-
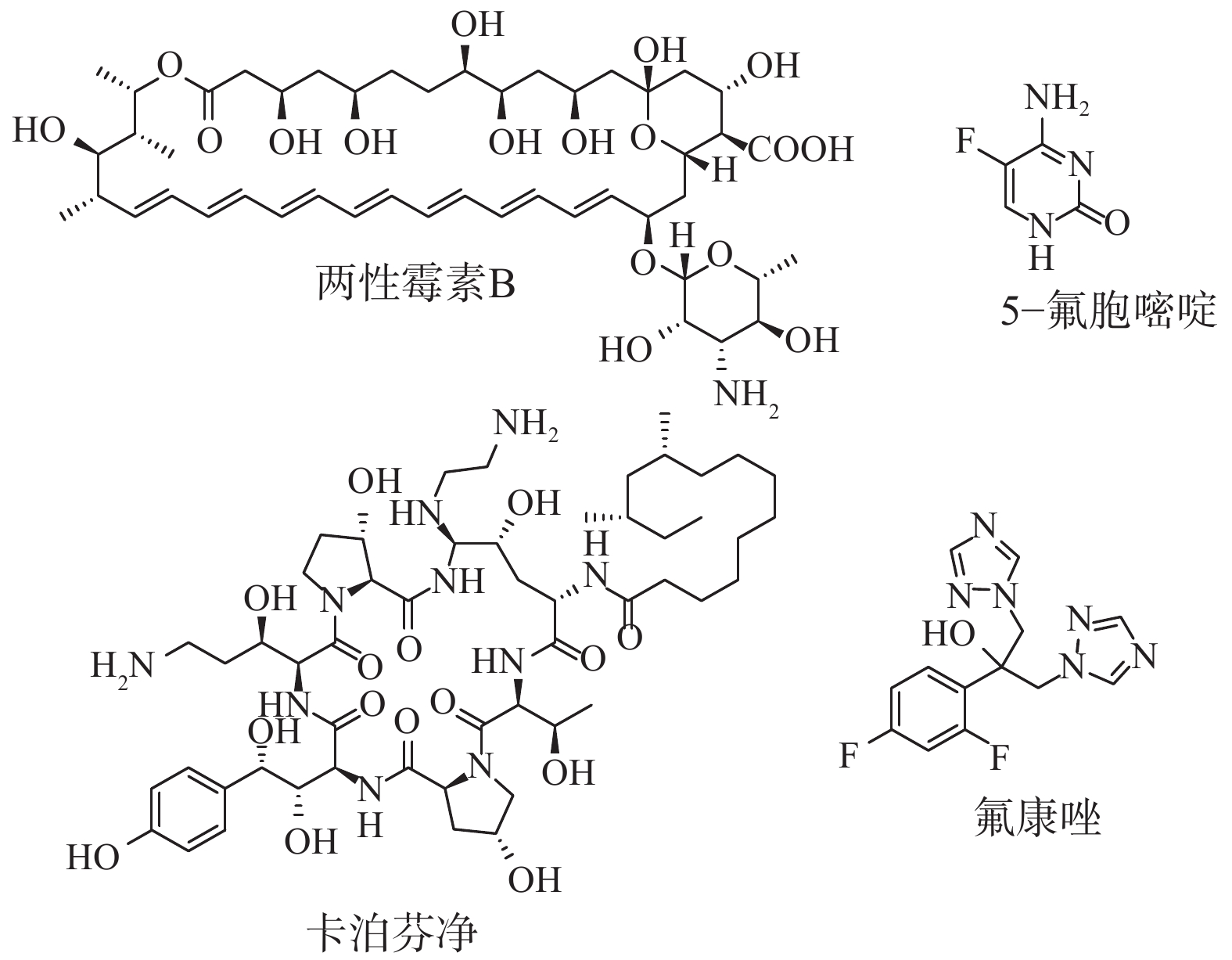
近年来,随着肿瘤、器官移植和获得性免疫缺陷综合征(AIDS)等导致的免疫功能低下人群的增加,侵袭性真菌感染(IFIs)的发病率和病死率逐年上升[1-2]。念珠菌、隐球菌和曲霉菌是IFIs最主要的致病菌,并且造成的病死率超过90%[3]。在念珠菌属中,白念珠菌(Candida. albicans)是院内血液感染最常见的致病菌原体,其在重症监护病房(ICU)患者中致病率超过17%,病死率高达40%[4-5]。临床上治疗IFIs的抗真菌药物主要包括:多烯类(两性霉素B)、核酸类(5-氟胞嘧啶)、唑类(氟康唑)和棘白菌素类(卡泊芬净)药物(图1)[6-7]。然而,由于临床上出现抗真菌药物严重的耐药性和毒副作用,IFIs的治疗效果相当有限。因此,迫切需要研发全新机制的抗真菌药物。
组蛋白乙酰化修饰(包括组蛋白乙酰化和去乙酰化)是表观遗传学研究的重要组成部分。组蛋白去乙酰化酶(HDACs)将组蛋白和其他蛋白上的赖氨酸末端乙酰基去除,对染色体重塑和基因的表达起着重要作用[8-9]。目前HDAC抑制剂主要集中于抗肿瘤研究方向,且已有多个上市药物应用于肿瘤的治疗。据研究报道,真菌中的HDACs,如烟曲霉[10]、白念珠菌[11-12]、酿酒酵母[13]和新生隐球菌的HDACs[14-15]参与了毒力相关的过程和形态变化。因此,抑制真菌HDACs可能是治疗IFIs的有效策略。
联合药物治疗是提高临床一线药物疗效并克服真菌耐药性的有效策略之一。真菌的耐药性涉及转录调节,其中染色体重塑和组蛋白修饰起主要作用。HDACs调节的组蛋白修饰在应激信号通路中起着至关重要的作用,这可能与真菌对各种环境(包括药物)的应激反应有关[16]。此外,已有研究报道,HDAC抑制剂与唑类药物联用具有协同增效作用[17-18]。例如,HDAC抑制剂MGCD290与氟康唑联用具有协同抗多种临床真菌分离株的作用[19]。
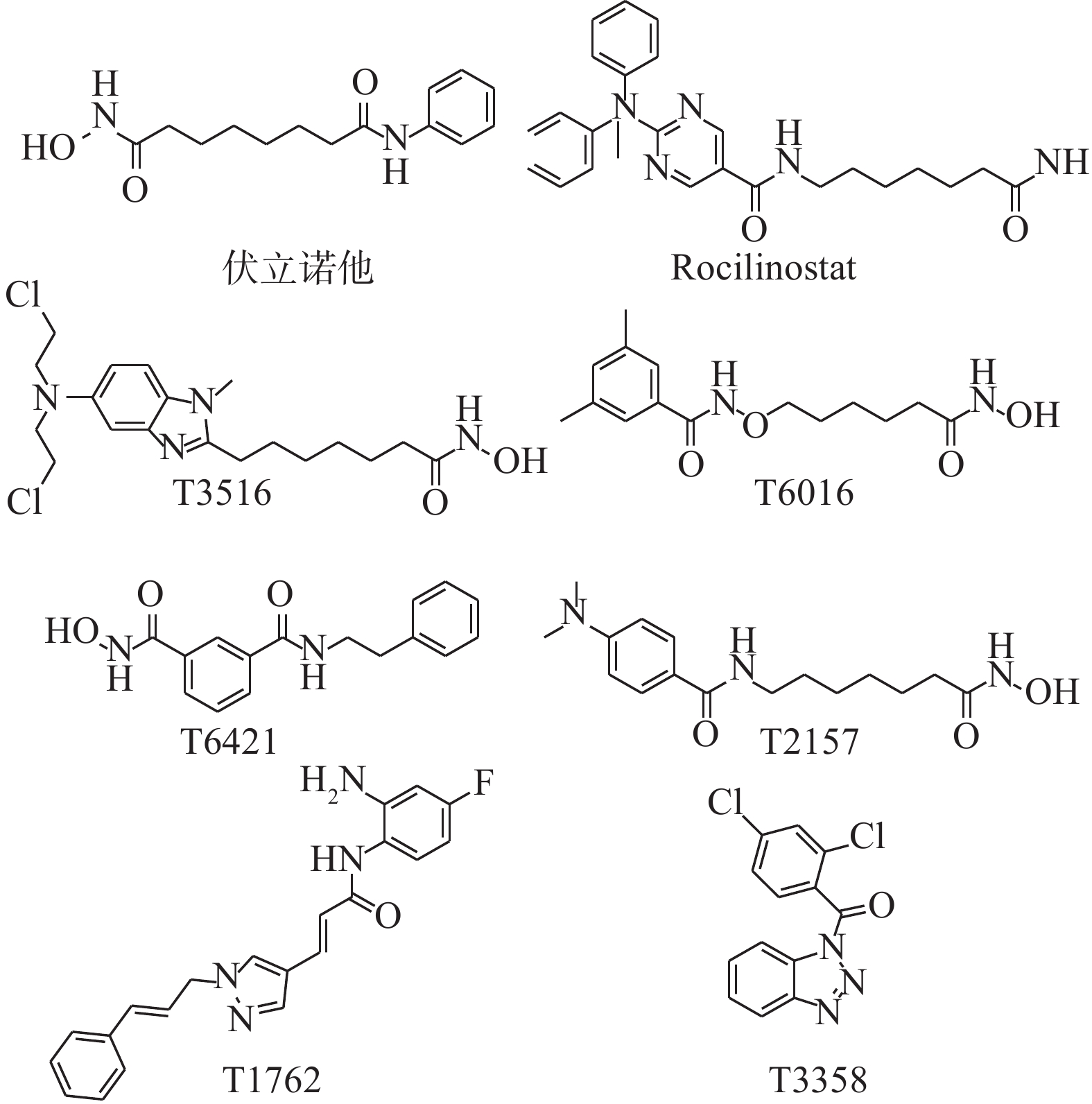
基于此,本研究首先对8个市售的HDAC抑制剂(图2)进行体外协同抗真菌活性测试,筛选结果显示化合物Rocilinostat与氟康唑联用具有优秀的体外协同抗耐药白念珠菌活性。后续考察其与不同唑类药物联用时对不同念珠菌属的体外协同抗真菌活性,以及对正常细胞的毒性作用,以期为抗真菌药物的研发提供依据。
HTML
-
临床分离的6株唑类耐药白念珠菌(编号:9893,10061,10060,9173,4108和0304103),2株唑类耐药热带念珠菌(编号:5008,10086),1株光滑念珠菌(编号:9073)和1株耳道念珠菌(编号:0029)由海军军医大学附属长征医院提供。菌株活化首先从−80 ℃中挑取菌株冻存液至YEPD液体培养基活化24 h,然后取10 μl菌悬液至1 ml YEPD中,并在30 ℃、200 r/min下培养16 h后待用。HUVEC细胞来源于中国科学院上海细胞库,并在新鲜配置的DMEM完全培养基中培养。
YEPD液体培养基:取10 g酵母浸膏、20 g葡萄糖、20 g蛋白胨溶解于1 000 ml三蒸水中,经高压蒸汽灭菌(121 ℃, 15 min)后,保存于4 ℃条件下备用。RPMI 1640培养基:取10 g RPMI 1640(Gibco)粉末、34.5 g吗啡啉丙磺酸、2 g NaHCO3、2.7 g NaOH溶解于1 000 ml三蒸水中,经0.22 μm的微孔滤膜过滤与灭菌后,置于4 ℃条件下保存和备用。DMEM完全培养基:按照89% DMEM基础培养基+10%胎牛血清+1%的双抗比例混匀制得,混匀后置于4 ℃条件下保存和备用。PBS缓冲液:10 × PBS 100 ml溶解于900 ml三蒸水中,经高压蒸汽灭菌(121 ℃, 15 min)后,置于4 ℃条件下保存和备用。
-
THZ-92A气浴恒温振荡器(上海博迅医疗生物仪器股份有限公司)、MJ-150-I霉菌培养箱(上海一恒科学仪器有限公司)、LW100T生物显微镜(北京测维光电技术有限公司)、HDC-15K高速离心机(上海泰坦科技股份有限公司)、C170二氧化碳培养箱(BINDER GmbH)、infinite M200多功能酶标仪(Tecan Austria GmbH)、高压蒸汽灭菌锅、无菌洁净工作台。
-
本实验参照美国临床和实验室标准协会(CLSI)公布的M27-A3方案中微量液基稀释法进行。首先,收集活化好的真菌细胞,PBS洗3次后用RPMI 1640培养基制成浓度为1×103 CFU/ml的菌悬液。按照每孔100 μl接种菌悬液至无菌96孔板中,1~9列加入倍半稀释的HDAC抑制剂,A~F行加入倍半稀释的氟康唑,其中G行只加氟康唑,第10列只加化合物,第11列为不加药的阴性对照组,后将96孔板置于35 °C条件下孵育48 h。测定每孔在630 nm处的吸光度A,依据公式:抑制率(%)=(A阳性对照孔−A化合物孔)/(A阳性对照孔−A阴性对照孔)× 100%,计算各孔对应的抑制率。如果某一孔和其左边孔对应的抑制率均大于80%,则该孔对应的化合物和FLC浓度分别作为FIC化合物和FIC氟康唑,利用协同指数公式:FICI =(FIC化合物./MIC80 化合物)+(FIC氟康唑/MIC80 氟康唑),计算各化合物对应的FICI。
-
收集活化好的白念珠菌0304103稀释在RPMI 1640培养液中,保持菌浓度为1×105 CFU/ml。取5 ml稀释的菌悬液和不同浓度的待测药物加入50 ml的离心管中, DMSO组作为空白对照组和32 μg/ml FLC作为阳性对照。随后将50 ml的离心管置于30 °C条件下振荡培养(200 r/min),在多个时间点吸取不同药物组的真菌混悬液(100 μl)于96孔板上,测量A630值并使用GraphPad Prism 7作图。
-
收集指数生长期的白念珠菌0304103细胞(湿重为100 mg),然后用3 mg snailase、12 μl 2-巯基乙醇和3 ml snailase反应缓冲液等新鲜配置的真菌裂解液来处理它们,以制备真菌原生质体。真菌原生质体分散在PBS(20 ml)中以获得混悬液,然后往96孔板每孔中加入100 μl的混悬液和不同浓度的化合物Rocilinostat,并在35 °C下培育12 h。接着往每个孔中加入30 μmol/L的HDAC底物,于37°C下孵育6 h。随后添加100 μl HDAC酶促终止溶液并在37°C下孵育2 h。最后,在每个孔中取出100 μl培养物添加到黑板中,用Ex=360 nm,Em=460 nm来监测荧光强度并记录下来用于计算HDAC酶的抑制率。
1.1. 实验试剂与菌株
1.2. 仪器
1.3. 棋盘式微量液基稀释法
1.4. 时间-生长曲线实验
1.5. 真菌细胞总HDAC酶活性测试实验
-
表1列出了HDAC抑制剂单独使用或与氟康唑联合使用的体外抗真菌活性筛选结果。MIC80为抑制80%真菌细胞生长的最低药物浓度。实验结果表明,8个HDAC抑制剂单独使用对耐药白念珠菌均无直接的抗真菌活性(MIC80>64 μg/ml);而化合物Rocilinostat(FICI=0.039)和伏立诺他(FICI=0.125)与FLC联用时均表现出良好的协同抗真菌活性。其中,化合物Rocilinostat的协同活性最佳,值得进一步研究。
抑制剂 抑制剂 氟康唑 FICI 单用 联用 单用 联用 伏立诺他 >64 4 >64 4 0.125 Rocilinostat >64 2 >64 0.5 0.039 T3516 >64 64 >64 64 2 T6016 >64 64 >64 64 2 T6421 >64 32 >64 32 1 T2157 >64 32 >64 32 1 T1726 >64 64 >64 64 2 T3358 >64 32 >64 64 1.5 注: FICI值≤ 0.5表示协同,FICI值> 4表示拮抗;0.5<FICI<4表示不相关。 -
为进一步考察Rocilinostat是否具广谱的抗真菌作用,挑选9株临床分离的念珠菌属菌株进行协同抗真菌活性测试。如表2所示,Rocilinostat与FLC联合使用时,对两株耐FLC的白念珠菌(C. albicans 9173,FICI=0.094; C. albicans 4108, FICI=0.5)和对FLC敏感的光滑念珠菌(C. glabrata 9073)表现出协同增效作用,而对热带念珠菌(C. tropicis)和耳道念珠菌(C. auris)没有协同抗真菌活性。当Rocilinostat与伏立康唑(VRC)联用时,对耐VRC的白念珠菌(C. albicans 10060, FICI=0.033)表现出优异的协同抗真菌活性 (表3)。
菌株 单用 联用 FICI Rocilinostat 氟康唑 Rocilinostat 氟康唑 9893 >64 >64 64 64 2 10061 >64 >64 64 64 2 10060 >64 >64 64 64 2 9173 >64 >64 4 2 0.094 4108 >64 >64 32 32 0.5 10186 >64 >64 64 64 2 5008 >64 >64 64 8 1.125 9073 32 4 32 8 0.375 0029 64 32 >64 32 1 菌株 单用 联用 FICI Rocilinostat 伏立康唑 Rocilinostat 伏立康唑 0304103 >64 >64 32 2 0.531 10061 >64 >64 32 0.125 0.502 10060 >64 >64 2 0.125 0.033 -
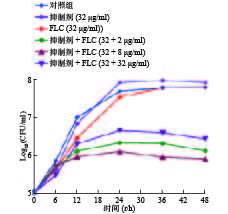
为进一步考察化合物Rocilinostat的协同抗真菌活性,我们又开展了时间-生长曲线实验。从图3结果可以看出,高浓度的氟康唑或Rocilinostat单独使用对真菌生长无抑制作用,而Rocilinostat与不同浓度的氟康唑联用能够有效抑制真菌的生长,且呈浓度依赖趋势 (图3中抑制剂为Rocilinostat)。
-
采用HUVEC(人脐静脉内皮细胞)对化合物Rocilinostat进行细胞毒性的评价。结果如表4显示,化合物Rocilinostat对正常细胞表现出低毒性,IC50值为52.17 μmol/L (22.60 μg/ml),相当于其发挥协同抗耐药真菌(C. albicans 0304103)活性MIC80值的44倍,表明Rocilinostat对真菌细胞具有较强的选择性作用。此外,我们还测试了化合物Rocilinostat对真菌总HDAC酶的抑制活性,结果表明,Rocilinostat对真菌HDAC酶抑制活性(IC50=0.41 μmol/L)优于泛HDAC抑制剂伏立诺他(IC50=1.03 μmol/L)。
化合物 HUVEC 白念珠菌(总HDAC酶) Rocilinostat 52.17 0.41 伏立诺他 — 1.03 注: “—”表示没有测试。
2.1. 化合物Rocilinostat与氟康唑联用具有协同抗真菌活性
2.2. Rocilinostat与氟康唑或伏立康唑联用对多种白念珠菌的抗真菌活性
2.3. Rocilinostat与氟康唑联用有效抑制真菌的生长
2.4. Rocilinostat对真菌细胞的选择性作用
-
本研究从市售的8个HDAC抑制剂中筛选出协同活性最佳的化合物Rocilinostat。进一步研究发现Rocilinostat与氟康唑联用对白念珠菌和光滑念珠菌具有协同增效作用。此外,化合物Rocilinostat与伏立康唑联用对临床分离的耐药白念珠菌株同样具有优秀的抗真菌活性。更值得关注的是,化合物Rocilinostat对正常细胞表现出低毒性,其对真菌细胞具有很好的选择性。因此,HDAC抑制剂Rocilinostat可以作为一种低毒、有效的唑类抗真菌药物增效剂,为抗真菌药物的发展提供了新的研究基础。








 DownLoad:
DownLoad: