-
疲劳是指机体由于某一生理变化过程不能维持其运动机能在某一特定水平或者不能维持其运动强度在某一预定水平[1]。疲劳是日常生活中非常普遍的生理现象,通常人们经过一定的体力或是脑力劳动后会感到疲乏无力,疲劳发生时可导致抑郁、焦虑等问题,通常被认为与认知障碍、睡眠质量差、身体功能障碍和能量失衡有关[2-3]。关于疲劳产生的机制有多种解释,主要包括能源衰竭学说、疲劳相关的代谢产物堆积和自由基学说等。其中,运动过程中产生的自由基攻击细胞和线粒体等生物膜,导致氧化与抗氧化作用失衡,被认为是运动性疲劳产生的主要原因之一[4-6]。目前临床上用于缓解疲劳的药物或疗法较少,因此,从疲劳产生机制出发,开发新型抗疲劳药物或潜在物质具有重大意义。
纳米硒(SeNPs)作为一种抗氧化剂,在治疗氧化应激相关疾病中具有潜在的应用前景。然而,SeNPs由于表面能高,通常稳定性较差,容易发生聚沉,形成灰色或黑色的元素硒[7]。功能化SeNPs具有较低的毒性和较高的生物兼容性及反应性,有助于提高疗效和临床应用价值[8]。虎奶菇[Pleurotus tuber-regium (Fr.) Singer]又称菌核侧耳、茯苓侧耳等,为担子菌纲口蘑科侧耳属真菌,性甘温,补气益血,主要分布在热带和亚热带地区,是一种珍贵的药食两用真菌[9],在民间常被用于治疗哮喘、感冒发烧、胃病等疾病[10]。研究表明,多糖是虎奶菇重要的活性成分之一,具有增强小鼠免疫力、提高抗氧化应激能力和抑制肝脏脂质过氧化等多种药理活性[11-12]。
我们前期的试验利用非洲野生虎奶菇菌核多糖作为封端剂,制备出大小可控、高稳定性的新型纳米硒——虎奶菇多糖功能化纳米硒(PTR-SeNPs)[13]。研究表明,PTR-SeNPs具有显著的保肝、抗细菌、抗真菌、抗肿瘤和促进骨形成等多种生物药理活性,表现出巨大的潜在临床应用价值和开发前景[14-18]。本文探究PTR-SeNPs对游泳运动性疲劳的拮抗作用,以期为PTR-SeNPs作为天然补硒剂在抗疲劳保健品或药品的开发和应用提供实验依据。
-
SPF级C57/BL6小鼠48只,雄性,6~8周龄,体质量20 g~25 g,由江苏赛业模式生物研究中心(太仓)有限公司提供,生产许可证号:SCXK(苏)2018-0003。于福建中医药大学动物实验中心SPF级动物房内饲养。
-
PTR-SeNPs(香港理工大学未来食品研究院食品科学及营养学系黄家兴教授制备并提供,初始摩尔浓度为1.30 mmol/L);生理盐水(福州海王福药制药有限公司);血清尿素氮(BUN)、乳酸(LD)、谷丙转氨酶(ALT)、谷草转氨酶(AST)、乳酸脱氢酶(LDH)、超氧化物歧化酶(SOD)、丙二醛(MDA)、过氧化氢(CAT)和糖原(Glycogen)测定试剂盒(南京建成生物工程研究所);BCA蛋白浓度测定试剂盒(碧云天生物技术有限公司);动物用异氟烷(深圳市瑞沃德生命科技有限公司)。
5417R低温高速离心机(德国Eppendorf公司);LXJ-IIB低速离心机(上海安亭科学仪器厂);Th900超低温冰箱(美国Thermo公司);DK-S24电热恒温水浴锅(上海精宏实验设备有限公司);AR2310电子天平(奥豪斯仪器有限公司);M-244超纯水机(德国Milipore公司);BCD-216SDN 4℃冰箱(中国Haier集团);Lab dancer S25涡旋仪(IKA公司);M200 Pro多功能酶标仪(瑞士Tecan公司);IMS-300全自动雪花制冰机(常熟市雪科电器有限公司);BioSpec 70/20 USR小动物核磁共振成像仪(瑞士布鲁克公司)。
-
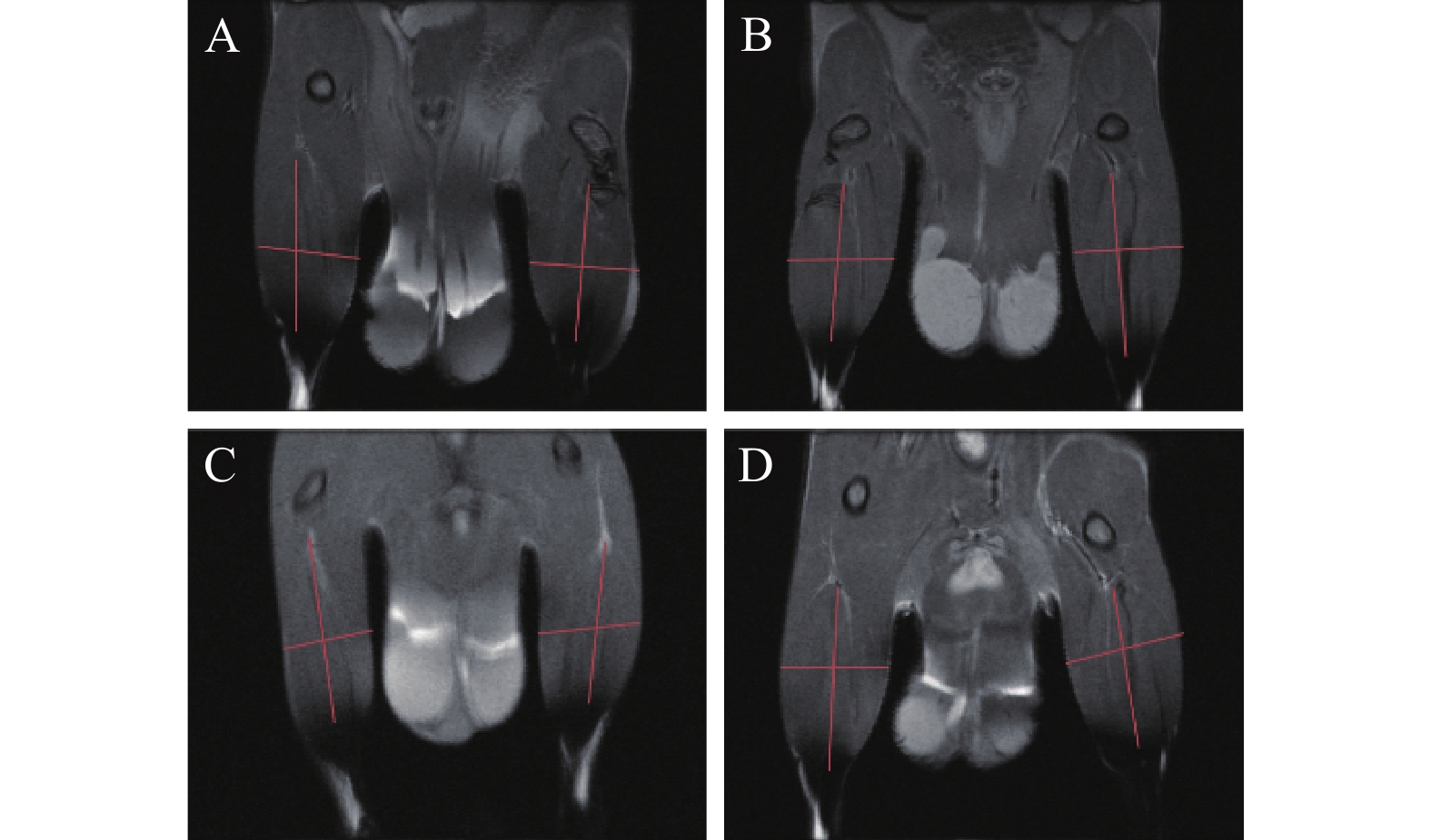
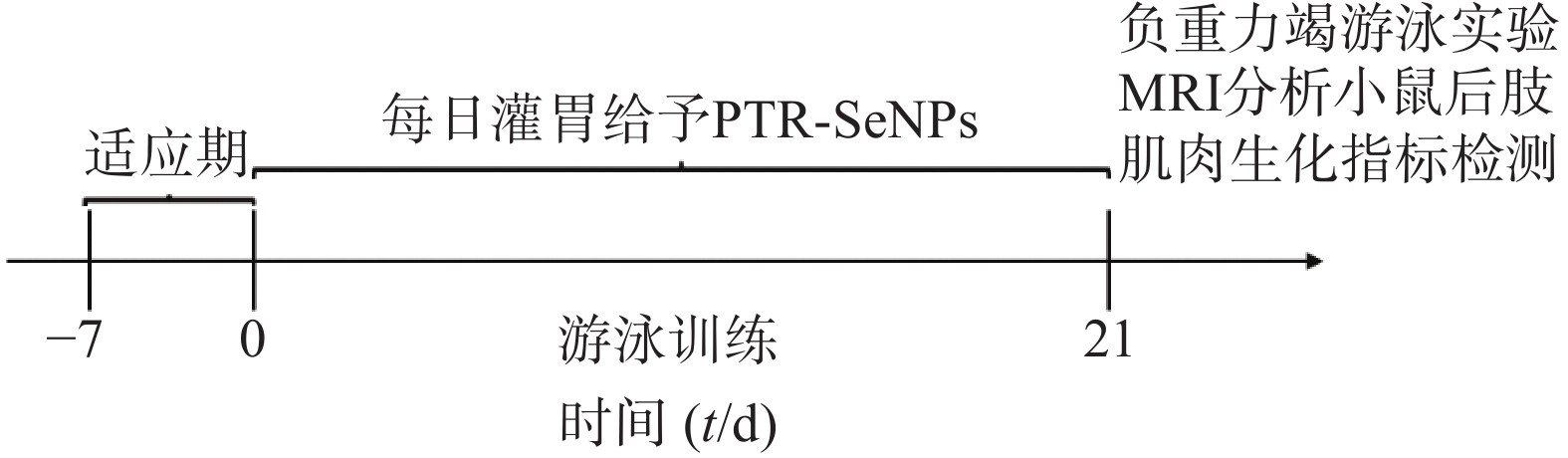
C57/BL6小鼠48只,雄性,6~8周龄,体质量20~25 g,在SPF级实验动物房饲养,室内温度为20℃~26℃,相对湿度40%~70%,噪声<60 dB,光照明暗交替12 h/d,所有小鼠适应性喂养1周,待处理。将C57/BL6小鼠分为4组,每组12只,分别为对照组、游泳训练模型组(EC组)、PTR-SeNPs低剂量组[2.5 μmol/(kg·bw),LPTR-SeNPs组]、PTR-SeNPs高剂量组[10 μmol/(kg·bw),HPTR-SeNPs组]。按所示剂量灌胃21 d,每天给药1次。第1~4天,给药30 min后,适应性游泳训练20 min,连续4 d,第5~7天每天比前1 d增加20 min,连续3 d,第8~20天每天游泳80 min,持续13 d。每日监测食物与水的摄取量,并定期记录小鼠体质量变化情况(第1、5、10、15和20天)。第21天给药30 min后,每组各取3只,游泳80 min后,MRI扫描小鼠后肢肌肉长度和宽度,其余小鼠进行负重力竭游泳训练后,眼球取血并摘取肝脏,进行疲劳相关生化指标检测(图1)。
-
第21天给药30 min后,每组各取3只,异氟烷气体麻醉小鼠,MRI扫描小鼠后肢肌肉,利用ImageJ软件分析各组的肌肉长度和宽度,计算各组与对照组之间的比值,即得各组的肌肉相对长度和宽度。
-
第21天给药30 min后,在尾部绑上重量为小鼠体质量5%的铅丝,将其放入水温为(30±2)℃、50 cm×40 cm×40 cm的水箱中进行负重力竭游泳测试。适当用玻璃棒搅拌水,或拨弄小鼠,使小鼠一直处于运动状态。小鼠的头部沉下水面8 s不能浮出即判断为力竭,记录每只小鼠从开始游泳至力竭的时间[19]。每只小鼠测试过后更换新的超纯水,避免小鼠留在水中的警戒物质对其他小鼠产生干扰。负重力竭游泳结束后立即进行眼球取血,脱颈处死,摘取肝脏,保存于−80℃,待测。
-
小鼠眼球取血,3 500 r/min,4℃离心15 min,取血清,参照试剂盒说明书检测血清中BLA、BUN、ALT、AST和LDH含量。
-
取适量肝组织,用生理盐水漂洗,吸净水分,参照试剂盒说明书检测肝脏中SOD、CAT活性和MDA、HG水平。
-
采用SPSS 23.0软件对实验数据统计分析,以(
$ \bar{x}\pm {s} $ )表示,采用ANOVA单因素方差分析数据,以P<0.05差异有统计学意义。 -
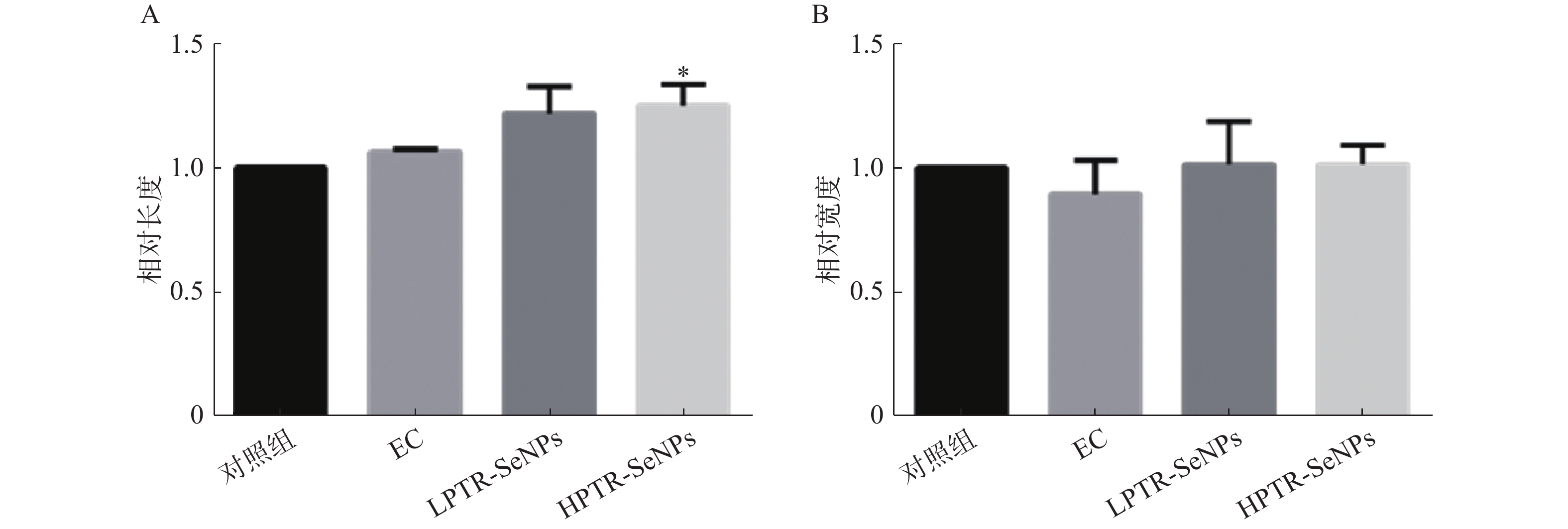
MRI扫描小鼠后肢肌肉(图2),ImageJ软件分析并计算各组小鼠后肢肌肉的相对长度和宽度,结果如图3所示。与对照组比较,HPTR-SeNPs能显著增加后肢肌肉相对长度(P<0.05)。结果表明,PTR-SeNPs可通过增加后肢肌肉长度,改变肌肉结构,可能与提高机体的耐力相关。
-
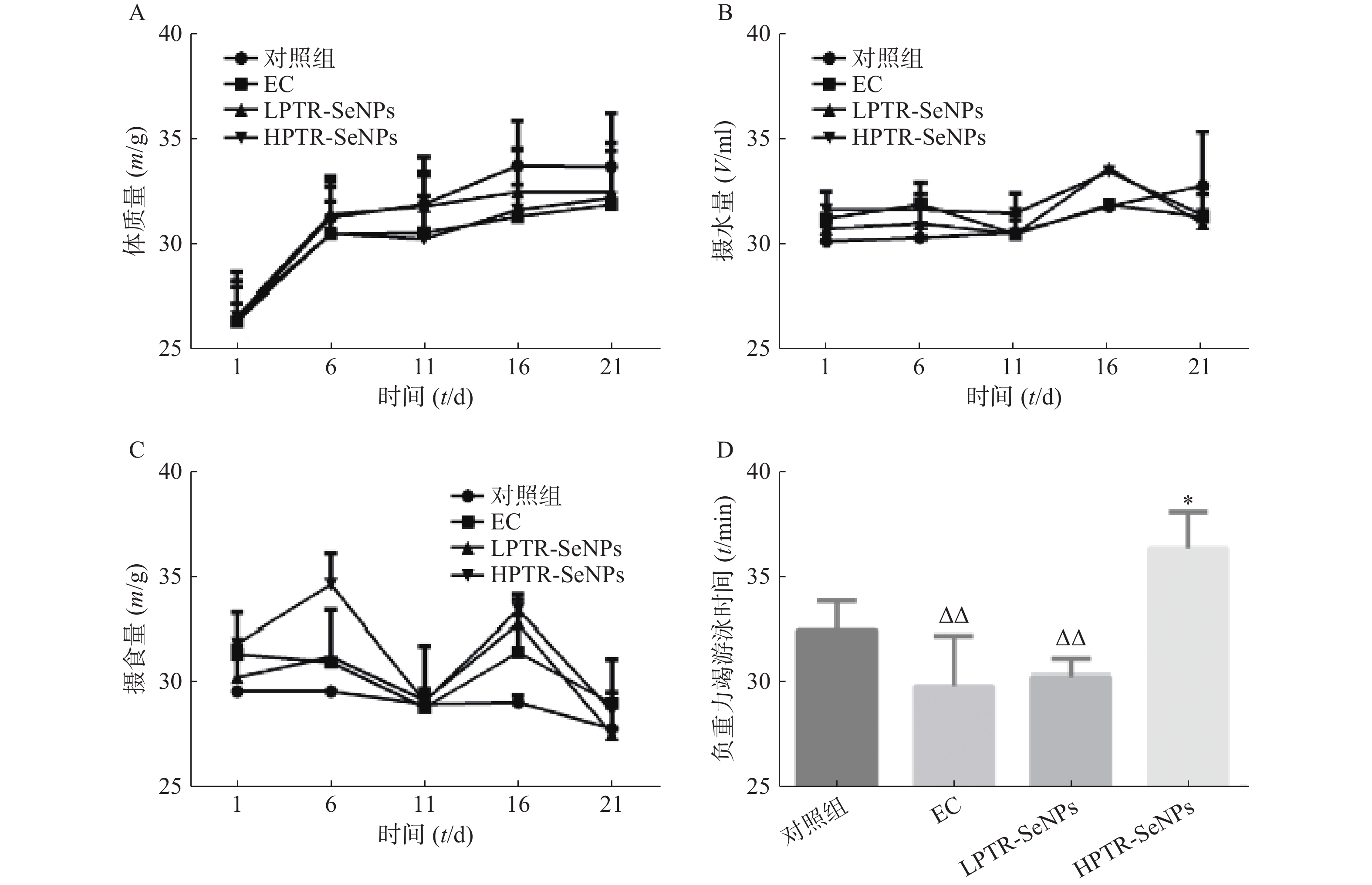
疲劳是机体复杂的生理变化过程,运动耐力可作为客观直接的判断标准[20]。由图4A-C可知,与对照组比较,给药21 d后,L/HPTR-SeNPs对运动训练小鼠的体质量、摄食和摄水量均无显著影响。小鼠连续灌胃给予PTR-SeNPs 21 d后,通过负重力竭游泳实验考察PTR-SeNPs对小鼠运动性疲劳的影响,结果如图4D所示,与对照组比较,HPTR-SeNPs显著提高小鼠的负重力竭游泳时间(P<0.05);与EC组或LPTR-SeNPs组比较,HPTR-SeNPs能显著提高小鼠的负重力竭游泳时间(P<0.01),说明PTR-SeNPs可以显著提高小鼠运动耐力,明显缓解小鼠疲劳症状。
-
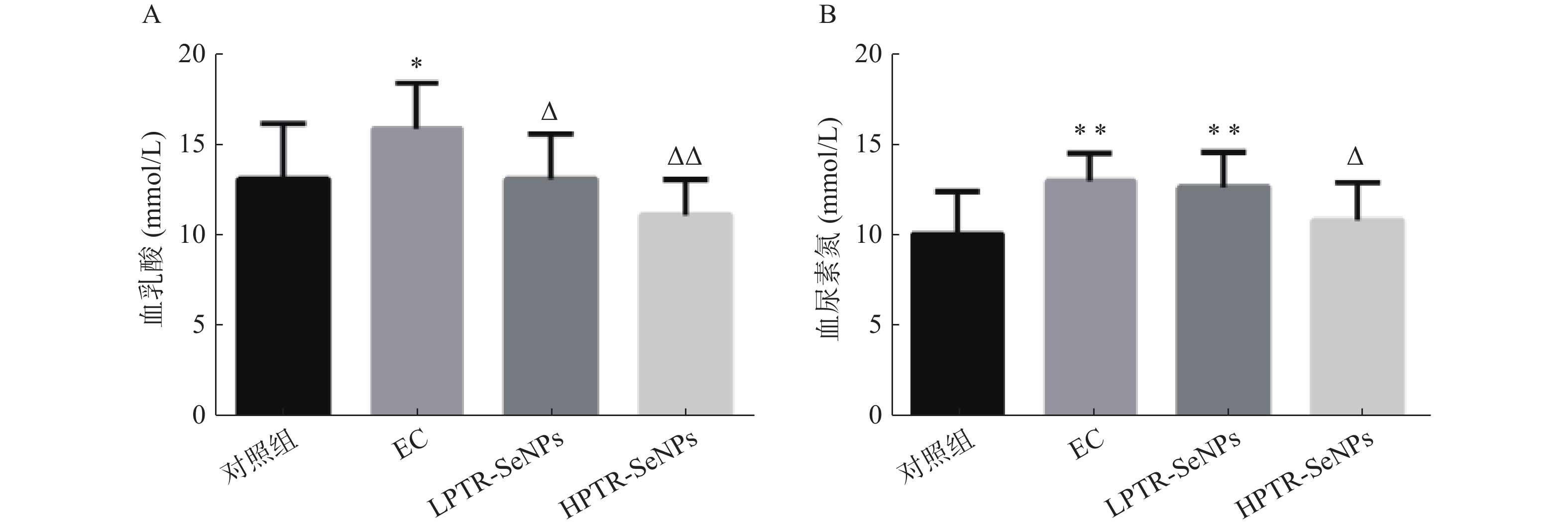
糖原作为机体主要的供能物质,运动产生疲劳时,大量ATP被消耗,此时机体糖酵解过程增强,糖酵解过程最终代谢产物为BLA,BLA在机体内大量堆积,使机体内环境呈酸性,细胞正常的能量转换和物质代谢受到影响,最终导致机体产生疲劳[21-23]。当机体能量消耗达到一定程度,体内的蛋白质开始分解,参与能量代谢,使得血清中BUN含量急剧升高[24]。小鼠血清BLA含量检测结果如图5A所示,与对照组比较,EC组BLA含量显著升高(P<0.05);与EC组比较,LPTR-SeNPs组与HPTR-SeNPs组BLA含量显著下降(P<0.05或P<0.01)。表明PTR-SeNPs可能通过促进葡萄糖更充分氧化分解,减少BLA的生成,或者加快BLA代谢,从而提供更为充足的ATP,减少运动小鼠BLA的蓄积。血清BLA含量检测结果如图5B 所示,与对照组比较,EC组和LPTR-SeNPs组BUN含量显著升高(P<0.01);而HPTR-SeNPs组的BUN含量则无显著性差异。与EC组比较,HPTR-SeNPs组BUN含量显著下降(P<0.05)。提示PTR-SeNPs可能通过提高葡萄糖的利用,减少蛋白质分解,降低疲劳小鼠血清BUN的生成。
-
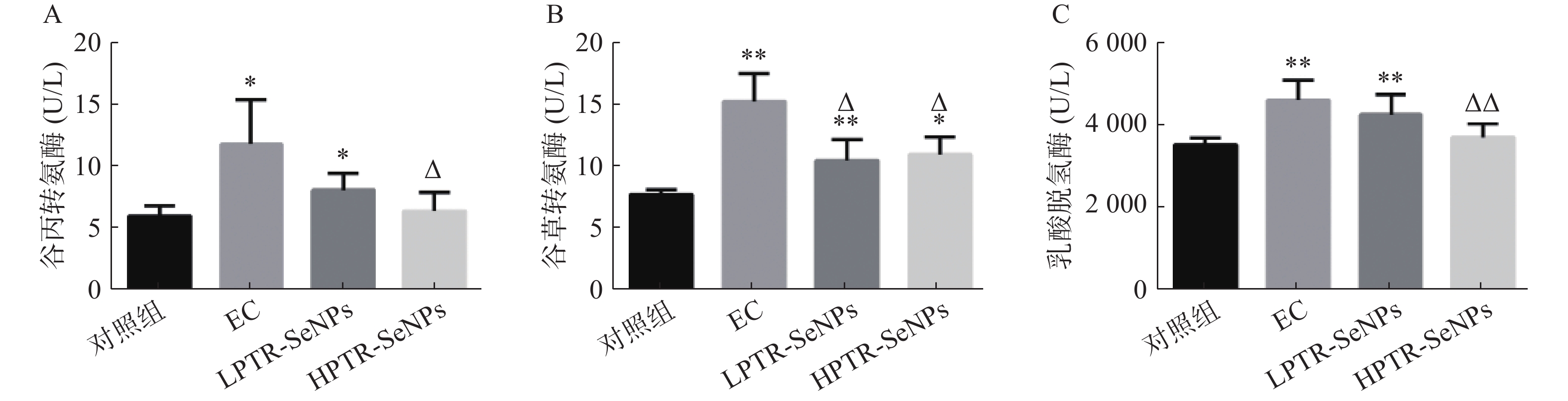
机体在进行剧烈运动时,会生成大量的自由基,使机体产生氧化应激,导致肝脏、心肌和肌肉等组织器官出现受损,血清中ALT、AST和LDH水平亦可作为评价小鼠在游泳应激过程中的敏感指标[25]。由图6可知,与对照组比较,EC组血清中的ALT、AST和LDH含量显著升高。LPTR-SeNPs组的ALT和LDH含量以及L/HPTR-SeNPs组AST含量均显著升高。与EC组比较,HPTR-SeNPs组ALT水平显著降低(P<0.05),见图6A;L/HPTR-SeNPs组的AST水平显著降低(P<0.05),见图6B;HPTR-SeNPs组的LDH活性水平显著降低(P<0.01),见图6C。结果提示PTR-SeNPs改善运动性疲劳过程中的肝脏生理功能。
-
机体内含有的多种抗氧化活性酶,如SOD和CAT,可减少体内活性氧自由基的含量,保护肌细胞。抗氧酶活性的提高能够延缓运动疲劳,MDA水平可作为判断机体内物质氧化程度的指标[26-27]。由图7可知,与对照组比较,HPTR-SeNPs组的CAT活力和HG含量显著升高(P<0.01)。与EC组比较,LPTR-SeNPs组小鼠肝脏中SOD活力和HG含量显著升高(P<0.05),HPTR-SeNPs组小鼠肝脏中CAT活力和HG含量显著升高(P<0.01)。表明PTR-SeNPs可以提高体内SOD、CAT活力,清除体内自由基,减缓小鼠剧烈运动后MDA堆积对肌细胞所造成的损害,同时提高机体HG含量。提示PTR-SeNPs能够显著提高机体的抗氧化应激能力和HG储备能力,从而有效改善运动性疲劳。表明PTR-SeNPs具有增强肝脏生理功能的作用,能有效拮抗游泳训练疲劳的发生。
-
目前,疲劳已严重影响到人们的身心健康和生活质量,受到了国内外专家的关注,并逐渐成为学者研究的热门课题。硒是生物体中必需的微量元素,在预防癌症、心血管疾病和提高免疫力中具有重要作用。近年来,SeNPs作为一种新型补硒药物出现,具有更好的生物利用度以及更小的毒性[2]。然而,SeNPs不稳定、极易聚集的性质,限制了其在临床上的应用。近年来,天然生物大分子作为SeNPs的修饰剂,可提高其稳定性和生物活性,并逐渐成为研究热点[15]。蘑菇多糖具有丰富的羟基,能有效地吸附在SeNPs上,避免其聚积和沉淀[28]。研究表明,蘑菇多糖修饰的SeNPs其稳定性大幅增强,且具有抗肿瘤、抗氧化、保肝和抗疲劳活性等多种生物活性[29]。
本研究结果表明,PTR-SeNPs能显著拮抗小鼠的游泳训练疲劳,其抗疲劳作用可能与改善肝脏生理功能、增加糖原储备、减少代谢物堆积及增强机体抗氧化能力有关。因此,深入探究PTR-SeNPs的抗疲劳活性作用机制,为其在抗疲劳药物及保健品的开发应用提供重要的实验参考依据。
Anti-fatigue activity of selenium nanoparticles functionalized by polysaccharides from Pleurotus tuber-regium sclerotium
-
摘要:
目的 通过检测小鼠后肢肌肉相对长度、负重游泳时间及其血清和肝脏相关指标,探究虎奶菇菌核多糖功能化纳米硒(PTR-SeNPs)的体内抗疲劳功效。 方法 48只雄性C57/BL6小鼠分为4组,每组12只,即对照组、游泳训练组(EC组)、PTR-SeNPs低剂量组(LPTR-SeNPs组)、PTR-SeNPs高剂量组(HPTR-SeNPs组),分别给予生理盐水(对照组与EC组)、LPTR-SeNPs[2.5 μmol/(kg·bw)]和HPTR-SeNPs[10 μmol/(kg·bw)],1次/d,连续灌胃21 d。通过磁共振成像系统分析PTR-SeNPs对小鼠游泳训练后肢肌肉结构的影响,同时测定小鼠的负重游泳时间并检测血清中血乳酸(BLA)、血尿素氮(BUN)、谷丙转氨酶(ALT)、谷草转氨酶(AST)和乳酸脱氢酶(LDH)含量及肝脏中肝糖原(HG)、丙二醛(MDA)水平和过氧化氢酶(CAT)、超氧化物歧化酶(SOD)活力。 结果 与对照组比较,EC组小鼠血清BLA、BUN、ALT、AST及LDH含量显著升高(P<0.05或P<0.01),HPTR-SeNPs组小鼠肝脏中CAT含量显著升高(P<0.01),小鼠后肢肌肉相对长度显著增长(P<0.05),负重力竭游泳时间提高(P<0.05),L/HPTR-SeNPs组MDA水平无明显差异;与EC组比较,HPTR-SeNPs组小鼠负重游泳时间显著延长(P<0.01),BLA及BUN含量均显著降低(P<0.05或P<0.01),L/HPTR-SeNPs组HG含量显著增加(P<0.05或P<0.01),HPTR-SeNPs组血清ALT、AST及LDH水平均显著降低(P<0.05或P<0.01),肝脏CAT活力显著升高(P<0.01),LPTR-SeNPs组血清AST活力显著降低(P<0.05)、肝脏SOD活力显著升高(P<0.05)。 结论 PTR-SeNPs具有改善肝脏生理功能、增加糖原储备、减少代谢物堆积及增强机体抗氧化能力,以及减缓疲劳的作用,具有开发成保健品或药品的潜力。 Abstract:Objective To investigate the anti-fatigue effect of PTR-SeNPs in vivo by measuring the muscle relative length of hindlimb, load-bearing swimming time as well as serum and liver indexes of mice. Methods 48 male C57/BL6 mice were randomly assigned into 4 groups with 12 mice in each group, including vehicle control group (control group), swimming training exercise group (EC group) with vehicle treatment, swimming training exercise with low dose of PTR-SeNPs group (LPTR-SeNPs) and high dose of PTR-SeNPs group (HPTR-SeNPs). The mice were intragastrically administrated with normal saline in both control group and EC group, as well as 2.5 and 10 μmol/(kg·bw) PTR-SeNPs in LHPTR-SeNPs group, respectively, once per day for consecutively 21 days. After swimming training exercise, the muscle structures in the hind limb of mice were examined by magnetic resonance imaging. Furthermore, the burdened swimming time was measured, the serum content of blood lactic acid (BLA), urea nitrogen (BUN), alanine aminotransferase (ALT), glutamic oxalate aminotransferase (AST) and lactate dehydrogenase (LDH), as well as the hepatic level of glycogen (HG), malondialdehyde (MDA) and activity of catalase (CAT) and superoxide dismutase (SOD) were determined. Results Compared with the control group, the serum contents of BLA, BUN, ALT, AST and LDH in EC group (P<0.05 or P<0.01) and hepatic CAT in HPTR-SeNPs group (P<0.01) were significantly increased. The muscle relative length of hind limbs and the burdened swimming time were extended by HPTR-SeNPs markedly (P<0.05). There was no significant difference in MDA level in LHPTR-SeNPs group. Compared with EC group, the burdened swimming time of mice was significantly prolonged (P<0.01), the contents of BLA and BUN were obviously decreased in the HPTR-SeNPs group (P<0.05 or P<0.01), the level of HG was significantly increased in the LHPTR-SeNPs groups (P<0.05 or P<0.01), the serum content of ALT, AST and LDH were markedly decreased in the HPTR-SeNPs group (P<0.05 or P<0.01). Hepatic SOD activity was remarkably increased in LPTR-SeNPs group (P<0.05), the level of CAT was evidently increased (P<0.01) and AST was decreased (P<0.05) in the HPTR-SeNPs group. Conclusion PTR-SeNPs could improve the liver physiological function, increase glycogen storage, reduce the accumulation of metabolites and enhance the body’s antioxidant capacity to ameliorate fatigue significantly, which could present the potential to be developed into health care products or drugs. -
Key words:
- PTR-SeNPs /
- swimming training exercise /
- antioxidant stress /
- anti-fatigue effect
-
肠道菌群是定植在人体内复杂而庞大的微生物群落,成人肠道中有多达上千种的细菌,基因数超过人类100多倍[1]。在已确定的细菌门类中,厚壁菌门和拟杆菌门占95%以上,很大程度上影响着整个菌群的功能[2]。在漫长的生物共演化过程中,这些肠道菌群与人体形成了相互依存的共生关系。肠道菌群及其代谢产物具有协助消化吸收食物、合成维生素和能量、保护肠道黏膜屏障等功能,在参与人体重要代谢、抵御外来致病菌侵袭以及调节免疫机制等方面发挥重要作用[3]。多项研究表明,大量慢性疾病的发生与体内肠道菌群结构的改变有关,包括炎性肠病、代谢性疾病、肥胖或营养不良、神经系统疾病和心血管疾病等[4-5]。
成骨细胞和破骨细胞参与骨形成和吸收的过程称为骨代谢,成人的骨代谢活动处于动态平衡状态,一旦因各种原因导致骨吸收与骨形成失衡,就会引起局部或整体骨代谢异常,引发骨质疏松症、关节病、类风湿等一系列骨代谢疾病[6]。其中,骨质疏松症是由多种原因引起,以骨量降低、骨组织微结构损坏为特征,导致骨脆性增加、骨折风险增高的常见全身性骨代谢疾病。近年来,肠道菌群调控骨代谢的研究受到广泛关注,已有较多研究发现肠道菌群与骨量减少及骨质疏松症的发病关系密切[4],肠道中的微生物可通过影响营养吸收、产生代谢产物、调控宿主内分泌及免疫系统等途径影响骨代谢。中药具有多成分、多靶点的作用机制,目前中药干预肠道菌群调控骨代谢研究亦有较多报道。本文将对肠道菌群影响骨代谢机制及中药经此途径调控骨代谢相关研究进展进行综述。
1. 肠道菌群与骨代谢关系研究
1.1 肠道菌群通过营养吸收影响骨代谢
肠道菌群可通过影响机体对营养物质的吸收来调控骨代谢。肠道菌群结构发生变化可改变肠道局部环境的pH值,影响大分子物质和矿物质的吸收。其中,乳酸杆菌和双歧杆菌浓度升高可促进钙、镁和磷等矿物质的吸收,从而增加骨矿物质密度[7]。此外,肠道菌群可通过胆汁酸代谢途径来调控骨代谢,其不仅参与原代胆汁酸的转化,而且可以使之脱羟基化产生次级胆汁酸参与代谢,从而促进维生素D的吸收,影响成骨细胞和破骨细胞的形成[8]。同时,肠道菌群可影响肠道胆汁酸库的组成,并调控结肠Foxp3调节性T细胞(Tregs)群体,进而调控肠道免疫反应影响骨代谢[9-10]。另外,有研究显示胆汁酸对于肠道中钙离子的吸收也有一定的影响[11]。可见,肠道菌群对宿主营养物质的吸收、合成具有重要作用,菌群中的有益菌可对骨骼健康产生积极影响。
1.2 肠道菌群代谢物短链脂肪酸调控骨代谢
短链脂肪酸(SCFAs) 是膳食纤维经肠道菌群发酵产生的代谢产物,主要包含乙酸、丙酸和丁酸。SCFAs的产生依赖于肠道菌群,其中梭状杆菌、双歧杆菌、乳酸菌等是产生SCFAs的主要菌群。反之,SCFAs可通过降低肠道pH值,抑制各种致病菌生长等作用,来维持肠道菌群的平衡,影响肠道菌群结构。两者或单独或协同在宿主的营养吸收、免疫、内分泌等多方面发挥重要作用[4]。
SCFAs可直接作用于骨细胞调控骨代谢。Lucas等[12] 研究发现,外源补充丙酸和丁酸可降低小鼠破骨细胞相关基因TRAF6和NFATc1表达,调节破骨细胞分化,抑制体外和体内的骨吸收而不影响骨形成,从而显著增强骨量。而Martinis等[13] 研究发现,SCFAs中的丁酸盐,可能通过诱导组蛋白乙酰化,调控miRNA表达,发挥调控骨结构、骨重吸收和成骨细胞分化间平衡的作用。总之,在肠菌代谢物直接作用于骨细胞的过程中,肠内SCFAs浓度的增加可有效抑制骨吸收,减缓骨流失。
1.3 肠道菌群调控氧化应激影响骨代谢
肠道菌群可通过调控机体氧化应激对骨代谢产生影响。益生菌能通过多种途径降低机体的氧化应激,如与金属离子Fe2+、Cu2+等发生螯合反应,产生谷胱甘肽、丁酸盐、叶酸等具有抗氧化活性的代谢产物,介导Nrf2-Keap1-ARE、NFκB、MAPK、PKC等抗氧化信号通路,调控机体抗氧化酶反应等[14]。若有害菌过度增殖,则会诱导血液中的内毒素引起明显的氧化应激反应。已有研究证实,过度的氧化应激在骨重塑过程中,会促进骨形成相关细胞如骨髓间充质干细胞、成骨细胞,并促进骨细胞的凋亡和破骨细胞的增殖及分化,导致骨形成速率相对骨吸收速率滞后,打破骨代谢的动态平衡[15]。
1.4 肠道菌群调控免疫影响骨代谢
1.4.1 肠道菌群影响炎症因子调控免疫
肠道菌群可通过影响炎症因子表达诱导机体免疫调控骨代谢。肠菌失调可直接刺激炎性细胞因子表达,导致破骨细胞过度活跃,加速骨流失,影响骨稳态。研究显示,与普通小鼠相比,无菌小鼠炎症因子、B细胞和T细胞的表达均减少,而骨小梁数目、骨密度则明显增加[16]。另有研究显示,特定种类的梭状芽孢杆菌比例增高,会促进肠道中CD4 T细胞向 Th17 细胞和 Treg 细胞的分化[17]。其中,Th17细胞数量增加可导致IL-17、TNF-α和IL-1水平上升,从而诱导 RANKL的表达,加速破骨细胞生成[18];Treg细胞则通过促炎细胞因子IL-4、IL-10、TGF-β等,间接促进破骨细胞活化加速骨吸收[19]。值得注意的是,肠菌代谢物丁酸盐可直接调节CD4 T、CD8 T和Tc17细胞的基因表达,诱导小鼠结肠Treg细胞分化,并通过为肠黏膜上皮细胞提供能量、改善肠道屏障等多角度影响宿主免疫系统[20];双歧杆菌、嗜热链球菌等多种益生菌,同样参与诱导Th17 细胞和 Treg 细胞的分化[21]。
1.4.2 肠道菌群影响肠黏膜屏障功能
肠道菌群还能通过维持肠道上皮黏膜屏障功能调控机体免疫影响骨代谢。肠黏膜屏障是由杯状细胞分泌的黏蛋白形成的黏液层,是肠道菌群的能量来源。健康的黏液层可减少肠内菌群与内层上皮细胞接触,具有抑制细菌黏附、限制免疫反应等作用[22]。研究显示,肠菌结构和代谢物的变化可直接造成黏蛋白减少,肠道通透性增加,导致肠道中有害菌及代谢物进入机体,引发免疫应答,最终刺激破骨细胞的激活,促进骨吸收[23]。另外,Wang等[24]研究发现,将老年性骨质疏松大鼠的粪菌移植到幼龄大鼠体内可诱发年轻大鼠骨质疏松。粪菌移植后,模型大鼠肠道菌群组成和结构发生改变,影响了黏蛋白表达,导致肠结构损伤、绒毛缩短、肠通透性增加,肠屏障功能严重受损;而骨钙素、CTX和P1NP显著升高,骨体积、骨体积密度、骨小梁数显著降低。通过KEGG预测发现,引起骨质流失的主要原因可能与模型大鼠RIG-I样受体信号通路显著增加有关。
可见,在肠道菌群调控免疫影响骨代谢的过程中,有害菌过度增殖或过量益生菌均可使肠菌结构发生改变,导致机体炎症因子表达上调,肠黏膜屏障功能受损,加速骨吸收。
1.5 肠道菌群通过调节内分泌影响骨代谢
1.5.1 肠道菌群与雌激素相互作用
肠道菌群可通过与雌激素代谢间的相互作用调控骨代谢。雌激素戒断会增加肠道通透性致使肠道菌群结构发生变化,降低菌群多样性;同时肠道菌群结构改变,菌群将减少β-葡萄糖醛酸酶的分泌,继而降低肠内雌激素吸收[25]。雌激素可通过与雌激素受体结合直接作用骨细胞、改变转录因子活性、降低NF-κB活性、调节自噬等多种途径影响骨代谢,是维持骨代谢平衡的重要物质[26]。Ma等[27]研究发现,切除双侧卵巢(OVX)的大鼠肠道菌群严重失调,菌群中厚壁菌门与拟杆菌门比值显著增加;其中瘤胃球菌属、梭菌属和粪球菌属与破骨性指标呈正相关,且与OVX大鼠的骨量丢失趋势一致;拟杆菌属和丁酸弧菌属则与骨量丢失呈负相关。黄俊俊等[28]在对OVX小鼠骨折愈合效果的研究中发现,补充益生菌可升高血钙及骨钙素水平,增加骨小梁数量和骨密度,促进小鼠骨折部位愈合。该实验为改善肠道菌群可正向调节雌激素缺乏所引起的骨丢失提供了有力的依据。
1.5.2 肠道菌群诱导甲状旁腺激素的合成代谢
肠道菌群可通过多种方式调控甲状旁腺激素代谢,对骨代谢产生影响。甲状旁腺激素是细胞外钙和磷酸盐水平的主要内分泌调节剂,可促进骨骼形成和骨吸收[29]。研究发现,在原发性和继发性甲状旁腺功能亢进症小鼠模型中,肠内分节丝状细菌过度增殖,是引起肠道Th17细胞增高,刺激甲状旁腺激素诱导骨吸收的主要原因[30]。Li等[31]则从肠菌代谢物丁酸盐信号通路角度展开研究,发现丁酸盐可通过诱导甲状旁腺激素的合成代谢,增加Tregs细胞的数量,并通过CD8 T细胞刺激成骨Wnt配体Wnt10b的产生,进而激活Wnt信号传导促进骨形成。
1.5.3 肠道菌群及SCFAs调控IGF-1水平
肠道菌群及其代谢物SCFAs均可通过干预宿主体内胰岛素样生长因子-1(IGF-1)水平影响骨代谢[32]。有研究显示,相较于普通小鼠,无菌小鼠的IGF-1 水平更低[33]。Yan等[34]研究发现,抗生素治疗可降低小鼠血清IGF-1,并抑制骨形成。给抗生素治疗的小鼠补充SCFAs后,IGF-1和骨量均可恢复至抗生素治疗前水平。另外,该研究表明无菌小鼠定植SPF肠道菌群的初期,会出现急性骨吸收增加导致骨量减少。定植1个月后,骨吸收消退,骨形成占据主导,测得定植小鼠肠内SCFAs浓度、血清IGF-1相较无菌小鼠均显著升高,提示菌群定植的时长也是影响骨代谢的关键因素。
1.5.4 肠道菌群调节5-羟色胺合成
肠道菌群还可通过诱导肠嗜铬细胞(EC)中5-羟色胺(5-HT)的合成调控骨代谢[35]。肠菌代谢产生的SCFAs可增加TPH1 mRNA表达并与EC细胞合成5-HT[36] 。5-HT是机体重要的激素和神经递质,其信号转导在骨骼发育和维护中起着重要的调节作用。研究发现,口服5-HT合成初始酶Tph-1抑制剂6周可升高OVX小鼠成骨细胞数量、骨形成率和血清骨钙素水平,显著改善骨质疏松症状[37]。而转录因子FOXO1可能是5-HT抑制成骨细胞增殖、降低成骨细胞与破骨细胞的比例的关键因素[38] 。
2. 中药干预肠道菌群调控骨代谢
传统中医认为骨质疏松症以肾精亏虚、骨枯髓减为本,以淤血痹阻、骨络失荣为标。病位主要在肝、肾、脾,临床治疗以温补肝肾为主,辅以健脾活血[39]。现代药理研究表明,补益类中药如淫羊藿、杜仲、黄芪等富含黄酮类、多糖等有效成分,对骨质疏松具有显著的疗效。近年研究发现,补益类中药发挥抗骨质疏松症作用的同时,可以调节机体的肠菌结构,并且可能通过干预肠道菌群结构和丰度、调节机体脂质和胆汁酸代谢、保护肠黏膜屏障功能、减少炎症因子释放、调节自噬等方面对骨质疏松的治疗发挥积极的作用。与此同时,有研究发现,黄连、葛根、马齿苋等清热药在调节肠道菌群结构,发挥解热抗炎作用的过程中,可以缓解宿主骨质疏松的症状。提示清热药可能通过调节宿主的肠道菌群发挥抗骨质疏松的作用。以下列举部分中药及复方的相关研究。
2.1 清热类中药
2.1.1 黄连
黄连主要成分黄连素是异喹啉生物碱类化合物,具有广谱抑菌作用,临床常用于治疗胃肠炎、细菌性痢疾等肠道感染。Jia等[40]研究发现,OVX-牙周炎大鼠在给予黄连素后,肠道菌群结构发生改变,肠菌代谢物丁酸盐显著增加,肠屏障功能明显好转,相关炎症因子IL-17A表达显著降低,缓解了牙周骨质流失。这一实验表明,黄连素可能通过改变肠菌结构,调节宿主免疫等方面对改善骨代谢产生积极影响。
2.1.2 葛根
葛根在传统论著中多用于表征发热、脾虚泄泻、消渴等病症。而现代药理研究发现,葛根中黄酮、萜类、有机酸等成分均具有广泛的药理活性。其中异黄酮类化合物葛根素具雌激素样作用,可用于绝经后骨质疏松的治疗。Li等[41]研究发现,葛根素可扭转OVX大鼠雌激素缺乏导致的肠菌失调,增加肠道内SCFAs含量,修复损伤的肠黏膜,降低结肠上皮通透性,减少TNF-α、IL-6、IL-1β等相关炎症因子释放,从而改善骨微环境。提示葛根素通过干预肠道菌群调节宿主免疫可能是其改善骨质疏松的途径之一。
2.1.3 马齿苋
马齿苋性寒味酸,具有清热燥湿、凉血解毒的作用,临床常用于湿热、热毒引起的痢疾。冯澜等[42]研究发现,马齿苋多糖可以调节脂多糖诱导的结肠炎小鼠肠道菌群结构,上调双歧杆菌和乳杆菌丰度, 降低肠道炎症因子释放,发挥抗炎、调节免疫的作用。另有研究发现,马齿苋可降低脂多糖诱导的溶骨小鼠的促炎症因子,抑制其破骨细胞分化,发挥抗骨质疏松的疗效[43]。提示在脂多糖诱导的炎症模型中,马齿苋可能通过干预肠道菌群调控宿主的免疫,进而影响骨代谢。
2.2 补益类中药
2.2.1 淫羊藿
淫羊藿是传统的补肝肾、强筋骨中药,现代药理学证实其有效成分淫羊藿苷可通过诱导骨形成、抑制骨吸收等途径改善骨质疏松症状[44]。Wang等[45]研究发现,淫羊藿苷可以改变OVX大鼠肠道菌群结构及粪便代谢物组成。其中乳酸菌等关键菌群丰度的上升,可显著上调机体胆汁酸、氨基酸和脂肪酸的代谢,从而提高OVX大鼠雌激素水平,产生抗骨质疏松作用。提示通过调节肠道菌群影响雌激素代谢可能是淫羊藿苷干预、防治骨质疏松的潜在途径。
2.2.2 女贞子
女贞子具有滋补肝肾的功效,临床上常配伍淫羊藿等药物治疗骨质疏松[46]。Chen等[47]研究发现,女贞子水提物可通过改变肠道菌群结构和丰度、促进肠道SCFAs生成等途径,改善OVX大鼠十二指肠绒毛形态,增加肠隐窝深度和吸收面积,从而增强肠道中钙结合蛋白D9k的表达,促进钙离子吸收,发挥抗骨质疏松作用。
2.2.3 杜仲
中药杜仲以干燥树皮入药,具有补肝肾、强筋骨、安胎的作用。现代研究表明,杜仲枝干、叶、花具有与树皮相似的化学成分和药理作用[48]。Zhao等[49]研究发现,杜仲叶醇提物可以在体外促进保加利亚乳杆菌的生长,有效抑制破骨细胞生成。在体内研究中,杜仲叶醇提物能够调节快速衰老模型小鼠的肠道菌群,具体表现在丰富肠菌多样性,调控厚壁菌门/拟杆菌门比例,增加小鼠血清及粪便中SCFAs浓度等方面,对抑制破骨细胞生成,提高骨密度,改善骨质疏松具有潜在影响。
2.2.4 黄芪
中药黄芪长于健脾补中,治疗脾、肺气虚证。现代药理学研究证实黄芪中富含皂苷、多糖等多种活性成分,具有广泛的药理活性。Liu等[50]研究发现,黄芪多糖可以显著改善地塞米松模型大鼠的肠菌结构,通过上调乳杆菌属、经黏液真杆菌属等益生菌群,下调梭状芽孢杆菌、普雷沃氏菌属等致病菌,显著降低酸性磷酸酶5和促炎细胞因子IL-2和TNF-α的生成,抑制破骨细胞生成,改善骨质疏松。
另外,有研究显示,肠道菌群可通过合成和代谢维生素B和维生素K促进成骨细胞向骨细胞转化,抑制破骨细胞生成,介导骨钙素羧化过程等发挥抗骨质疏松作用 [51-52]。因此,在研究中药干预肠道菌群结构的同时,菌群对宿主体内药物的吸收和代谢效果,可能影响到药物抗骨质疏松的疗效,同样值得关注。
2.3 中药复方
2.3.1 仙灵骨葆胶囊
仙灵骨葆胶囊具有滋补肝肾,活血通络,强筋壮骨的功效,临床常用于治疗肝肾不足,瘀血阻络所致的骨质疏松症。Tang等[53]基于LC-MS和宏基因组测序的方法,对仙灵骨葆胶囊在肠道菌群作用下的代谢、转化进行了定量分析。研究发现,仙灵骨葆胶囊可以改变OVX大鼠的肠道菌群结构,加速药物脱糖基化反应,有利于活性成分淫羊藿苷、补骨脂素和异补骨脂素等的吸收代谢;同时,仙灵骨葆胶囊可以促进拟杆菌、普氏菌和益生菌乳酸杆菌的生长,帮助调节机体脂质和胆汁酸代谢,改善骨质疏松。可见,在仙灵骨葆胶囊吸收代谢的过程中,肠道菌群发挥了重要作用;同时,仙灵骨葆胶囊可能通过干预肠道菌群对宿主的骨代谢产生潜在的作用。
2.3.2 补肾化痰方
熊孟欣[54]在结合多样性和宏基因组测序发现,补肾化痰方在改善OVX大鼠骨质疏松的同时,纠正了OVX大鼠失调的肠道菌群。研究显示,给药组肠道菌群中厚壁菌门丰度增加,拟杆菌门、变形菌门丰度减少,肠菌结构与空白组趋于一致。该变化有助于升高粪便和血清中SCFAs浓度,从而影响AMPK/m TOR/ULK1自噬信号通路调控, 促进OVX大鼠体内自噬。
2.3.3 健骨颗粒
健骨颗粒由淫羊藿、骨碎补、山茱萸等10味药组成,是临床上治疗绝经后骨质疏松的常用方剂。Sun等[55]研究发现,健骨颗粒能够改善雌激素缺乏引起的肠菌紊乱,恢复肠道菌群丰度,并通过GM-SCFAs-Treg/Th17轴,调节骨免疫相关细胞因子,有效减少骨质流失,改善OVX大鼠骨质疏松。
2.3.4 葛根芩连汤
葛根芩连汤由葛根、黄芩、黄连、甘草四味药组成,具解表清里之功效,善治急性肠炎、细菌性痢疾等,在临床上对胃肠道疾病、糖尿病及脂代谢疾病均有良好的疗效。长期糖脂代谢异常往往伴随骨代谢失调,王雅婷等[56]在对糖尿病继发骨质疏松大鼠的研究中发现,葛根芩连汤在治疗糖尿病的同时,模型大鼠的骨密度、骨钙含量、极限载荷等均有所回升。而多项研究表明,葛根芩连汤可以通过改善肠道菌群的结构、多样性,抑制致病菌,增加肠内SCFAs浓度等方面干预肠道菌群调控宿主的糖脂代谢,对糖尿病继发骨质疏松症的治疗发挥积极的作用 [57-58]。
3. 小结与展望
肠道菌群与骨代谢之间存在着密切的关联,肠菌结构改变可诱发机体骨代谢异常,导致骨量降低,造成骨质疏松症患病风险增加,是原发性骨质疏松症的重要致病机制之一。目前,对于肠道菌群调控骨代谢的研究大多停留在16S rRNA菌群种属多样性差异分析以及表型的检测,以此来预测其潜在的途径和机制。随着微生物组学测序技术的不断发展,在未来的研究中,可以通过但不限于宏基因组学、靶向代谢组学等分析手段,深入探索肠道菌群中不同门类细菌对骨代谢的影响,确定与骨骼健康密切相关的关键菌种,挖掘相关靶点和分子机制,为肠道菌群调控骨代谢提供更多理论依据。
此外,越来越多的研究证实药物-微生物组的互作机制对菌群平衡和药物疗效有重要的影响,因此,深入研究传统抗骨质疏松中药及复方与肠道菌群的互作机制,发现药物干预肠道菌群调控骨代谢的潜在靶点和通路,通过调节肠道菌群组成,优化药物对骨代谢相关疾病的治疗效果;挖掘清热药通过干预肠道菌群调控宿主脂质代谢和炎症反应发挥抗骨质疏松作用的潜力,或将为改善骨骼健康、防治骨代谢相关疾病提供新思路,为骨质疏松症治疗药物的研究提供新的方向。
-
-
[1] 代朋乙, 黄昌林. 运动性疲劳研究进展[J]. 解放军医学杂志, 2016, 41(11):955-964. doi: 10.11855/j.issn.0577-7402.2016.11.14 [2] 徐艺园, 王凯, 王佳音, 等. 疲劳产生相关机制研究进展[J]. 科学技术创新, 2019, (23):50-51. doi: 10.3969/j.issn.1673-1328.2019.23.030 [3] LIU G Y, YANG X, ZHANG J X, et al. Synthesis, stability and anti-fatigue activity of selenium nanoparticles stabilized byLycium barbarum polysaccharides[J]. Int J Biol Macromol, 2021, 179:418-428. doi: 10.1016/j.ijbiomac.2021.03.018 [4] WANG X, QU Y D, ZHANG Y F, et al. Antifatigue potential activity of Sarcodon imbricatus in acute excise-treated and chronic fatigue syndrome in mice via regulation of Nrf2-mediated oxidative stress[J]. Oxidative Med Cell Longev, 2018, 2018:9140896. [5] TANG W, JIN L, XIE L, et al. Structural characterization and antifatigue effect in vivo of maca (Lepidium meyenii walp) polysaccharide[J]. J Food Sci, 2017, 82(3):757-764. doi: 10.1111/1750-3841.13619 [6] ZHANG X Y, JING S, LIN H J, et al. Anti-fatigue effect of anwulignan via the NRF2 and PGC-1α signaling pathway in mice[J]. Food Funct, 2019, 10(12):7755-7766. doi: 10.1039/C9FO01182J [7] YAN J K, QIU W Y, WANG Y Y, et al. Fabrication and stabili-zation of biocompatible selenium nanoparticles by carboxylic curdlans with various molecular properties[J]. Carbohydr Polym, 2018, 179:19-27. doi: 10.1016/j.carbpol.2017.09.063 [8] MENON S, SHANMUGAM V K. Chemopreventive mechanism of action by oxidative stress and toxicity induced surface decorated selenium nanoparticles[J]. J Trace Elem Med Biol, 2020, 62:126549. doi: 10.1016/j.jtemb.2020.126549 [9] 徐军军, 李治堃, 黄飞龙, 等. 响应面法优化水溶性虎奶菇多糖提取工艺及其对胃癌细胞的抑制作用[J]. 食品工业科技, 2020, 41(7):185-189,202. doi: 10.13386/j.issn1002-0306.2020.07.031 [10] 王慧宾, 王爽, 翁雅青, 等. 虎奶菇中多糖的提取工艺、结构分析和生物活性研究进展[J]. 江西科学, 2019, 37(6):836-838,850. doi: 10.13990/j.issn1001-3679.2019.06.004 [11] WU G H, HU T, HUANG Z L, et al. Characterization of water and alkali-soluble polysaccharides from Pleurotus tuber-regium Sclerotia[J]. Carbohydr Polym, 2013, 96(1):284-290. doi: 10.1016/j.carbpol.2013.03.036 [12] WONG K H, LAI C K M, CHEUNG P C K. Stimulation of human innate immune cells by medicinal mushroom sclerotial polysaccharides[J]. Int J Med Mushr, 2009, 11(3):215-223. doi: 10.1615/IntJMedMushr.v11.i3.10 [13] CHEN T F, WONG K H, WU H L, et al. Pleurotus tuber-regium polysaccharide functionalized nano-selenium hydrosol with anti-tumor activity and preparation method thereof[P]. USA: US9072669B2, 2013-01-31. [14] YIP J, LIU L, WONG K H, et al. Investigation of antifungal and antibacterial effects of fabric padded with highly stable selenium nanoparticles[J]. J Appl Polym Sci, 2014, 131(17):40728. [15] WU H, LI X, LIU W, et al. Surface decoration of selenium nanoparticles by mushroom polysaccharides–protein complexes to achieve enhanced cellular uptake and antiproliferative activity[J]. J Mater Chem, 2012, 22(19):9602-9610. doi: 10.1039/c2jm16828f [16] ZHANG Z H, DU Y X, LIU T, et al. Systematic acute and subchronic toxicity evaluation of polysaccharide-protein complex-functionalized selenium nanoparticles with anticancer potency[J]. Biomater Sci, 2019, 7(12):5112-5123. doi: 10.1039/C9BM01104H [17] YU S M, LUK K H, CHEUNG S T, et al. Polysaccharide-protein complex-decorated selenium nanosystem as an efficient bone-formation therapeutic[J]. J Mater Chem B, 2018, 6(32):5215-5219. doi: 10.1039/C8TB01084F [18] 吴建国, 马利, 杨彬君, 等. 虎奶菇多糖纳米硒对叔丁基过氧化氢诱导的HepG2细胞氧化损伤的保护作用[J]. 福建中医药, 2017, 48(1):45-46. doi: 10.3969/j.issn.1000-338X.2017.01.021 [19] YUAN T, WU D, SUN K Y, et al. Anti-fatigue activity of aqueous extracts of Sonchus arvensis L. in exercise trained mice[J]. Molecules, 2019, 24(6):1168. doi: 10.3390/molecules24061168 [20] 彭梅, 张振东, 杨娟. 土党参多糖对小鼠的抗疲劳作用[J]. 食品科学, 2011, 32(19):224-226. [21] 闫曙光, 任杰, 潘亚磊, 等. 黄芪建中汤抗疲劳机制研究[J]. 世界科学技术-中医药现代化, 2020, 22(3):799-803. [22] 白海军, 王颖. 发酵法制备芸豆渣可溶性膳食纤维及其抗运动性疲劳作用研究[J]. 食品工业科技, 2022, 43(13):367-372. [23] 姚乐辉. 化橘红多糖抗氧化能力及抗疲劳作用的研究[J]. 粮食与油脂, 2019, 32(4):95-100. doi: 10.3969/j.issn.1008-9578.2019.04.026 [24] 谢飞飞. 远志多糖对力竭运动小鼠体内抗疲劳和体外抗氧化作用研究[J]. 食品工业科技, 2021, 42(6):332-336. doi: 10.13386/j.issn1002-0306.2020050292 [25] 王转丁, 杨晶晶, 李留安, 等. 不同强度游泳应激对小鼠血清ALT、AST和ALP活性的影响[J]. 黑龙江畜牧兽医, 2013, (21):164-166. doi: 10.13881/j.cnki.hljxmsy.2013.21.063 [26] 秦汝兰, 关颖丽, 吕重宁. 山刺玫果多糖抗疲劳及抗氧自由基水平机制研究[J]. 食品工业科技, 2019, 40(22):311-315. doi: 10.13386/j.issn1002-0306.2019.22.054 [27] 龚云, 陈晨. 基于知识图谱的国内运动性疲劳研究进展分析[J]. 西北成人教育学院学报, 2022, (1):67-74. [28] ZHANG Y F, WANG J G, ZHANG L N. Creation of highly stable selenium nanoparticles capped with hyperbranched polysaccharide in water[J]. Langmuir, 2010, 26(22):17617-17623. doi: 10.1021/la1033959 [29] SHI X D, TIAN Y Q, WU J L, et al. Synthesis, characterization, and biological activity of selenium nanoparticles conjugated with polysaccharides[J]. Crit Rev Food Sci Nutr, 2021, 61(13):2225-2236. doi: 10.1080/10408398.2020.1774497 -





 下载:
下载: