-
病理性心肌纤维化(myocardial fibrosis, MF)是常见的心血管病理生理表现,存在于多种心血管疾病,具体机制尚不明确,减轻心肌纤维化可能成为缓解房颤等多种心血管疾病治疗的有效靶点[1-4],但目前心肌纤维化的治疗尚缺乏有效治疗策略。研究表明,Rho/ROCK/JNK途径调节多种关键细胞功能,可以介导细胞凋亡、增殖、分化等,而抑制JNK和TGFβ/Smad途径可改善2型糖尿病大鼠的心肌纤维化,使RhoA/ROCK/JNK成为预防心肌纤维化的潜在治疗靶点[5- 6]。黄芪甲苷(astragaloside Ⅳ, AS-Ⅳ)是从中药黄芪中提取出的主要活性成分,具有抗氧化、抗炎症、降低血糖等多重药理作用, 并且AS-Ⅳ通过抑制ROS/Caspase-1/GSDMD信号通路可减轻心肌梗死诱导的心肌纤维化和心脏重塑[7-9]。本研究采用异丙肾上腺素诱导小鼠心肌纤维化,探讨黄芪甲苷对心肌纤维化的改善作用以及对ROCK/JNK信号的调控作用。
-
6周龄健康雄性C57/BL小鼠,体质量(20.5±1.1) g,购自空军军医大学实验动物中心[许可证号:SYXK(陕)2019-001],实验经空军军医大学伦理委员会批准(No.20220262);Atg5、Beclin-1、LC3 Ⅰ/Ⅱ、ROCK、JNK、GAPDH抗体、黄芪甲苷(美国Sigma-Aldrich公司);ROCK抑制剂Y-33075(上海阿拉丁生化科技股份有限公司);异丙肾上腺素(ISO,天津泰泽兴业生物科技有限公司);BNP试剂盒、结缔组织生长因子(CTGF)试剂盒(美国R&D Systems公司);超氧化物阴离子荧光检测探针(DHE,美国ThermoFisher公司);多功能酶标仪(美国Thermo公司);Olympus FV2000激光共聚焦显微镜(日本奥林巴斯公司);小动物超声仪(美国VisualSonics公司)。
-
将60只C57/BL小鼠按照随机数字表法随机分成4组,每组15只,即对照组、心肌纤维化组(MF组)、黄芪甲苷组、黄芪甲苷联合ROCK抑制剂Y-33075组(黄芪甲苷+Y-33075组)。各组以普通饲料喂养,MF组、黄芪甲苷组、黄芪甲苷+Y-33075组于小鼠肩胛间皮下注射异丙肾上腺素(ISO),首次剂量为 5 mg/(kg·d),后以2.5 mg/(kg·d),重复给药持续30 d,诱导心肌纤维化模型。对照组于同一部位注射相同体积生理盐水。给药组在造模同时给与药物治疗,黄芪甲苷组按100 mg/(kg·d)腹腔注射黄芪甲苷,黄芪甲苷+Y-33075组分别按100 mg/(kg·d)和10 mg/(kg·d)腹腔注射黄芪甲苷和Y-33075,MF组和对照组腹腔注射等量生理盐水,重复给药持续30 d。
-
各组小鼠经2%异氟烷麻醉后,四肢插上超声仪电极,探头在乳头肌水平位置用M型取样线检测,测量并计算得出左心室射血分数(LVEF)、左心室短轴缩短率(LVFS)、左心室舒张期前壁厚度(LVAWd)、左心室舒张末期容积(LVEDV)等指标。
-
固定小鼠,剪开并剥离小鼠颈部皮肤、皮下组织,暴露小鼠颈动脉,剪开快速取血2 ml至EP管中,3000 r/min离心15 min,取上层清亮血清。按照LDH、BNP、CTGF检测试剂盒说明书检测。
-
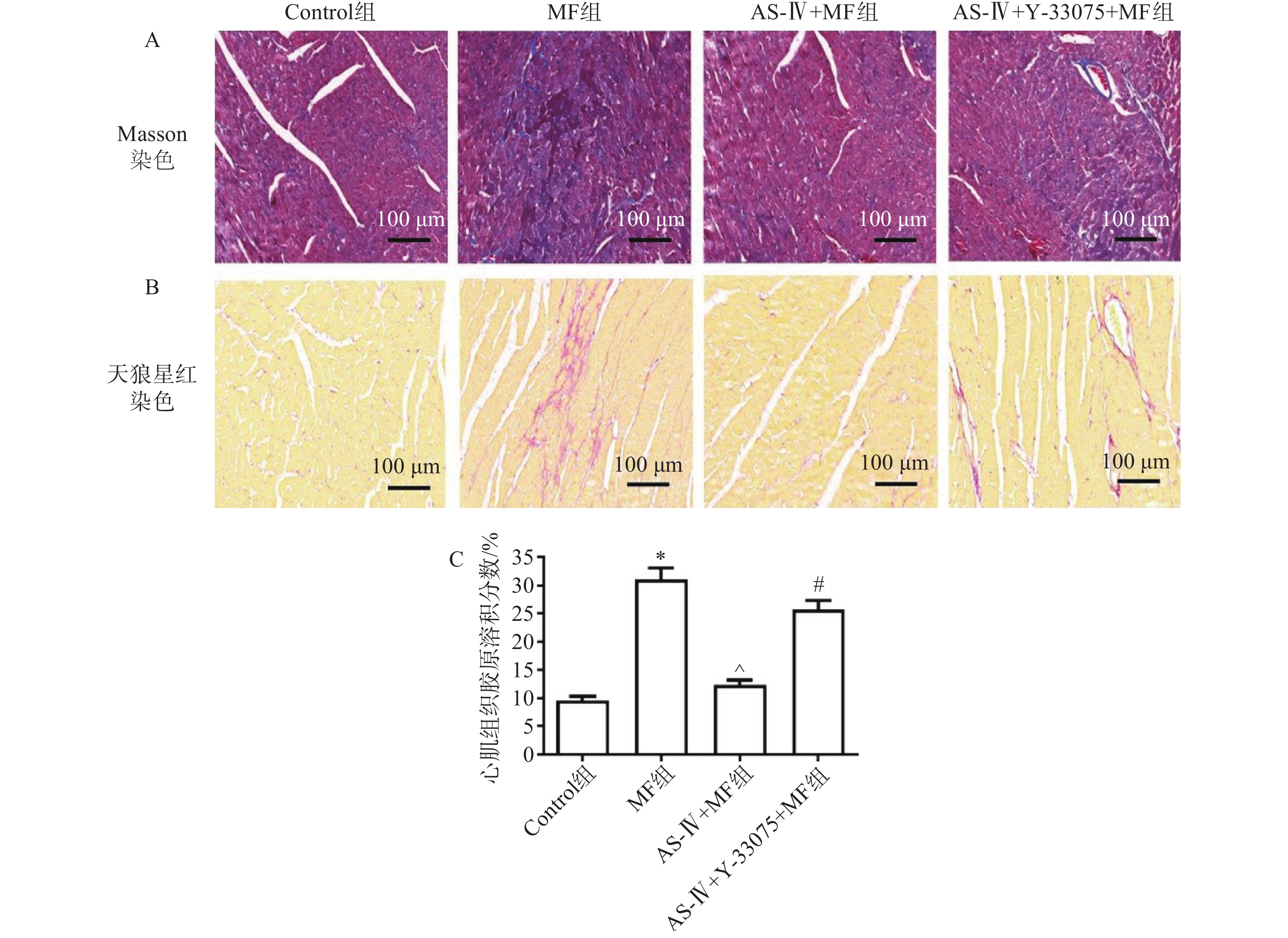
迅速剪下心脏,冷PBS缓冲液冲洗3遍,洗净血细胞后,以多聚甲醛(40 g/L)固定72 h,石蜡包埋、切片,行Masson、天狼星红染色,在光学显微镜下拍摄染色后的Masson、天狼星红图像并保存,使用Image J软件测定Masson染色中胶原阳性蓝色染色面积与组织总面积比值,即胶原容积分数。
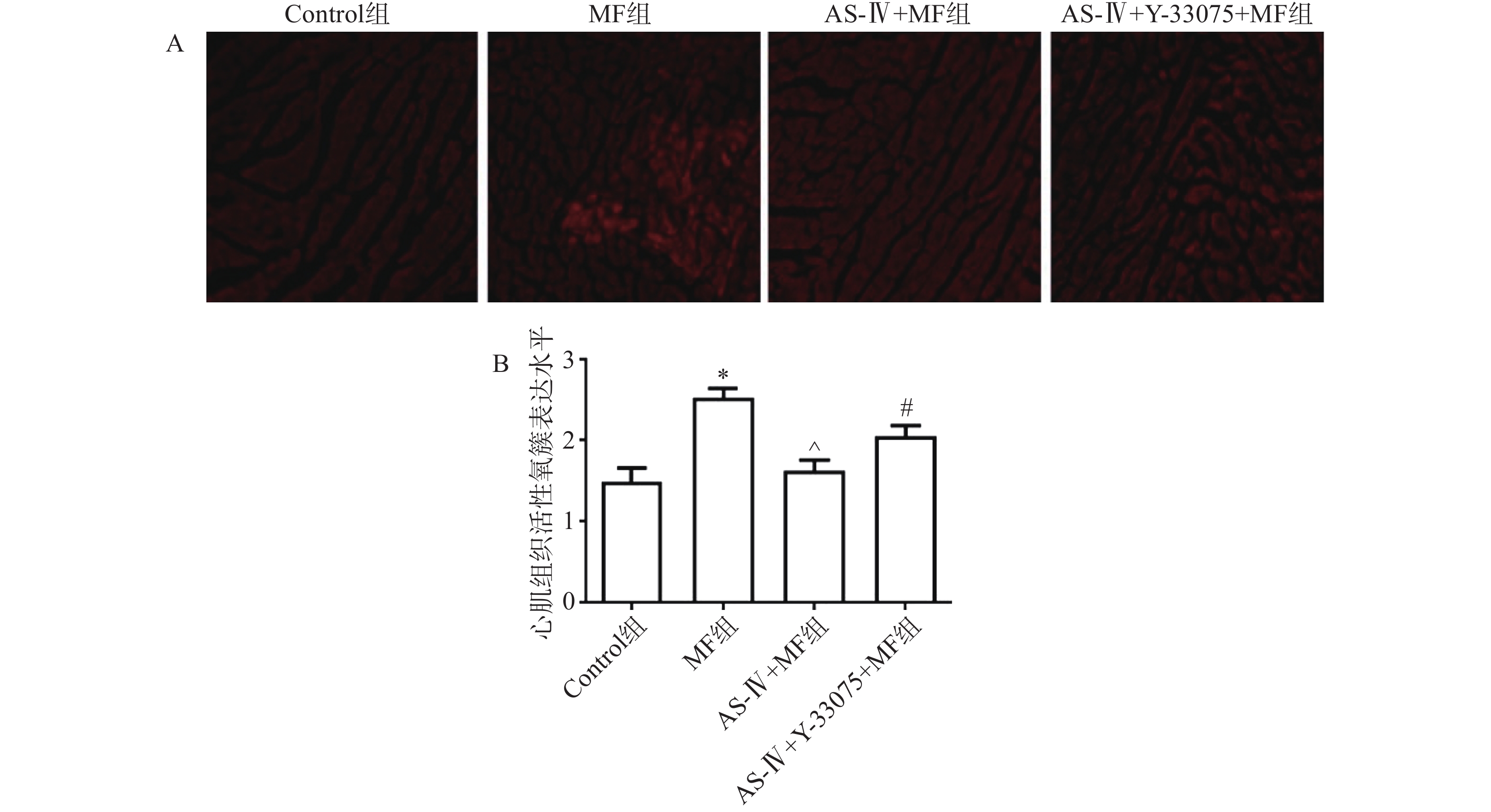
心脏在液氮中快速冷冻,并切成3 μm厚的切片。冷冻组织切片在黑暗潮湿的小室中用DHE反应混合物染色。使用Olympus FV1000激光共聚焦显微镜检查每个样品。使用Image J软件测定切片中二氢乙锭的荧光强度,以评估心肌组织中活性氧簇(ROS)的产生。
-
测定BNP、CTGF、Atg5的表达评估心肌组织纤维化及自噬水平。用TRIzol试剂从组织中分离总RNA,逆转录实验生成cDNA,用BIO-RAD分光光度法测定RNA和cDNA的浓度和纯度,PrimerPremier 6.0软件设计引物并合成(上海吉凯基因有限公司)。在iQ™5 RT-PCR系统中进行PCR扩增,采用2−ΔΔct法对靶基因表达量进行相对定量。各检测基因引物序列由美国复能基因公司提供(见表1)。
基因 上游序列(5'-3') 下游序列(5'-3') BNP TAGCCAGTCTCCAGAACAAATCC AAACAACCTCAGCCCGTCA CTGF CAGCATGGACGTTCGTCTG AACCACGGTTTGGTCCTTGG Atg5 CAGAAGCTGTTCCGTCCTGT CCGTGAATCATCACCTGGCT GAPDH AGAACATCATCCCTGCATCC AGTTGCTGTTGAAGTCGC -
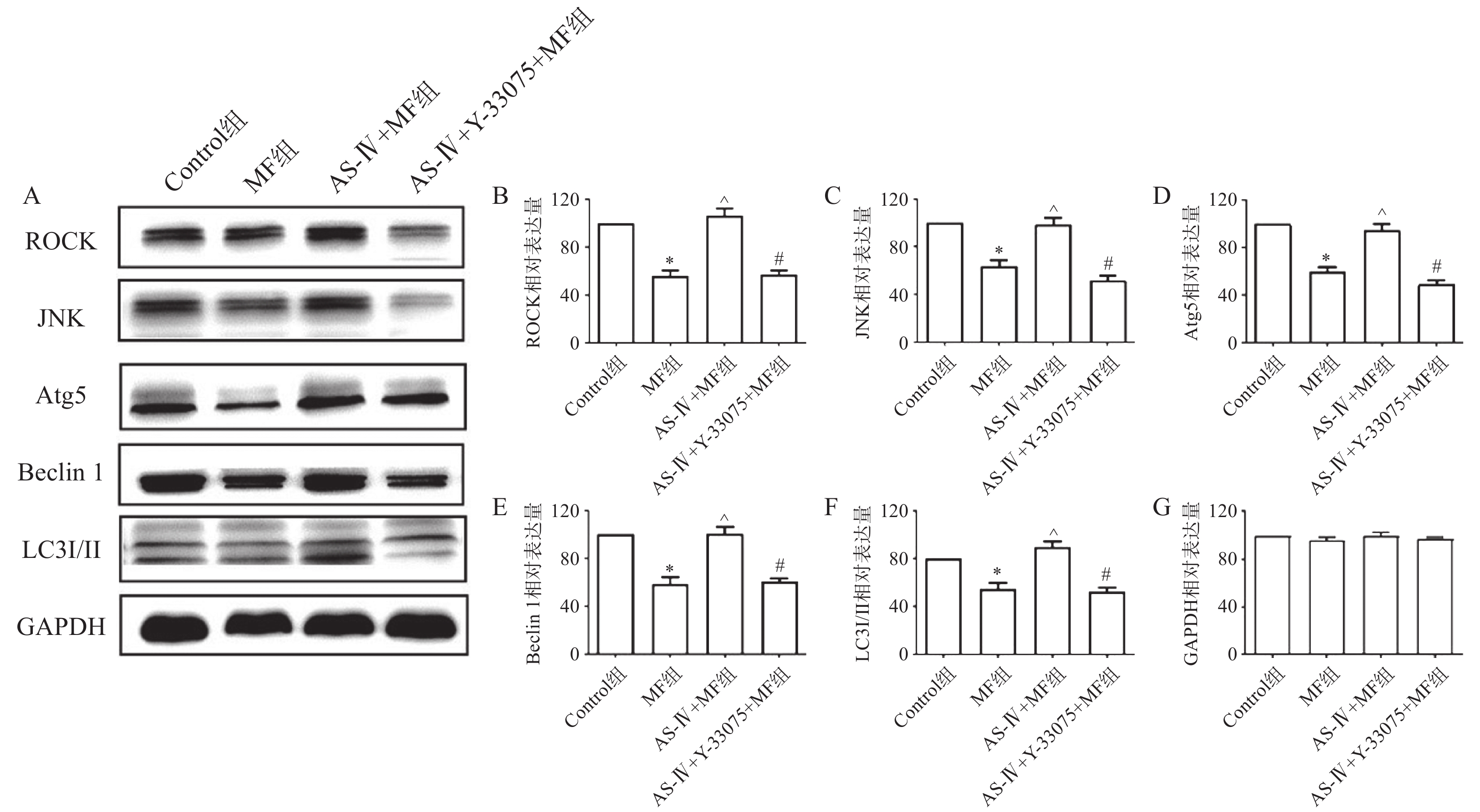
各组小鼠心肌组织取相同质量放入离心管中,加入预冷PBS,剪碎心肌组织,4℃ 3000 r/min离心10 min,弃上清,沉淀中加入含蛋白酶抑制剂的RIPA强裂解液,冰上充分研磨并裂解30 min,4℃ 12000 r/min离心30 min,取上清;BCA法蛋白定量;电泳并用湿转法将蛋白转移至PVDF膜,5%脱脂奶粉室温下封闭2 h,裁剪目的条带,放入对应ROCK(1∶1000)、JNK(1∶1000)、Beclin 1(1∶1000)、LC3 Ⅰ/Ⅱ(1∶1000)、Atg5(1∶1000)和GAPDH抗体(1∶5000)中,4℃摇床孵育过夜;TBST洗脱3次,5 min/次,将目的条带放入山羊抗兔或抗鼠二抗(1∶5000)中室温摇床孵育2 h,TBST洗脱3次,5 min/次,ECL化学发光液避光检测,Image Lab统计灰度值。
-
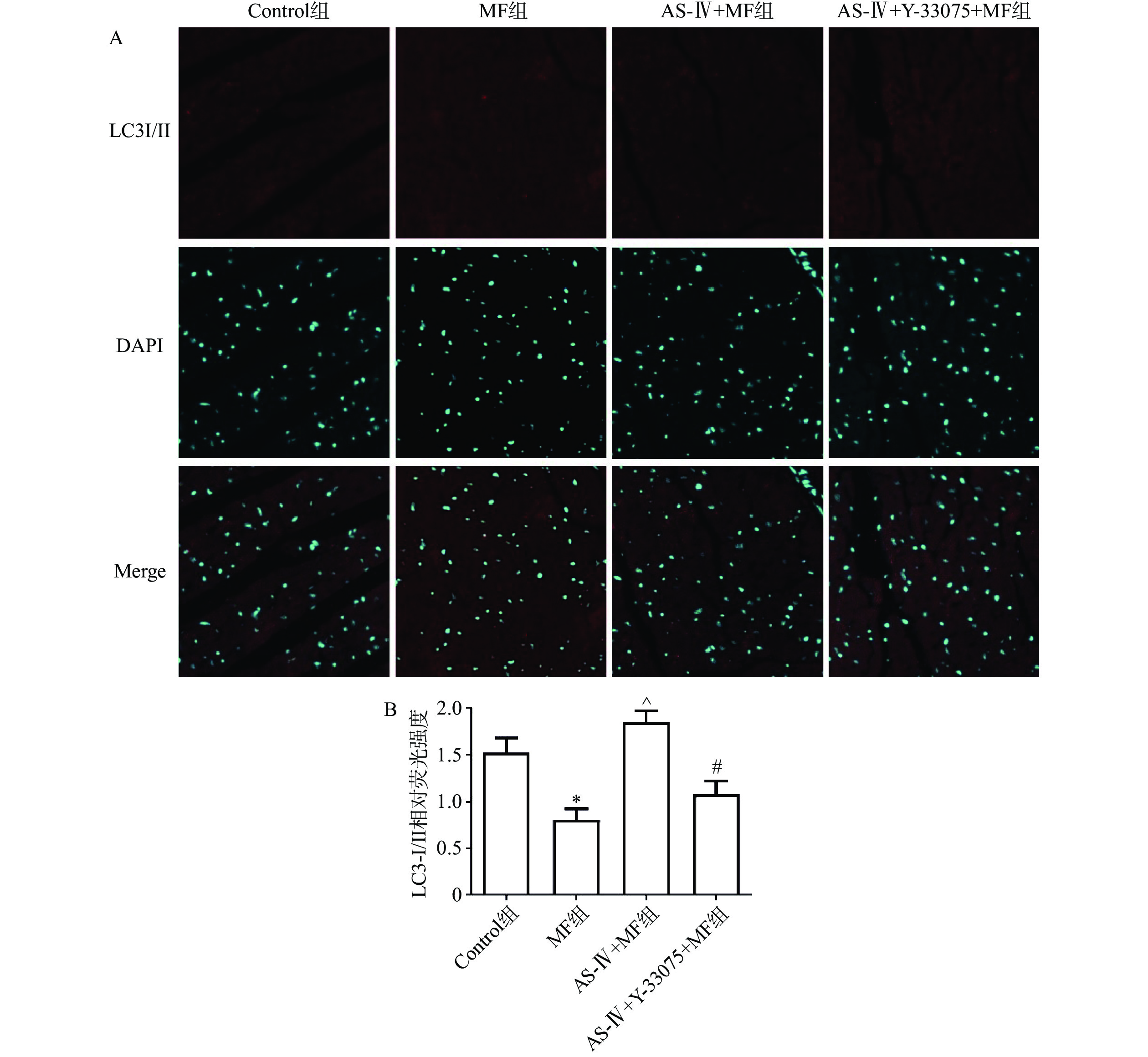
取小鼠左室前壁组织,石蜡包埋、切片、脱蜡、复水,用PBS缓冲溶液洗5次,每次3 min,Triton-X100(2 g/L)处理15 min,PBS液冲洗3次,每次3 min,山羊血清室温封闭1.5 h,PBS液洗3次,每次3 min,每个切片加LC3 Ⅰ/Ⅱ抗体(1∶100)50 μl,4 ℃孵育15 h,PBS液洗5次,每次3 min,暗室内每张切片加荧光二抗(1∶500) 50 μl,PBS液洗5次(3 min/次),避光条件下每张切片加DAPI溶液50 μl,PBS液洗5次,每次3 min,用荧光防淬灭液封片,采集图像。
-
数据采用GraphPad Prism 9.0软件统计和分析。计量资料以(均数±标准差)表示,多组间比较采用单因素方差分析,随后采用Bonferroni校正及post hoc t检验,P<0.05为差异具有统计学意义。
-
与对照组比较,MF组心脏功能明显降低,LVEF、LVEDV显著减小,LVAWd显著增加(P<0.05);与MF组比较,黄芪甲苷组心脏功能得到明显改善,LVAWd显著减少,LVEF、LVEDV显著增加(P<0.05);和黄芪甲苷组相比,黄芪甲苷+Y-33075组的LVEF、LVEDV显著降低(P<0.05)。超声结果表明,黄芪甲苷可以改善MF诱发的心脏功能障碍,Y-33075可阻断黄芪甲苷的心肌保护作用(表2)。
检测项目 对照组 MF组 黄芪甲苷组 黄芪甲苷+
Y-33075组LVEF (%) 72.31±6.27 35.43±8.12* 55.25±7.43^ 40.28±11.36# LVAWd (mm) 1.51±0.03 1.94±0.25* 1.62±0.20^ 1.71±0.18# LVEDV(μl) 57.92±7.23 49.78±9.36* 56.32±6.28^ 50.79±5.46# *P<0.05,与对照组比较; ^P<0.05,与MF组比较;#P<0.05,与黄芪甲苷组比较 与对照组相比,MF组血清中LDH、BNP、CTGF水平显著增加(P<0.05),心肌组织BNP、CTGF的mRNA表达显著增加(P<0.05),Atg5的mRNA表达降低(P<0.05);与MF组相比,黄芪甲苷组血清LDH、BNP、CTGF水平显著降低(P<0.05),心肌组织BNP、CTGF的mRNA表达减少(P<0.05),Atg5的mRNA表达增加(P<0.05);黄芪甲苷+Y-33075组血清中LDH、BNP、CTGF水平比黄芪甲苷组显著增加(P<0.05),心肌组织BNP、CTGF的mRNA表达明显增加(P<0.05),Atg5的mRNA表达降低(P<0.05),具体见表3、表4。
检测项目 对照组 MF组 黄芪甲苷组 黄芪甲苷+Y-33075组 LDH(U/L) 395.25±30.38 1102.43±62.35* 500.89±19.48^ 974.21±101.72# BNP(ng/L) 80.29±12.90 200.61±31.92* 137.33±19.28^ 194.72±26.31# CTGF(ng/L) 121.91±20.25 213.37±29.58* 163.05±10.29^ 189.03±25.42# *P<0.05,与对照组比较;^P<0.05,与MF组比较;#P<0.05,与黄芪甲苷组比较 项目 对照组 MF组 黄芪甲苷组 黄芪甲苷+Y-33075组 BNP 1 4.02±0.59* 1.59±0.33^ 3.98±0.49# CTGF 1 1.62±0.23* 0.91±0.37^ 1.54±0.29# Atg5 1 0.53±0.09* 2.56±0.61^ 0.69±0.30# *P<0.05,与对照组比较;^P<0.05,与MF组比较;#P<0.05,与黄芪甲苷组比较 -
心肌组织Masson、天狼星红染色检测各组小鼠心肌组织结构、胶原含量及纤维化水平。对照组小鼠心肌组织无纤维化,胶原含量低;MF组可见心肌结构排列紊乱,肌纤维断裂明显,胶原含量高;与MF组相比,黄芪甲苷组肌纤维排列整齐,纤维化程度改善,胶原含量降低;与黄芪甲苷组相比,黄芪甲苷+Y-33075组心肌纤维化程度明显增加,排列紊乱,胶原含量增高(图1)。
黄芪甲苷能够显著减轻脓毒症小鼠心肌组织氧化应激损伤。与对照组相比,MF组小鼠肾组织中ROS生成量显著增加(P<0.05);与MF组相比,黄芪甲苷组小鼠心肌组织中ROS生成量显著降低(P< 0.05);而与黄芪甲苷组相比,黄芪甲苷+Y-33075组小鼠ROS生成量显著增加(P < 0.05,图2)。
-
蛋白印迹检测结果提示,与对照组比较,MF组心肌组织中自噬相关蛋白ROCK、JNK、Atg5、Beclin 1、LC3 Ⅰ/Ⅱ表达水平降低(P<0.05);与MF组比较,黄芪甲苷组心肌组织ROCK、JNK、Atg5、Beclin 1、LC3 Ⅰ/Ⅱ表达水平增高(P<0.05);而与黄芪甲苷组比较,黄芪甲苷+Y-33075组心肌组织ROCK、JNK、Atg5、Beclin 1、LC3 Ⅰ/Ⅱ表达水平降低(P<0.05),提示黄芪甲苷可以改善MF所致心肌细胞自噬水平的降低,而Y-33075能阻断黄芪甲苷的这一作用(图3)。
心肌LC3- I/II免疫荧光染色结果显示,与对照组相比,MF组LC3-I/II蛋白免疫荧光染色数量显著减少(P>0.05);与MF组相比,黄芪甲苷组LC3-I/II蛋白染色的数量显著增加(P<0.05);与黄芪甲苷组相比,黄芪甲苷+Y-33075组LC3-I/II蛋白免疫荧光染色数量显著减少(P<0.05),结果也提示黄芪甲苷可以改善MF诱发的心肌自噬功能障碍,而Y-33075可阻断黄芪甲苷的这一作用(图4)。
-
病理性心肌纤维化是由多种因素引起的,这些因素可导致胶原纤维过度沉积,胶原浓度和体积分数显著增强,心肌胶原类型和结构排列紊乱,是房颤等多种慢性心血管疾病进展中的重要病理过程,然而心肌纤维化在分子水平的具体机制尚不清楚[10-13]。研究表明,多种重要机制参与了病理性心肌纤维化的发展过程,其中包括炎症反应、氧化应激、纤维化、细胞死亡等。近年来发现,自噬和泛素-蛋白酶体系统是维持细胞中蛋白稳态的重要途径,研究表明自噬在心肌纤维化过程中起着重要作用[14-16]。然而,自噬与心肌纤维化的相互作用机制尚不十分清楚,自噬及其调节可能成为治疗心肌纤维化的潜在策略。
近年来多项研究发现,一种来源于豆科植物蒙古黄芪或膜荚黄芪的黄酮类化合物黄芪甲苷,在心血管相关疾病中发挥了重要的保护作用[13-14]。然而,黄芪甲苷能否减轻心肌纤维化及其潜在分子机制还有待进一步探究。
本研究旨在探讨自噬与小鼠心肌纤维化的关系,并阐明了黄芪甲苷通过上调自噬改善MF的保护机制。根据本研究结果发现,心肌细胞自噬抑制参与了MF进展,同时,黄芪甲苷可激活ROCK/JNK通路促进细胞自噬改善MF,结果从一定从程度上阐明了MF的可能作用机制,并辅助探寻预防和治疗心肌纤维化的潜在靶点。
Rho蛋白激酶(ROCK)是丝氨酸蛋白激酶家族成员, Rho/ROCK途径调节多种关键细胞功能,如基因转录、细胞黏附、神经发育、细胞死亡[5-6]。Rho/ROCK的相关研究有助于更好地阐明多种疾病的病理生理过程,而抑制Rho/ROCK也可能成为潜在治疗靶点[17-19]。细胞自噬在心肌纤维化中起着关键作用,ROCK/.JNK具有自噬调控作用[17]。黄芪甲苷的心脏保护作用是通过激活细胞自噬介导[20-21]。本实验结果表明,在MF模型中,ROCK和JNK表达均下调,而黄芪甲苷组ROCK和JNK表达恢复。Y-33075是ROCK的抑制剂,Y-33075处理后可通过阻断ROCK减弱黄芪甲苷对MF的改善作用。因此,通过抑制ROCK/JNK通路,部分消除了黄芪甲苷对MF的改善作用以及黄芪甲苷调控细胞自噬的影响。
综上,本研究的结果表明黄芪甲苷通过激活ROCK/JNK通路促进自噬发挥保护作用,并发现MF与ROCK/JNK通路介导的自噬有关。然而,自噬在MF等不同疾病的特定阶段的具体作用机制仍不十分明确,自噬、MF以及两者之间的关系需要深入研究与进一步探索。
Alleviation of isoproterenol-induced myocardial fibrosis in mice by autophagy regulated by Astragaloside Ⅳ through activating ROCK/JNK pathway
doi: 10.12206/j.issn.2097-2024.202212056
- Received Date: 2022-12-28
- Rev Recd Date: 2023-06-04
- Publish Date: 2023-08-25
-
Key words:
- myocardial fibrosis /
- autophagy /
- ROCK/JNK /
- astragaloside Ⅳ /
- mice
Abstract:
| Citation: | WU Feifei, ZHANG Xiaoqi, LIAN Jing, YANG Jing, ZHAI Mengen, QIAO Rui, XU Chennian, YANG Tingting. Alleviation of isoproterenol-induced myocardial fibrosis in mice by autophagy regulated by Astragaloside Ⅳ through activating ROCK/JNK pathway[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(8): 478-484. doi: 10.12206/j.issn.2097-2024.202212056 |








 DownLoad:
DownLoad: