-
颅内感染尤其是多重耐药菌感染具有进展快、反应重、治疗难度大、病死率高的特点,故提高颅内感染治愈率受到患者及临床的广泛关注。替加环素(TGC)作为首个甘氨酰四环素类抗菌药物,具有抗菌谱广、抗耐药活性强的特点,对临床上常见耐药菌(如鲍曼不动杆菌、产碳青霉烯酶肺炎克雷伯菌、耐甲氧西林金黄色葡萄球菌等)均有较好的疗效[1]。在耐药菌治疗药物不足的情况下,临床医生将TGC用于颅内感染的治疗,此外,越来越多的案例报道称,TGC可有效清除致病菌[2-3]。
TGC成功治疗颅内感染与脑脊液中有效药物浓度密切相关,TGC浓度监测对于提高颅内感染治愈率、精准指导临床合理用药具有重要意义[4]。本研究将利用二维液相色谱高效的自动化在线样品处理能力,建立脑脊液中TGC浓度测定方法。该方法与已报道的质谱法相比,具有操作简便、成本低、抗干扰能力强、基质效应小等优势,脑脊液样品无需复杂的前处理,高速离心后直接进样,短时间内即可完成萃取、转移及分析,更易于临床推广应用。
-
二维高效液相色谱由FLC-2701全自动二维液相色谱耦合仪(湖南德米特仪器有限公司)和LC-20A色谱模块(日本岛津公司)构成;−80 ℃冰箱(美国赛默飞科技公司);十万分之一电子天平(德国赛多利斯仪器公司);涡旋混匀器(美国SI仪器公司);低温高速离心机(美国贝克曼仪器公司)。
-
TGC对照品(北京百灵威科技公司,批号: 10-MWV-62-1);乙腈、甲醇、磷酸为色谱纯;高氯酸、氨水为分析纯;超纯水自制。
-
入住神经外科且未使用TGC的患者,住院期间需开展脑脊液生化检查时留取或从留置腰大池/脑室引流管中适量抽取。
-
精密称取TGC对照品1.29 mg,用20 mg/ml精氨酸水溶液定容至100 ml容量瓶中,即得12.90 μg/ml的TGC标准溶液。
-
预处理方法考察时,取脑脊液样品500 μl,直接离心、分别加入3倍体积甲醇、乙腈、10%高氯酸沉淀蛋白,涡旋混匀5 min,13 000 r/min离心10 min,取上清液进样分析。最终确定预处理方法为: 脑脊液样品13 000 r/min离心10 min后直接进样,采用外标法定量分析。
-
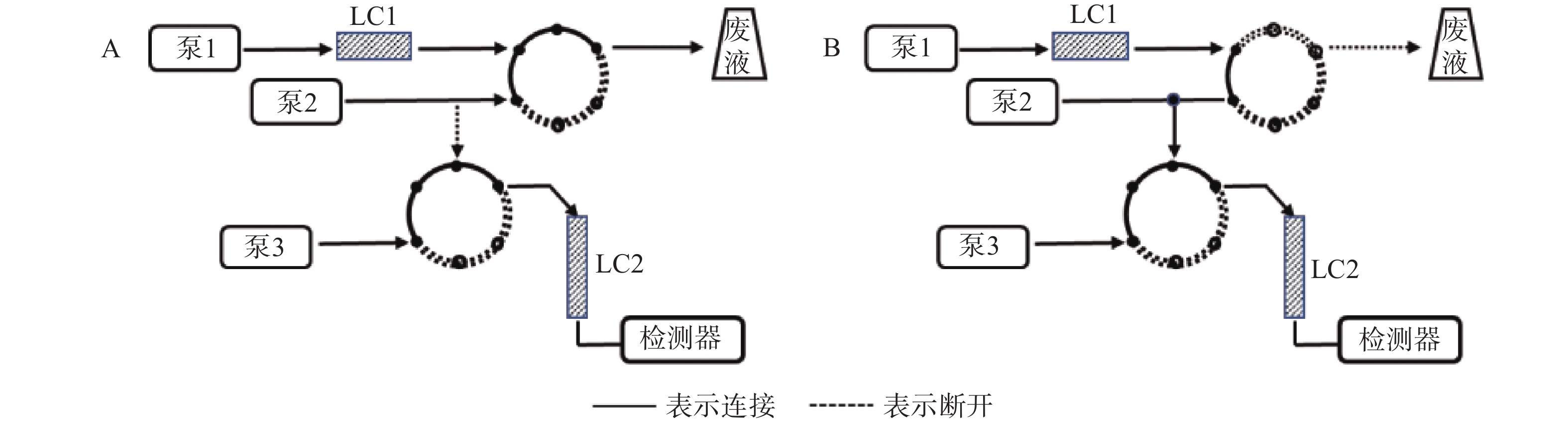
第一维色谱柱LC1为Aston SNX5苯基色谱柱(50 mm×4.6 mm,5 μm),流动相为磷酸铵(氨水调pH7.5)-甲醇 (45∶55,V/V),第二维色谱柱LC2为Aston SC5 C18色谱柱(275 mm×4.6 mm,5 μm),流动相为磷酸铵(氨水调pH7.4)-磷酸铵(氨水调pH3.0)-乙腈(30∶50∶20,V/V/V),辅助流动相为纯化水。检测波长340 nm,色谱柱温40 ℃,进样量:200 μl,工作原理图见图1,时间程序见表1。
表 1 TGC检测时间程序
时间(t/min) 流速(ml/min) 功能 LC1 LC2 辅助流动相 0.01~0.80 1.00 1.20 2.00 脑脊液样品于LC1中富集 0.81~3.49 1.00 1.20 0.01 目标物在LC1中初步分离 3.50~5.50 1.00 1.20 0.01 LC1与LC2串联,目标物洗脱至LC2 5.51~11.00 1.50 1.20 0.01 目标物在LC2中洗脱分离,清洗泵反向冲洗LC1 -
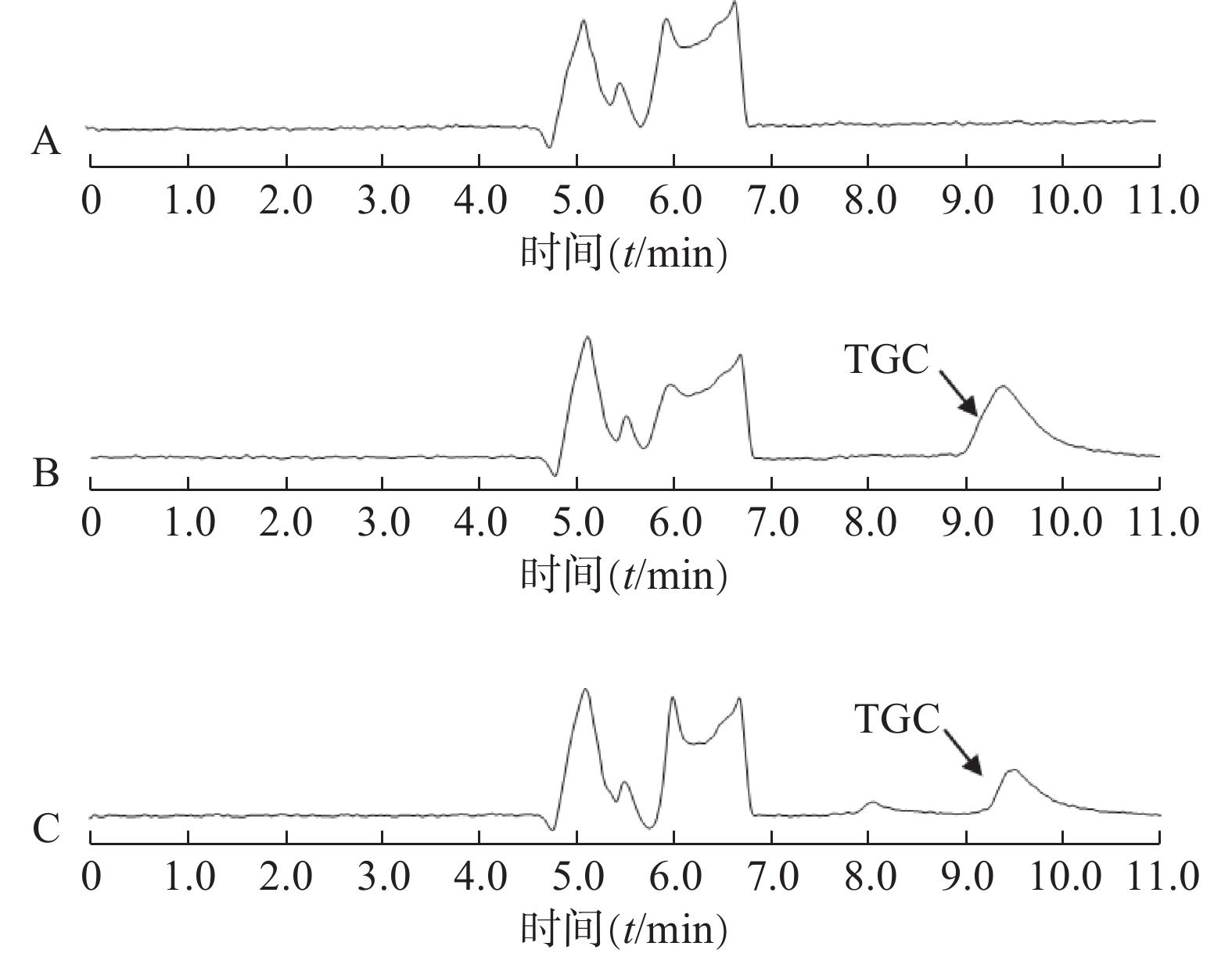
分别制备空白脑脊液样品、含TGC的标准脑脊液样品、患者用药后的脑脊液样品,进行二维液相分析,得到色图谱,观察有无内源性干扰,如图2所示。结果表明,TGC的出峰时间9.38 min,脑脊液的内源性成分对TGC的测定无干扰。
-
取不同体积TGC标准溶液,加入空白脑脊液定容,配制成浓度为64.5、129.0、387.0、645.0、1 290.0 ng/ml的系列对照溶液,按“2.2”项下操作后进样分析。以TGC浓度为自变量(X),脑脊液中TGC的峰面积为因变量(Y)进行线性回归,得回归方程为:Y=116.32X-3 223.6(r=0.999 8)。结果表明TGC浓度在64.5~1 290.0 ng/ml范围内线性关系良好,最低定量限为64.5 ng/ml。
-
分别配制含TGC低、中、高及定量下限(225.0、450.0、900.0、64.5 ng/ml)4个浓度的质控(QC)脑脊液样本,按“2.2”项下操作后进样分析。1 d内每个浓度平行测定5份QC样品,连续测定3 d。将测得的峰面积代入“2.4.2”项下,计算各质控样品的浓度,并根据样本实际浓度计算本法的精密度与准确度。结果如表2所示,日内、日间RSD均小于5%,准确度均在98.80%~106.51%之间。可见本实验所建立的二维液相色谱法测定脑脊液中TGC的精密度和准确度符合《中国药典》(2015年版)检测要求[5]。
表 2 脑脊液中测定TGC浓度的精密度与准确度(%)
浓度(ng/ml) 日内(n=5) 日间(n=3) 准确度 RSD 准确度 RSD 225.0 100.31 ± 2.83 2.82 98.80 ± 0.95 0.96 450.0 104.58 ± 2.19 2.09 103.51 ± 2.72 2.63 900.0 106.51 ± 2.06 1.94 101.82 ± 4.28 4.20 64.5 105.04 ± 2.47 2.35 99.01 ± 2.05 2.08 -
配制“2.4.3”项下低、中、高及定量下限4个浓度的QC样品,每个浓度平行3份,分别考察室温放置4 h、进样器放置6 h、反复冻融3次、冰冻放置1周后的稳定性。结果如表3所示,表明上述条件对TGC的检测结果无明显影响。
表 3 脑脊液中TGC在不同温度条件下的稳定性
样品浓度(ng/ml) 室温放置4 h 进样器放置6 h 反复冻融3次 冰冻放置1周 平均浓度(ng/ml) RSD(%) 平均浓度(ng/ml) RSD(%) 平均浓度(ng/ml) RSD(%) 平均浓度(ng/ml) RSD(%) 64.5 68.67 ± 2.93 4.27 65.52 ± 1.52 2.32 65.01 ± 1.43 2.20 68.56 ± 3.06 4.46 225 223.16 ± 5.06 2.27 221.62 ± 2.45 1.11 224.72 ± 4.50 2.00 222.99 ± 5.40 2.42 450 452.44 ± 9.11 2.01 459.04 ± 13.45 2.93 464.03 ± 8.39 1.81 459.68 ± 8.25 1.80 900 885.14 ± 15.09 1.70 881.32 ± 13.36 1.52 888.13 ± 13.80 1.55 899.79 ± 10.13 1.13 -
本实验选取2例接受TGC治疗的多重耐药菌所致的重症颅内感染患者,脑脊液标本药敏试验均报告对TGC敏感。患者1接受说明书推荐剂量,即首剂静脉滴注100 mg,后以50 mg每12 h维持治疗。患者2同样接受静脉给药,剂量为受试者1的2倍。达到稳定药物浓度后,留取两位受试者的脑脊液,按“2.2”项下操作后进样分析。受试者脑脊液中TGC浓度分别为187.42、275.41 ng/ml。
-
甲醇、乙腈、10%高氯酸是常用蛋白沉淀剂,本研究在空白脑脊液中分别加入3倍体积上述沉淀剂,离心后与不加蛋白沉淀剂组比较,沉淀量无显著差异,且所得上清液与沉淀完全分离。现多项研究认为,因脑脊液中蛋白质含量低,故直接离心更利于生物样品的微量分析[6]。另直接离心法进样,脑脊液中TGC浓度无稀释,也无其他前处理方法造成的误差,更利于临床样本的分析。
-
TGC对G-菌(如流感嗜血杆菌、大肠杆菌、对鲍曼不动杆菌等)MIC90为2~4 mg/L[7-8]。本试验中,无论是常规给药还是加倍剂量给药的患者,稳态脑脊液浓度显然均未起到杀菌作用,导致抗感染治疗效果不佳。这可能与以下因素有关:①TGC相对分子质量大而且水溶性强,很难进入到脑脊液中。有研究报道在血脑屏障正常的志愿者中,脑脊液中TGC浓度仅为血浆的11%。而对于脑膜炎患者,血脑屏障通透性可增至33%~52%,这可能也是静脉注射TGC成功治疗颅内感染的主要原因[9-10],但血脑屏障破坏的程度个体差异较大。②重症感染患者常伴有全身炎症反应、肝肾功能异常、多器官功能障碍综合征等,血药浓度低于正常水平,在同等穿透率的情况下,药物从血液进入脑脊液中的剂量减少[11]。③TGC的蛋白结合率较高,为71%~87%[12]。颅内感染时,脑脊液蛋白水平提高,使游离的药物浓度降低[13]。④ 脑脊液引流量与药物清除率呈正相关,引流量越大,脑脊液中的药物浓度越低[14]。
目前,TGC治疗方案大多参照说明书或文献报道推荐剂量,未考虑血脑屏障、脑脊液引流量、给药途径等患者自身因素的影响,使颅内TGC的浓度不足、疗效不佳。本研究建立的脑脊液中抗菌药物TGC的二维液相色谱分析方法简便、准确、稳定,对促进TGC的合理使用、改善临床治疗结局具有重要意义。临床可根据脑脊液中TGC的浓度,及时调整给药剂量或给药途径,提高治愈率,节省医疗费用。此外,也为研究TGC在颅内的药动学及量效关系奠定了基础。
Determination of tigecycline in human cerebrospinal fluid by two-dimensional liquid chromatography and its clinical application
-
摘要:
目的 建立二维液相色谱法检测人脑脊液中替加环素浓度,用于颅内感染患者的药物浓度监测。 方法 采用外标法定量,第一维色谱柱为Aston SNX5苯基色谱柱(50 mm×4.6 mm,5 μm),流动相为磷酸铵(氨水调pH7.5)-甲醇(45:55,V/V),流速1.0 ml/min。第二维色谱柱为Aston SC5 C18色谱柱(275 mm×4.6 mm,5 μm),流动相为磷酸铵(氨水调pH7.4)-磷酸铵(氨水调pH3.0)-乙腈(30:50:20,V/V/V),流速1.2 ml/min。检测波长340 nm,色谱柱温40 ℃,进样量:200 μl。 结果 脑脊液中替加环素64.5~1 290.0 ng/ml内与峰面积呈良好线性关系(r=0.999 8);日内、日间精密度RSD<5%,准确度为98.80%~106.51%。 结论 该方法操作简便、快速、准确、灵敏,适用于临床人脑脊液替加环素的浓度监测,可为颅内感染患者的个体化给药提供科学依据。 Abstract:Objective To establish a two-dimensional high-performance liquid chromatography method for the determination of tigecycline in human cerebrospinal fluid, which can be used for the drug monitoring in patients with intracranial infection. Methods The quantification was carried out by an external standard method. The first-dimension column was a Aston SNX5 phenyl chromatographic column (50 mm×4.6 mm, 5 μm) with ammonium phosphate (pH was adjusted with ammonium hydroxide to 7.5)-methanol (45∶55, V/V) as the mobile phase and the flow rate was 1.2 ml/min. The second-dimension chromatographic column was Aston SC5 C18 (275 mm×4.6 mm, 5 μm), with ammonium phosphate (pH was adjusted with ammonium hydroxide to 7.4)-ammonium phosphate (pH was adjusted with ammonium hydroxide to 3.0)- acetonitrile (30∶50∶20, V/V/V) as the mobile phase and the flow rate was 1.0 ml/min. The detection wavelength was 340 nm. The temperature was 40 ℃ and the injection volume was 200 μl. Results The calibration curve of tigecycline showed good linearity from 64.5 to 1 290.0 ng/ml in human cerebrospinal fluid (r=0.999 8). The RSD of intra and inter-day precision were less than 5.0% with the detection accuracy of 98.80%−106.51%. Conclusion This method is simple, quick, accurate, specific and sensitive. It meets the requirements of tigecycline determination in clinical human cerebrospinal fluid, which offers the individualized therapeutic assurance for patients with intracranial infection. -
我国已经进入老年化社会,骨质疏松症的发病率也不断上升,因此,对骨质疏松疾病的预防、治疗和预后研究是骨科领域的重点[1]。骨质疏松的治疗方式以药物治疗为主,包括雌激素、双磷酸盐、降钙素等,实践证明上述药物能够提高患者的骨密度,但是不良反应较多,容易诱发免疫反应及血管扩张,不利于预后的恢复[2]。近年来,诸多研究中使用复方骨肽注射液对胸腰椎骨质疏松性骨折患者进行治疗,效果比较明确,能够作用于人体刺激新骨形成,且刺激成骨细胞增殖,调节钙、磷代谢,维持骨代谢的平衡,增加骨密度,进而可以改善关节周围的组织营养微循环,有效发挥抗炎镇痛效用,明显促进了患者的身体恢复[3-4]。但关于复方骨肽注射液应用于腰椎骨质疏松的腰椎功能恢复以及整体治疗有效率评价的研究还鲜有报道。本研究选取了96例骨质疏松患者为研究对象,通过探究复方骨肽注射液在骨质疏松治疗中对患者中医症状评分、骨代谢指标、骨质疏松程度、骨密度、疼痛程度以及腰椎功能恢复情况,为临床上应用与推广复方骨肽注射液提供科学理论依据。
1. 资料与方法
1.1 临床资料
选取2018年1月至2020年1月海警总队医院收治的96例胸腰椎骨质疏松性骨折患者为研究对象。所有患者随机被分为甲组(接受钙尔奇D治疗联合复方骨肽注射液)和乙组(接受钙尔奇D治疗),各48例。纳入条件:①患者符合胸腰椎骨质疏松的诊断标准[5];②患者的资料完整,且同意参与临床研究;③患者均有运动后加重腰背疼痛症状;④患者对本研究的药物不过敏。排除条件:①患者伴有其他因素导致的腰椎骨折;②患者及其家属拒绝签署知情同意书;③患者伴有影响骨代谢的疾病;④患者存在精神障碍,不适合参与研究。
甲组患者中,男性20例,女性28例,年龄58~80岁,平均(71.23±3.05)岁;病程3~11年,平均(6.01±0.58)年。乙组中,男性21例,女性27例,年龄59~80岁,平均(71.44±3.69)岁;病程3~12年,平均(6.36±0.57)年。两组的资料在性别、年龄、病程上差异无统计学意义(P>0.05),具有可比性。本研究经本院伦理委员会批准,患者和家属均对研究知情,自愿参与研究,已签署知情同意书。
骨质疏松症诊断标准[5]:根据世界卫生组织1994年制定的相关标准进行判定:①检测出骨密度较同年龄、性别、种族的成人骨峰值低于1个标准为正常;②检查出骨密度低于1~2.5个标准,判定为骨量减少;③若检测出骨密度降低超过2.5个标准,则为骨质疏松;在判定严重骨质疏松时,若患者符合以上标准,且同时伴有一处骨折即可。
1.2 方法
乙组患者在确诊疾病后,接受钙尔奇D(批准文号:国药准字H10950029,惠氏制药有限公司)治疗,口服600 mg/次,2次/d。
甲组在乙组的基础上,接受复方骨肽注射液(批准文号:国药准字H32020004,南京新百药业有限公司)静脉滴注,用法:将10 ml药液加入250 ml 0.9%氯化钠注射液中,1次/d。两组连续治疗45 d后,进行各项指标的对比。
1.3 观察指标
(1)统计患者治疗前、后的腰背痛手术评分[6],依据腰背痛中医症状评分表对患者实施判定,按照中华骨科学会脊柱学组的腰背痛评分标准:≥16分为优,11~15分为良,6~10分为可,0~5分为差。内容包含:大小便无力与会阴麻木感、腰背部疼痛感、下肢疼痛与麻木感、工作与生活能力、下肢功能、临床症状等,评分越高,则意味着身体恢复越好。
(2)对比两组的骨代谢生化指标,抽取患者空腹静脉血3 ml,离心处理后,采集血清样本,使用免疫分析法检测骨碱性磷酸酶(BALP)和Ⅰ型前胶原氨基端前肽(PIINP)水平。
(3)分析两组经过治疗后的骨质疏松程度[7],按照日本慈惠医大标准进行评估,腰3椎体骨小梁可分为4个等级,即0~3级,其中,0级代表骨小梁正常,3级代表纵行的骨小梁变模糊,而水平的骨量消失明显。
(4)使用骨密度检测仪,对两组用药前、后的骨密度指标进行对比。
(5)在患者治疗前、后采用视觉模拟评分表(VAS)对两组的腰背疼痛情况进行评估[8],使用方法:在一张纸上画一条长为10 cm的横线,一端用0表示(无痛),另一端用10表示(剧烈疼痛),指导患者凭借主观感受进行标记;使用腰椎疾患治疗成绩评分表[9](JOA)对患者的腰椎恢复情况进行对比,包含自觉症状、临床检查、日常活动等,总分29分,得分越高,意味着患者的腰椎功能恢复越好。
1.4 统计学分析
用SPSS 25.0软件分析数据,连续性资料采用(
$\bar x \pm s$ )描述,t检验进行分析,分类变量采用[n(%)]描述,χ2检验进行分析,单向有序资料采用秩和检验进行分析。所有统计数据以P<0.05表示差异有统计学意义。2. 结果
2.1 两组患者中医症状评分比较
两组患者,治疗后评分均高于治疗前(P<0.05);治疗后,两组患者中医症状评分差异有统计学意义,甲组优于乙组(P<0.05,表1)。
表 1 两组患者中医症状评分比较(n=48,$\bar x \pm s$ ,分)组别 治疗前 治疗后 t P 甲组 11.36±1.48 19.44±2.44*# 19.616 <0.05 乙组 11.66±1.69 14.33±2.01* 7.044 <0.05 t 0.767 11.199 P >0.01 <0.05 *P<0.05,与治疗前比较;#P<0.05,与乙组治疗后比较。 2.2 两组患者骨代谢指标水平比较
治疗后,两组患者骨代谢指标水平较治疗前下降,差异有统计学意义(P<0.05);其中,甲组患者治疗后BALP、PIINP水平低于乙组(P<0.05,表2)。
表 2 两组患者骨代谢水平指标比较(n=48,$\bar x \pm s$ )骨代谢指标 甲组 乙组 治疗前 治疗后 治疗前 治疗后 BALP(U/L) 368.34±25.07 211.69±19.55*# 368.96±24.88 289.23±20.52* PIINP(ng/ml) 58.44±13.55 38.11±5.02*# 58.66±13.58 46.63±4.58* t 85.518 59.581 75.845 79.942 P <0.05 <0.05 <0.05 <0.05 *P<0.05,与同组治疗前比较;#P<0.05,与乙组治疗后比较。 2.3 两组患者骨质疏松程度比较
治疗后,两组患者同等级骨质疏松程度差异有统计学意义(P<0.05),甲组3级骨质疏松患者比例低于乙组(P<0.05,表3)。
表 3 两组患者骨质疏松程度比较[例数(%)]组别 0级 1级 2级 3级 甲组(n=48) 28(58.33) 13(27.08) 5(10.42) 2(4.17)* 乙组(n=48) 20(41.67) 11(22.92) 9(18.75) 8(16.67) *P<0.05,与乙组3级骨质疏松患者比较。 2.4 两组患者治疗前后的骨密度比较
治疗后,两组患者骨密度差异有统计学意义(P<0.05),甲组的骨密度水平高于乙组(表4)。
表 4 两组患者治疗前、后的骨密度比较(n=48,$\bar x \pm s$ ,g/cm2)组别 治疗前 治疗后 t P 甲组 0.82±0.23 0.97±0.22* 3.625 <0.05 乙组 0.81±0.22 0.87±0.18 1.462 >0.01 t 0.218 2.437 − − P >0.01 <0.05 − − *P<0.05,与同组治疗前比较。 2.5 两组患者VAS、JOA评分
治疗后,甲组的VAS评分低于乙组(P<0.05),而JOA评分高于乙组(P<0.05),见表5。
表 5 两组患者的VAS、JOA评分(n=45,$\bar x \pm s$ ,分)组别 VAS JOA 治疗前 治疗后 治疗前 治疗后 甲组 7.38±0.35 3.19±0.41*# 16.01±2.32 25.66±1.44* 乙组 7.44±0.32 4.01±0.39* 15.66±3.15 21.22±1.88* t 0.877 10.040 0.620 12.990 P >0.01 <0.05 <0.05 <0.05 *P<0.05,与同组治疗前比较;#P<0.05,与乙组治疗后比较。 3. 讨论
3.1 骨质疏松疾病介绍
骨质疏松是以骨组织微结构破坏、骨量低而导致骨骼脆性增加为主要特征的一种全身性疾病,在老年人中高发[9]。绝经后女性中也有较大部分可发生骨质疏松,与绝经后骨吸收速度明显高于骨质生成的速度,引发了骨质的丢失有关[10]。
3.2 钙尔奇D在骨质疏松患者中的作用
机体内的胶原蛋白三重螺旋分子结构会跟随人的年龄增长而水解,当遭到重大破坏时,钙盐无法有效沉积,可导致骨矿流失增加,引起骨代谢异常发生。钙尔奇D中含有的钙质属于骨矿化的底物,可以满足机体对钙的需求,继而提高骨钙内环境的稳定性,也利于骨形成速度的增加,明显缓解骨质疏松的临床症状,在一定程度上避免了骨折的发生[11]。当前钙尔奇D已经在临床上被广泛应用,例如,治疗妊娠期妇女缺钙、佝偻病等。有研究[12]指出,钙尔奇D可以促进骨胶原的合成,有效抑制胶原的降解,显著增加骨密度,且对骨微结构进行改善。
3.3 复方骨肽注射液在骨质疏松患者中的作用
骨肽注射液中含有多肽类骨代谢因子、无机钙、有机钙、氨基酸、微量元素等,且同时含有多种骨生长因子,作用于人体后,可促进成骨细胞的增殖,也能够促进骨代谢水平的改善,随着时间的推移,新骨形成,且钙、磷代谢、骨代谢平衡改善明显,明显促进骨折的愈合[13]。另外,该药物能够将关节周围的组织营养微循环加以改善,能起到消炎镇痛的效用。
3.4 联合用药对骨密度、骨代谢的作用
骨密度是当前临床上用以评估骨骼情况的重要指标,其反映了骨质疏松的程度,且可以预测骨折的危险性,血清骨钙素属于成骨细胞合成以及分泌的元素,具有不受骨吸收因素影响的特点,稳定性较强,因此,临床上应用该指标对成骨细胞活动、骨细胞活动状态进行判定[14]。有研究[15]指出,机体的骨更新率高时,则血清骨钙素水平增加明显,而老年患者由于骨质疏松等原因,为低转换型,故而血清骨钙素水平增加的幅度一般。BALP、PIINP是反应骨代谢的生化指标,能够评价骨质疏松的程度,其中,PIINP是骨组织中的唯一胶原,占骨基质的90%以上,能够有效反映骨细胞活动及骨形成;而BALP属于ALP同工酶之一,属于成骨细胞,可以较为敏感地反映骨质疏松情况。
本研究中,两组患者骨密度、骨代谢水平治疗后均较治疗前改善(P<0.05),乙组患者BALP、PINP、骨密度水平均较甲组高(P<0.05)。钙尔奇D可以促进胶原合成,抑制胶原的降解,继而增加了骨密度,改善了骨结构。复方骨肽注射液作为一种复方制剂,含有较多有益成分,且含丰富的骨生长因子,对骨代谢水平具有较好的调节作用,两者联合能够更好地刺激骨细胞增殖,加快细胞的生长,故而促进了骨密度增加。
3.5 联合用药有效缓解骨质疏松的临床症状
骨痛是骨质疏松患者常见的临床症状之一,严重骨痛的患者被迫减少了日常活动,不仅影响生活质量,还可加重骨质疏松程度。本研究中,两组患者在中医症状评分、VAS、JOA评分上差异有统计学意义(P<0.05),复方骨肽注射液联合钙尔奇D治疗较单纯钙尔奇D治疗,对骨质疏松患者中医症状评分、VAS、JOA评分改善更好。在维生素D补充的基础上联合骨肽注射液治疗,较大程度地改善了骨密度以及骨代谢水平,缓解了临床症状,促进腰椎功能的恢复。此外,复方骨肽注射液中的成分还能够发挥消肿止痛作用,减轻疼痛程度,减少对生活自理能力的影响,有利于患者预后康复。
综上所述,复方骨肽注射液治疗骨质疏松患者具有较好效果,能够改善骨代谢、骨密度指标及骨质疏松程度,有利于缓解临床症状,可在骨质疏松治疗中应用推广。
-
表 1 TGC检测时间程序
时间(t/min) 流速(ml/min) 功能 LC1 LC2 辅助流动相 0.01~0.80 1.00 1.20 2.00 脑脊液样品于LC1中富集 0.81~3.49 1.00 1.20 0.01 目标物在LC1中初步分离 3.50~5.50 1.00 1.20 0.01 LC1与LC2串联,目标物洗脱至LC2 5.51~11.00 1.50 1.20 0.01 目标物在LC2中洗脱分离,清洗泵反向冲洗LC1 表 2 脑脊液中测定TGC浓度的精密度与准确度(%)
浓度(ng/ml) 日内(n=5) 日间(n=3) 准确度 RSD 准确度 RSD 225.0 100.31 ± 2.83 2.82 98.80 ± 0.95 0.96 450.0 104.58 ± 2.19 2.09 103.51 ± 2.72 2.63 900.0 106.51 ± 2.06 1.94 101.82 ± 4.28 4.20 64.5 105.04 ± 2.47 2.35 99.01 ± 2.05 2.08 表 3 脑脊液中TGC在不同温度条件下的稳定性
样品浓度(ng/ml) 室温放置4 h 进样器放置6 h 反复冻融3次 冰冻放置1周 平均浓度(ng/ml) RSD(%) 平均浓度(ng/ml) RSD(%) 平均浓度(ng/ml) RSD(%) 平均浓度(ng/ml) RSD(%) 64.5 68.67 ± 2.93 4.27 65.52 ± 1.52 2.32 65.01 ± 1.43 2.20 68.56 ± 3.06 4.46 225 223.16 ± 5.06 2.27 221.62 ± 2.45 1.11 224.72 ± 4.50 2.00 222.99 ± 5.40 2.42 450 452.44 ± 9.11 2.01 459.04 ± 13.45 2.93 464.03 ± 8.39 1.81 459.68 ± 8.25 1.80 900 885.14 ± 15.09 1.70 881.32 ± 13.36 1.52 888.13 ± 13.80 1.55 899.79 ± 10.13 1.13 -
[1] 陈洁, 胡章勇. 替加环素治疗多重耐药菌感染的药物监测[J]. 生命的化学, 2019, 39(3):521-526. [2] 梅升辉, 朱乐亭, 杨莉, 等. 替加环素治疗颅内耐药菌感染的研究进展[J]. 中国药房, 2016, 27(14):2005-2008. doi: 10.6039/j.issn.1001-0408.2016.14.45 [3] SIPAHI O R, MERMER S, DEMIRDAL T, et al. Tigecycline in the treatment of multidrug-resistant Acinetobacter baumannii meningitis: Results of the Ege study[J]. Clin Neurol Neurosurg,2018,172:31-38. doi: 10.1016/j.clineuro.2018.06.008 [4] WU Y X, CHEN K, ZHAO J W, et al. Intraventricular administration of tigecycline for the treatment of multidrug-resistant bacterial meningitis after craniotomy: a case report[J]. J Chemother,2018,30(1):49-52. doi: 10.1080/1120009X.2017.1338846 [5] 国家药典委员会. 中华人民共和国药典2015年版四部[S]. 北京: 中国医药科技出版社, 2015: 364. [6] 缪文清, 汪硕闻, 范先煜, 等. HPLC法测定人脑脊液中万古霉素浓度及在颅内感染中的应用[J]. 中国药师, 2019, 22(3):399-403. doi: 10.3969/j.issn.1008-049X.2019.03.003 [7] 嵇金如, 沈萍, 魏泽庆, 等. 国产和进口替加环素体外抗菌活性比较[J]. 中国感染与化疗杂志, 2015, 15(4):330-334. doi: 10.3969/j.issn.1009-7708.2015.04.006 [8] DOWZICKY M J, PARK C H. Update on antimicrobial susceptibility rates among gram-negative and gram-positive organisms in the United States: results from the Tigecycline Evaluation and Surveillance Trial (TEST) 2005 to 2007[J]. Clin Ther,2008,30(11):2040-2050. doi: 10.1016/j.clinthera.2008.11.006 [9] RODVOLD K A, GOTFRIED M H, CWIK M, et al. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose[J]. J Antimicrob Chemother,2006,58(6):1221-1229. doi: 10.1093/jac/dkl403 [10] POLAT M, OZKAYA-PARLAKAY A. Tigecycline salvage therapy for ventriculoperitoneal shunt meningitis due to extensi- vely drug-resistant Acinetobacter baumannii[J]. Eur J Pediatr,2019,178(1):117-118. doi: 10.1007/s00431-018-3271-2 [11] 邵华, 宋一帆, 何杰, 等. 高剂量替加环素在感染性休克患者体内的药物监测及药代动力学[J]. 中国药科大学学报, 2017, 48(6):721-726. doi: 10.11665/j.issn.1000-5048.20170614 [12] MURALIDHARAN G, MICALIZZI M, SPETH J, et al. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects[J]. Antimicrob Agents Chemother,2005,49(1):220-229. doi: 10.1128/AAC.49.1.220-229.2005 [13] MAZUMDER S, RAMYA B S, BILIGI D S. Utility of urine reagent strips in cerebrospinal fluid analysis: an aid to bedside diagnosis of meningitis[J]. Indian J Pathol Microbiol,2018,61(3):356-359. doi: 10.4103/IJPM.IJPM_821_16 [14] LI X G, SUN S S, LING X, et al. Plasma and cerebrospinal fluid population pharmacokinetics of vancomycin in postoperative neurosurgical patients after combined intravenous and intraventricular administration[J]. Eur J Clin Pharmacol,2017,73(12):1599-1607. doi: 10.1007/s00228-017-2313-4 期刊类型引用(3)
1. 李长秀,李振山,张晗,高菲,李晋,王婧,侯大鹏,刘燕琳. 基于蒙特卡洛模拟和药动学/药效学模型评价替加环素脑室注射治疗泛耐药鲍曼不动杆菌颅内感染的给药方案. 中华神经医学杂志. 2024(04): 379-386 .  百度学术
百度学术2. 刘晓芹,李涛,董华,李文. 新型二维色谱系统建立重症医院获得性肺炎患者中替加环素含量的测定方法研究. 中国实用医药. 2023(19): 170-172 .  百度学术
百度学术3. 夏利新,毛知兰,苏秋平,罗旭蔚,谭莲,颜加岭,刘文辉. 颅内鲍曼不动杆菌感染患者脑脊液中替加环素的药物浓度监测. 中国处方药. 2021(11): 46-48 .  百度学术
百度学术其他类型引用(0)
-







 下载:
下载:



 下载:
下载: