-
在长期空间飞行特殊环境中,保持航天员的健康是航天任务成功的首要目标。因此,安全有效的药物治疗和药品保障对航天员的健康至关重要。然而用于长期太空飞行的药物的稳定性变化,不能用陆地环境稳定性评价结果来判断[1],在陆地上药物稳定性主要受处方、温度、光线、湿度和水分及包装材料等因素影响。而航天环境受微重力、硬真空、湿度变化、温差和辐射影响[2],这些都可能导致药物的不稳定,其主要影响因素为空间辐射。空间辐射主要包括空间天然产生的非电离辐射和由人工产生的电离辐射。当药物暴露于任何电离辐射时,辐射导致药物分子发生价层电子跃迁,与赋形剂发生相互作用,导致药物的结构发生一定变化,从而丧失药理活性。
酒石酸唑吡坦(zolpidem tartrate)主要通过作用于中枢神经系统的苯二氮䓬受体,增强γ -氨基丁酸(GABA)系统的抑制机制,具有较强的镇静、催眠作用[3-4]。在载人航天特殊作业环境中,酒石酸唑吡坦可以用于治疗睡眠障碍,缓解由其导致的焦虑等情绪,是“太空药箱”的常用药品之一[2]。
为了考察酒石酸唑吡坦片在太空环境中的稳定性,以60Co辐射为辐射源模拟太空的射线环境。辐射技术是20世纪兴起的一种灭菌及保鲜技术,是以Χ射线、γ射线等电离辐射产生的高能射线抑制病原生物的生长而达到杀虫、杀菌等目的[5-6]。根据原中华人民共和国卫生部发布的《60Co辐射中药灭菌剂量标准》[7]和《美国药典》42[8] (USP42)对辐射灭菌的规定,选定了8、25、80 kGy这3个剂量,建立UHPLC法测定不同剂量辐射下的酒石酸唑吡坦片的含量变化。实验通过60Co辐射因素对航天药物稳定性的影响,从而预测太空环境中药品的有效期,为今后航天药品的制剂工艺、包装储存等提供必要的研究资料。
-
LC-2030 PLUS高效液相色谱仪(日本岛津公司)包括LC-20ADXR溶剂输送泵,SPD-20A紫外检测器,SIL-20AXR 自动进样器和CTO-20A柱温箱;60Co辐照装置(海军军医大学辐照中心)。
酒石酸唑吡坦片 (思诺思,杭州赛诺菲制药有限公司,批号ET098),酒石酸唑吡坦对照品(纯度99.8%,武汉赛维尔生物科技有限公司,批号201921);甲醇(Merck公司),磷酸(上海安谱实验科技股份有限公司),三乙胺(上海安谱实验科技股份有限公司),均为色谱纯;水为自制纯化水(上海和泰仪器有限公司)。
-
将酒石酸唑吡坦片(含包装)置于辐照环境中,设定辐射剂量,按照接收剂量为0、8、25、80 kGy进行60Co辐射,每天受辐照一次,于1个月后取样。
-
精密称定酒石酸唑吡坦对照品约10 mg,置10 ml容量瓶中,加流动相溶解并稀释至刻度,摇匀,得酒石酸唑吡坦对照品储备液;再精密量取1 ml,置 100 ml容量瓶中,加流动相稀释至刻度,摇匀,得浓度为10 μg/ml的对照品溶液。
-
取0、8、25、80 kGy辐射后的酒石酸唑吡坦片各10片,精密称定并研细成药粉。精密称取细粉适量(约相当于酒石酸唑吡坦10 mg)置于10 ml量瓶中,加流动相溶解并稀释至刻度,摇匀,过滤,精密量取续滤液1 ml,置100 ml量瓶中,加流动相稀释至刻度,摇匀,用0.45 μm微孔滤膜过滤,即得。
-
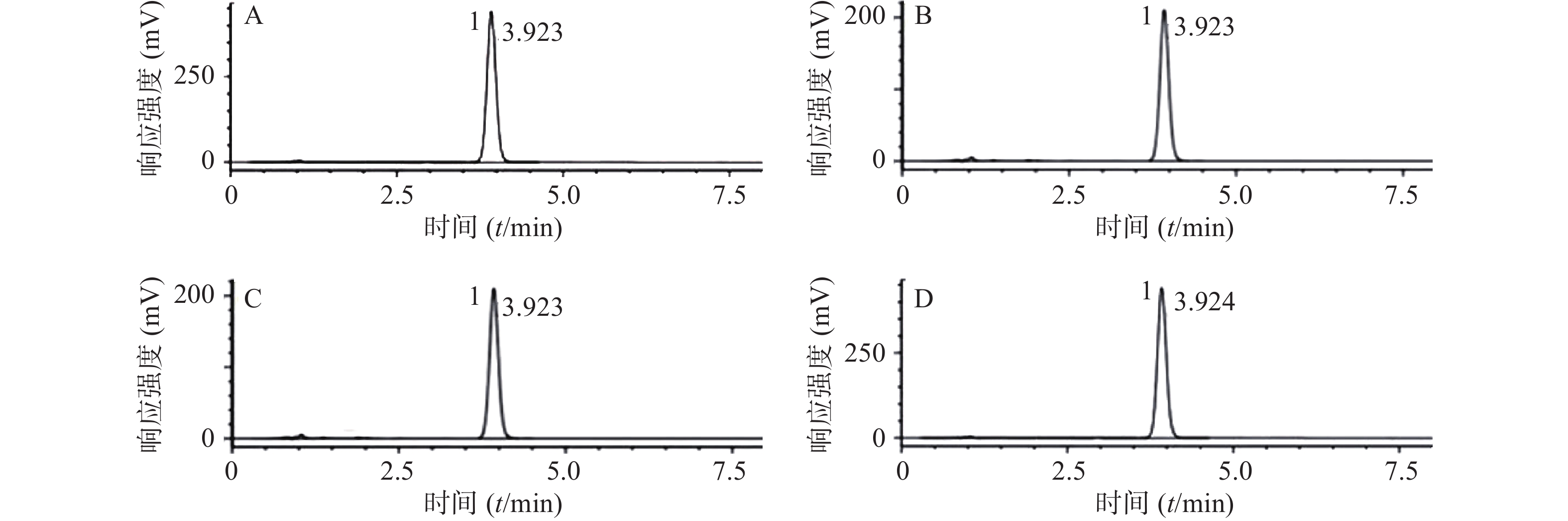
色谱柱为Inertsil C18柱(3.0 mm×150 mm,3.0 μm),流动相为乙腈-甲醇-0.05 mol/L磷酸溶液(用三乙胺调节pH值至5.5) (18∶26∶56),检测波长为254 nm,流速为0.7 ml/min,柱温30℃,进样量为10 μl,色谱图中酒石酸唑吡坦的保留时间为3.92 min,理论塔板数按酒石酸唑吡坦峰计算大于5 000。精密称取标准品配制浓度约为10 μg/ml的对照品溶液,在上述色谱条件下重复进样6次,测定峰面积,计算峰面积RSD为0.06%,表明仪器精密度良好。每个辐射强度取1批供试品,按照“2.3”项下配制供试品溶液,在上述色谱条件下进样分析,对照品溶液及供试品溶液的色谱结果见图1。
-
精密量取对酒石酸唑吡坦对照品储备液适量置100 ml量瓶中,加流动相稀释至刻度,摇匀,得浓度分别为5、10、20、40、80 μg/ml的工作曲线溶液。按“2.4”项下色谱条件分别进样10 μl进行测定,记录色谱图。以酒石酸唑吡坦对照品浓度 (X,μg/ml)为横坐标,相应峰面积(Y)为纵坐标,绘制标准曲线,得回归方程为Y=1.03
$ \times $ 105X+1.5×104, r=0.9996,结果表明酒石酸唑吡坦在5~80 μg/ml浓度范围内,线性关系良好。 -
精密称取同一批未受辐射的酒石酸唑吡坦片样品粉末(约相当于酒石酸唑吡坦10 mg)6份,照“2.3”项下制成供试品溶液,按“2.4”项下色谱条件分别进样10 μl测定,记录峰面积,计算含量,其平均含量104.2%, RSD为0.87%,表明该方法重复性良好。
-
取辐射强度为8 kGy 的供试品溶液,按“2.3”项下方法制备供试品溶液,按含量测定方法测定,在0、1、2、5、8、12 h分别进样10 μ1,记录峰面积,峰面积RSD为0.06%,表明溶液在12 h内稳定。
-
精密称取同一批未辐射酒石酸唑吡坦片样品粉末(约相当于酒石酸唑吡坦10 mg)3份,分别置于10 ml量瓶中,再精密称取酒石酸唑吡坦对照品约8、10、12 mg各3份,分别置于上述3个10 ml量瓶中,加流动相溶解并稀释至刻度,摇匀,过滤,精密量取续滤液1 ml,置 100 ml量瓶中,加流动相稀释至刻度,摇匀,每个浓度制备3份。按“2. 4”项下色谱条件进样测定,记录峰面积,计算平均加样回收率,结果见表1。
称取量
(m/mg)原有量
(m/mg)加入量
(m/mg)测得量
(m/mg)回收率
(%)平均回收率
(%)RSD
(%)140.27 10.99 12.81 23.82 100.2 98.2 1.72 140.24 10.99 12.80 23.81 100.2 140.24 10.99 12.81 23.81 100.1 140.28 10.99 10.84 21.42 96.2 140.30 10.99 10.82 21.42 96.4 140.27 10.99 10.83 21.41 96.2 140.32 10.99 8.78 19.62 98.3 140.21 10.98 8.79 19.61 98.1 140.22 10.98 8.78 19.60 98.1 -
每个辐射强度取1批供试品,按“2.3”项下方法制备供试品溶液,平行3份,在“2.4”项的色谱条件下进样分析,记录色谱峰面积,按照外标法计算酒石酸唑吡坦含量,其 0、8、25和80 kGy辐射量下酒石酸唑吡坦平均含量分别为105.1%、106.4%、102.7%和105.4%(表2)。
辐射剂量(kGy) 含量(%) 平均含量(%) 0 103.1 105.1 103.2 109.2 8 107.1 106.4 109.1 103.2 25 107.2 102.7 105.0 96.1 80 100.1 105.4 107.2 109.0 -
研究表明测定酒石酸唑吡坦原料药和片剂含量的方法主要有分光光度法[9]、高效液相色谱法[10]和毛细管区带电泳法[11]等。本实验采用超高效液相色谱法,相比于传统的高效液相色谱法,其色谱柱粒径更小,柱效更高, 出峰较快,大大提高了灵敏度和检测效率。
在建立超高效液相色谱法方法过程中,曾选用乙腈-0.05 mol/L磷酸溶液和甲醇-0.05 mol/L磷酸溶液,但是色谱峰不能完全分离,干扰性大。最终选用乙腈-甲醇-0.05mol/L磷酸溶液(用三乙胺调节pH值至 5.5) (18∶26∶56),试验结果表明,流动相中加入适量三乙胺调节其pH值, 在pH4.0~6.5的范围内,分离度随pH值增大而增大[12], pH值<4.5时,酒石酸唑吡坦峰形较差,pH值>6.0时,酒石酸唑吡坦峰拖尾严重。经优化,选择pH5.5,其唑吡坦峰的对称性好,理论塔板数高,柱效更高。
-
实验选定了8、25、80 kGy不同辐射剂量下对酒石酸唑吡坦片稳定性影响进行考察,结果显示随着辐射剂量逐步增大,在其他条件相同的情况下,酒石酸唑吡坦片的主药含量并没有明显变化,酒石酸唑吡坦片能保持相对稳定。药物含量指在制剂中药物量是否符合要求,在药物含量不变的情况下,药物的有效性还与剂型的相关特性有关,如溶出度等。有研究表明,药物受到一定剂量的辐射后,药物的溶出度会发生改变[13]。后续将从不同辐射剂量的酒石酸唑吡坦片的溶出度、水分等做进一步研究。
Determination of zolpidem tartrate tablets after radiation by UHPLC
doi: 10.12206/j.issn.1006-0111.202110019
- Received Date: 2021-10-11
- Rev Recd Date: 2022-01-04
- Available Online: 2022-01-20
- Publish Date: 2022-01-25
-
Key words:
- zolpidem tartrate /
- ultra high performance liquid chromatography /
- content determination /
- radiation
Abstract:
| Citation: | ZHANG Wen, HUANG Xinhui, YANG Xingrui, ZHOU Tingting, GAO Jianyi, LI Yongzhi. Determination of zolpidem tartrate tablets after radiation by UHPLC[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(1): 62-65. doi: 10.12206/j.issn.1006-0111.202110019 |









 DownLoad:
DownLoad: