但偶感乏力,活动后症状加重,遂于当地医院就诊,血常规提示:白细胞 72.97×109 /L; 血红蛋白 49.2 g/L; 血小板 101×109 /L;骨髓涂片示幼稚细胞占61%,医师对症予以2 U O型悬浮红细胞改善贫血,建议行骨穿及MICM检查并转诊专科治疗。后为进一步治疗,于2023年2月15日由急诊以“贫血待查,乏力”收治入火箭军特色医学中心(本院)。否认其他传染病史和慢性病史,否认药物、食物过敏史。
-
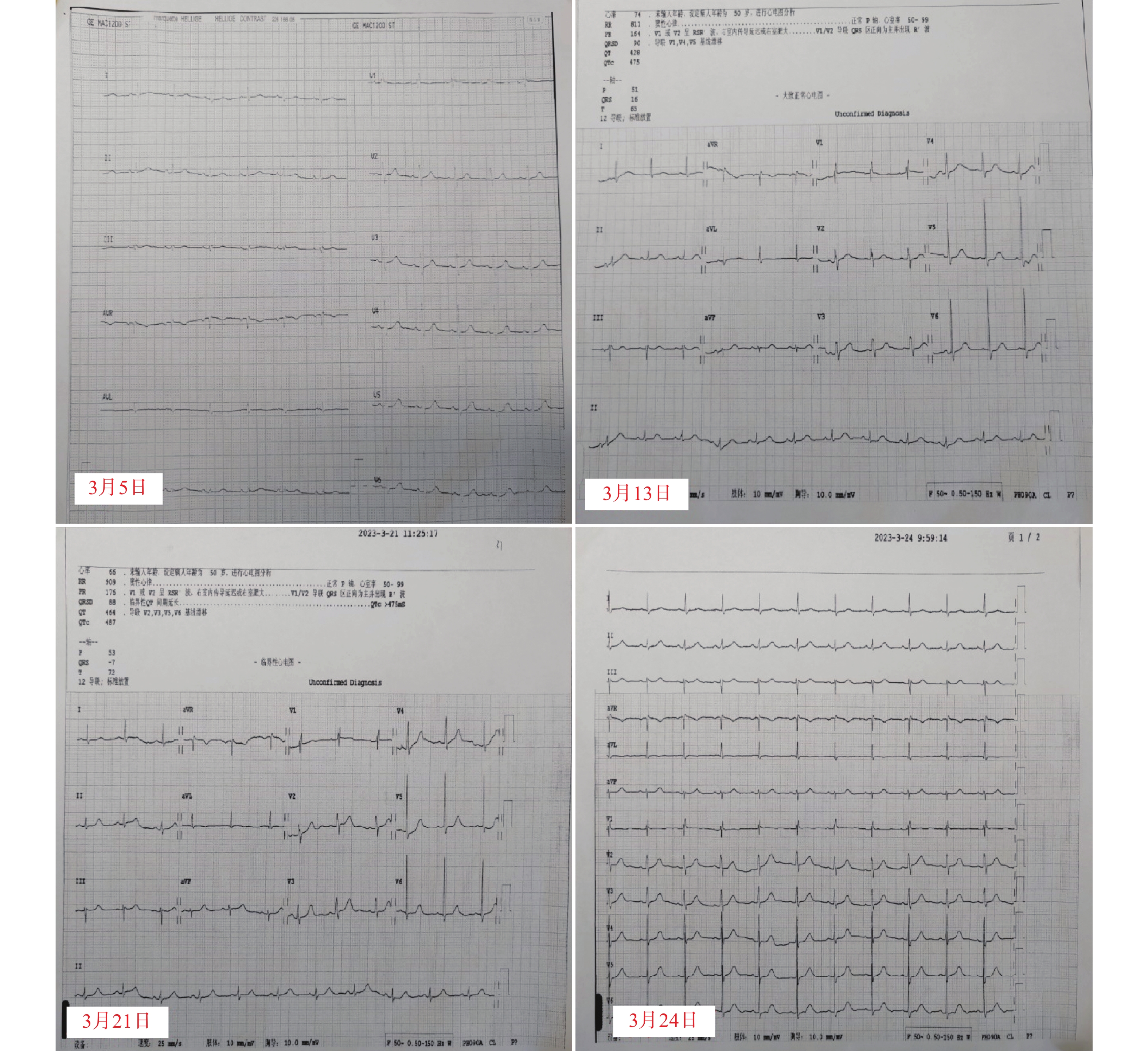
入院后完善各项常规检查。血常规:白细胞 102.73×109 /L; 血红蛋白 49.2 g/L; 血小板 85×109 /L;生化:血钾3.32 mmol/L;骨髓涂片示幼稚细胞占75%,根据骨髓免疫分型及细胞形态学检查结果确诊为急性髓系白血病,FAB分型为急性单核细胞白血病(AML-M5)。医师于2月20日行维奈克拉(口服,第1天100 mg,第2天200 mg,第3~28天400 mg)+阿扎胞苷(皮下注射,第1~7天75 mg/m2)方案治疗。3月5日基因检测结果回报,患者FLT3基因突变阳性,遂于3月6日起,加用富马酸吉瑞替尼片(口服,120 mg d1~28)。药师建议,可在患者吉瑞替尼开始用药前、首次用药第8天和第15天进行心电图检查。根据心电图检测回报,患者在用药前、用药第8、15天的QTc数值分别为: 402、475、487 ms,因患者QTc间期延长超过60 ms,药师建议立即停用可疑药品吉瑞替尼。停药第3天复查心电图,结果显示QTc数值为424 ms,恢复至正常范围内。心电图监测结果如图1所示。
-
国内尚未对QTc间期延长做统一定论,一般将QTc数值>440 ms作为QTc间期延长的界值,但也存在部分正常人QTc数值超过这个正常值范围[10]。美国心脏协会、美国心脏病学学会认为男性QTc 470 ms,女性480 ms为正常值。但不论男女,QTc>500 ms或QTc变化数值较基线值变化超过60 ms都属于明显异常,且在美国卫生及公共服务部发布的常见不良事件评价标准(CTCAE v5.0)中,将QTc≥501 ms或比基线期>60 ms定义为QTc间期延长的3级不良反应事件。本例患者用药期间QTC数值变化近90 ms,QTc间期明显延长,及时查明可疑药品,予以对症处理十分必要。
患者自述既往无心脏病史,吉瑞替尼用药前心电图结果正常,可排除患者自身疾病影响。根据国家药品监督管理局、药品不良反应中心对于判定不良反应事件相关性分析原则[11]:①患者吉瑞替尼用药前QTc结果正常,3月6日用药后,QTc数值不断上升,因此不良反应发生与用药存在时间关系;②通过查阅说明书和相关文献可知,吉瑞替尼具有引起患者QTc间期延长的风险;③患者停药后,QTc数值逐渐恢复正常;④本周期未再使用吉瑞替尼;⑤患者同期应用的其他治疗药物,例如:维奈克拉、伏立康唑、美罗培南均为长期用药,且吉瑞替尼停药后这些药物仍在使用。综上所述,可判断患者QTc间期延长的不良反应很可能是由吉瑞替尼引起的。
-
QTc间期通常是指心室除极化和复极化的总耗时,也就是自QRS波起点至T波终点所需的时间[12]。长QTc间期综合征(LQTS),是一种心室复极时程延长、不均一性增大的疾病。心电图上表现为 QTc间期延长、T波和(或)U波异常、早搏后的代偿间歇,且心率减慢时可发生尖端扭转型室性心动过速(TdP),严重时可诱发猝死。临床表现为心动过缓、晕厥、搐搦,包括先天性和获取性两类[10]。该患者既往无心脏疾病史及家族遗传史,且入院时QTc数值正常,可排除先天性LQTS。获取性LQTS与药物、心脏疾病、代谢异常等因素相关,其中常见的可引起LQTS药物有:抗心律失常药、抗生素、抗精神失常药、抗肿瘤药等[13-14]。
吉瑞替尼致QTc间期延长机制研究较少,通常认为药源性LQTS的发生与抑制电压依赖性K+通道电流有关,主要为延迟整流K+通道(IK)。其中,快速激活(Ikr)者的α亚基由hERG基因编码,是整个动作电位复极时相的主要电流。若Ikr通道受阻,则可造成心脏动作电位时程中QTc间期延长,心室复极化延迟,进而诱发TdP [15-16]。患者入院时生化结果示:血钾3.32 mmol/L,低于正常值范围。钾离子对于维持心肌细胞自律性、传导性和兴奋性具有重要意义。低血钾时,Ikr通道外向复极电流减弱,动作电位复极延迟,多表现为心脏自律性增强及传导减慢,是诱发LQTS的重要因素。又因为吉瑞替尼在体内主要经CYP3A酶代谢,是CYP酶系中易受合用药物影响的亚型,而患者化疗期间,出现Ⅳ度骨髓抑制(白细胞0.48×109 /L,血红蛋白82 g/L,血小板17×109 /L,中性粒细胞绝对值0.03×109 /L),间断发热,医师给予注射用美罗培南1 g 1/8 h 静滴+伏立康唑200 mg 1/12 h 静滴(用药时间3月13日至3月26日)对症治疗。伏立康唑属于强效CYP3A/P-gp抑制剂,可有效抑制CYP3A酶活性,使得患者体内吉瑞替尼代谢减慢,血液浓度可增加1.5倍左右。因此,不良反应发生率随之上升。本例患者使用吉瑞替尼后出现QTc间期延长的3级不良反应,可能与患者血钾水平低、联合使用CYP3A/P-gp抑制剂引起药物暴露水平升高有关。
-
一旦发生药源性LQTS,首先要判断可疑药品,立即停用明确或可能进一步诱发尖端扭转型室性心动过速的药物,并进行连续的QTc间期监测,积极对症治疗。临床药师可通过详细询问患者既往病史,仔细审查用药医嘱,判断引起不良反应的可能药品,通过关联性评价确定引发不良反应的可能性大小,分析用药医嘱中是否存在合并用药的相互作用增加患者QTc间期延长风险。同时评估是否存在其他诱发或加重患者LQTS发生风险的高危因素。本例患者联用伏立康唑会增加吉瑞替尼血药浓度,故临床药师建议吉瑞替尼用药过程中,避免与强效CYP3A/P-gp抑制剂合用,同时及时补钾,改变低血钾状态,防止TdP发生。该患者吉瑞替尼停药后,密切监护其心电图变化情况,3 d后QTc数值恢复至正常范围内(424 ms)。临床药师建议可酌情恢复用药,以80 mg的减量剂量继续用药,恢复用药后患者未再出现心电图明显异常。目前患者规律服用该药,病情控制平稳。建议在后续2个周期治疗开始前进行心电图检查,同时提醒患者若出现心慌、胸闷、晕厥要立即到医院就诊。若不能自行恢复,可静脉注射硫酸镁治疗[10,17],同时在后续2个周期治疗开始前进行心电图检查。
-
国内外对于吉瑞替尼引起QTc间期延长的报道十分罕见,QTc间期延长的不良反应往往不易被发现,最终对患者产生严重危害。临床药师通过了解已知或可能的存在诱发LQTS或TdP风险相关的药物,个体化评估药源性QTc间期延长风险及临床获益情况,判断合并用药是否存在相互作用情况,及其他并发的高危因素,并通过适当减少用药剂量、及时纠正电解质紊乱等方法,来降低不良反应发生概率,提高用药安全性。
Participation of clinical pharmacists in QTc interval prolongation induced by gilteritinib
doi: 10.12206/j.issn.2097-2024.202309050
- Received Date: 2023-09-20
- Rev Recd Date: 2023-11-22
- Available Online: 2024-06-24
- Publish Date: 2024-06-25
-
Key words:
- gilteritinib /
- QTc interval prolongation /
- adverse drug reaction /
- clinical pharmacists
Abstract:
| Citation: | CUI Xiaolin, FU Xiaofei, DU Yanhong, LIU Juan, ZHU Qian, LIU Ziqi. Participation of clinical pharmacists in QTc interval prolongation induced by gilteritinib[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(6): 263-266. doi: 10.12206/j.issn.2097-2024.202309050 |








 DownLoad:
DownLoad: