-
环肽主要源于植物、真菌,以及海洋天然产物和绝大多数生物有机体中[1]。环肽是具有特殊的生物活性和较强的药理活性的一类化合物[2-3]。而且大多数环肽结构比直链肽更加稳定,且脂溶性高、穿膜性强、体内半衰期长等优点[4-5],从而使环肽在抗肿瘤、抗病毒、天然领域中起着重要的作用[6-9]。
Auyuittuqamide A是从极地海洋天然产物微孢子菌属(RKAG186)中分离得到的一种环肽化合物,它是由10个氨基酸残基组成。Russell等[10]从Sesquicillium microsporum中分离得到四个环十肽auyuittuqamide A-D。该类肽有一定的天然活性和对人体低毒性[11-12]。auyuittuqamide A经过核磁共振光谱法和串联质谱法证明了这类化合物的结构。用Marfey法[13]确定了氨基酸的绝对结构。然而,天然产物中分离提取的auyuittuqamide A的含量很低,很难推进构效关系和下一步的研究与应用。因此,采用固相合成法合成环十肽具有成本低、时间短、操作简单等优点[14-16]。
本文用2–氯三苯甲基氯(CTC)树脂为固相载体,通过HOBT/DIC为缩合体系,依次缩合氨基酸,完成全保护直链肽的合成。再以PyBOP/HOBT/DIPEA为缩合体系,在DCM溶液中液相环合[17-19],随后利用TFA进行保护基的脱除,进而得到环十肽auyuittuqamide A(图1)。
-
Fmoc-L–亮氨酸(Fmoc-Leu-OH)、Fmoc–甘氨酸(Fmoc-Gly-OH)、Fmoc-L–缬氨酸(Fmoc-Val-OH)、Fmoc-O–叔丁基–L–丝氨酸(Fmoc-Ser(tBu)-OH)、Fmoc-L–异亮氨酸(Fmoc-Ile-OH)、N-(9–芴甲氧羰酰基)-N–甲基–L–苯丙氨酸(Fmoc-N-Me-L-Phe-OH)、Fmoc-N–甲基–L–缬氨酸(Fmoc-N-Me-L-Val-OH)、N-Fmoc-N–甲基–O–叔丁基–L–苏氨酸(Fmoc-N-Me-Thr(tBu)-OH)[希施生物科技(上海)有限公司];2–氯三苯甲基氯(CTC)树脂(上海吉尔生化有限公司);N,N–二异丙基乙胺(DIPEA)、六氟磷酸苯并三唑–1–基–氧基三吡咯烷基磷(PyBOP)、1–羟基苯并三氮唑(HOBT)、1,3–二异丙基碳二亚胺(DIC)、三氟乙醇(TFE)、三氟乙酸(TFA)购自北京百灵威科技有限公司;N,N–二甲基甲酰胺(DMF)、二氯甲烷(DCM)(国药集团化学试剂有限公司);乙腈为色谱纯。
CHA-S气浴恒温振荡器(江苏金坛国胜实验仪器厂);SK7200BT超声波清洗器(上海科导超声仪器有限公司);LD5-2A低速离心机(北京京立离心机有限公司);低温恒温反应浴(槽)、SHZ-D(Ⅲ)循环水式真空泵(上海东玺制冷仪器设备有限公司);WSZ-50A轨道式振荡器(上海-恒科技有限公司);Waters2695/E2695高效液相色谱仪(美国沃特世公司);LC-1型反向制备液相色谱仪(北京创新恒通科技有限公司);6538 UHD Accurate Mass Q-TOF LC/MS质谱仪(美国安捷伦公司)。
-
按图2路线,固相合成天然环肽auyuittuqamide A。
-
用称量纸称取2–氯三苯甲基氯(CTC)树脂1 g(载样量为0.45 mmol/g)于多肽固相合成反应管中,加入DCM(10 ml)和DMF(10 ml)溶胀树脂,20 min后抽干。用DMF和DCM冲洗5遍,加Fmoc-Gly-OH(600 mg,2 mmol)和DIPEA(666 μl)的DMF溶液入反应管中,反应管固定于CHA-S气浴恒温振荡器中常温震荡4 h。再用DCM和DMF各洗涤5遍,抽干之后再加入20%哌啶的DMF溶液(10 ml),重复2遍,每一遍10 min,从而脱去氨基酸上的Fmoc保护基,再依次使用DMF和DCM各洗涤5遍。之后将已经配置好的Fmoc–氨基酸–OH(2 mmol,5倍当量),HOBT(2 mmol,5倍当量),DIC(2 mmol,5倍当量)的DMF溶液加入到反应管中,反应管固定于CHA-S气浴恒温振荡器中常温震荡2 h,反应完成之后,再使用DMF和DCM冲洗5遍。重复上述步骤,依次偶联氨基酸Fmoc-N-Me-L-Val-OH、Fmoc-Val-OH、Fmoc-Ser(tBu)-OH、Fmoc-Ile-OH、Fmoc-Gly-OH、Fmoc-N-Me-L-Phe-OH、Fmoc-Val-OH、Fmoc-N-Me-Thr(tBu)-OH、Fmoc-Leu-OH。
-
所有氨基酸完成偶联之后,获得直链肽–树脂复合物,使用无水乙醚将其挥干,加入TFE/DCM(1∶4,V/V)混合溶液10 ml,放置在轨道式振荡器,常温震荡4 h。过滤并收集滤液,滤液用旋转蒸发仪蒸干,获得直链肽粗品,粗品用制备型RP-HPLC进行纯化,再使用冻干机将其干燥,获得纯品直链肽。
-
在0 ℃的条件下将直链肽溶于50 ml的DCM溶液缓慢滴加入HOBT(3 eq)、PyBOP(5 eq)、DIPEA(10 eq)溶于DCM 50 ml中,滴加完成之后,于常温搅拌反应过夜。反应结束后,使用旋转蒸发仪蒸干反应溶剂,获得目标粗品,再将粗品用制备型RP-HPLC纯化,再使用冻干机将其干燥,获得纯品环肽。
-
先配置脱除侧链保护基溶液12 ml(TFA/DCM=1/3),将溶液加入到环肽冻干后的纯品中,固定于轨道式振荡器,常温震荡反应4 h。
-
色谱条件:Ryoung C18色谱柱(20mm×250 mm,10 μm);流动相A:水+0.1%TFA,流动相B:乙腈+0.1%TFA,梯度洗脱:(0~5 min, 40% B;5~60 min,40%~70% B);流速:20ml/min; 紫外检测波长214 nm。
-
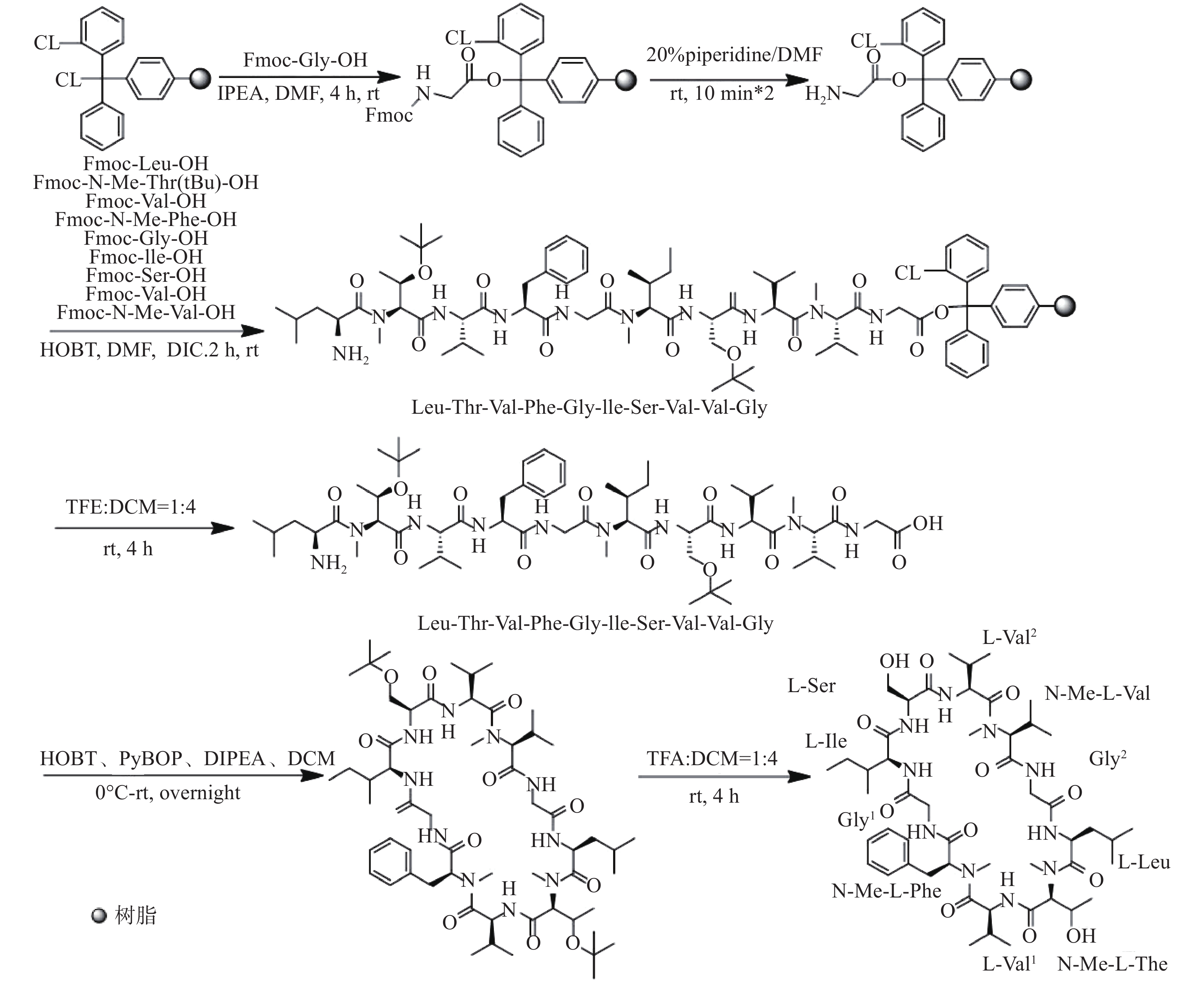
本步是将直链肽从树脂上切割,得到油状物粗品360 mg,再将粗品进行纯化,得到纯品100 mg,收率为:[实际值100mg/(理论值1144×0.45 mmol)]×100%=19.42%,HPLC图谱如图3所示,HR-Q-TOF-MS质谱图中对应的[M+H]+峰1145.7444,[M+Na]+峰1167.7266显示的分子量与直链肽auyuittuqamide A相吻合。
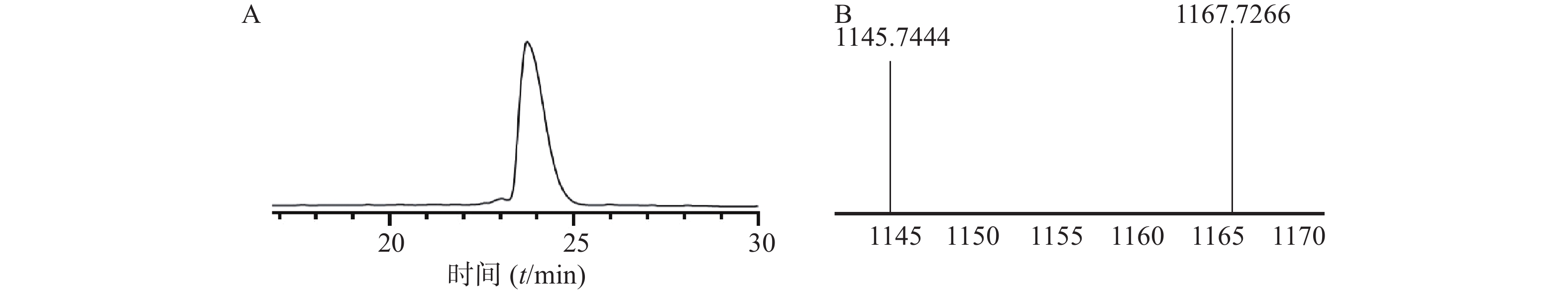
本步是将直链肽纯品进行环合,得到油状物粗品,再将粗品进行纯化,得到纯品30 mg,此步收率为:[实际值30mg/(理论值1126×0.45 mmol)]×100%=5.92%,HPLC图谱如图4所示,HR-Q-TOF-MS质谱图中对应的[M+H]+峰1127.7346,[M+Na]+峰1149.7159显示的分子量与auyuittuqamide A直链肽环合后分子量相吻合。
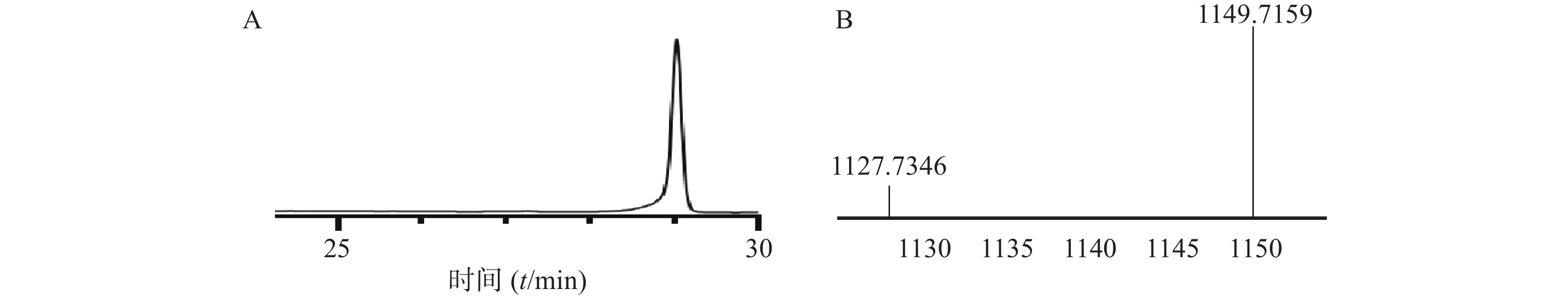
本步是将环合后纯品脱除侧链保护基,得到油状物粗品,再用乙腈和水溶解,最后冻干得到纯品25 mg,收率为:[实际值25mg/(理论值1014×0.45mmol)]×100%=5.48%,HPLC图谱如图5所示,HR-Q-TOF-MS质谱图中对应的[M+H]+峰1015.6115,[M+Na]+峰1037.5937显示的分子量与环肽auyuittuqamide A分子量相吻合。
-
本研究通过500MHz核磁共振氢谱对环肽进一步表征,确认与文献中auyuittuqamide A的核磁共振氢谱相符。1H-NMR (500MHz, d-DMSO) δ: 8.91 (d, J = 9.85 Hz, 1H), 8.70 (d, J = 9.5Hz, 1H), 8.55 (d, J = 6.95 Hz, 1H), 7.75 (t, J = 5.5 Hz, 1H), 7.47−7.43 (m, 3H), 7.27−7.17 (m, 5H), 5.01 (d, J = 9.75 Hz, 1H), 4.88 (m, 1H), 4.59−4.52 (m, 2H), 4.45 (t, J = 9.15 Hz, 1H), 4.39 (t, J = 8.8 Hz, 1H), 3.32 (dd, J = 4.3 Hz, 1H), 3.88−3.82 (m, 2H), 3.78 (t, J = 6.5 Hz, 2H), 3.74 (d, J = 4.45 Hz, 1H), 3.33−3.27 (m, 4H), 3.22 (s, 3H), 3.17−3.13 (m, 4H), 2.98−2.93 (m, 1H), 2.48−2.45 (m, 7H), 1.97 (m, 1H), 1.87−1.82 (m, 2H), 1.64−1.60 (m, 2H), 1.20−1.13 (m, 3H), 1.01 (d, J = 6.5 Hz, 3H), 0.86 (d, J = 5.75 Hz, 3H), 0.82−0.78 (m, 17H), 0.76−0.72 (m, 5H), 0.70 (d, J = 6.45 Hz, 3H)。
-
本法采用先固相合成直链肽,再液相进行环合获得目标产物auyuittuqamide A。并且采用先环合,再脱除侧链保护基,从而有效地避免了侧链裸露的羟基对环合时的影响[20]。使用高效液相色谱进行纯化,得到纯品纯度大于95%,收率为5.48%的目标产物auyuittuqamide A。本研究首次完成了对auyuittuqamide A的全合成,本法优点:更为省时,方法简单,易于操作,且经济实用。缺点:产率还有待提高,方法需进一步优化。总的来说,本方法为该类环肽化合物的全合成提供了参考。
The total synthesis of natural cyclopeptide auyuittuqamide A
-
摘要:
目的 用Fmoc固相直链合成和液相环合的方法合成天然环肽auyuittuqamide A。 方法 以2–氯三苯甲基氯(CTC)树脂为固相载体,1,3–二异丙基碳二亚胺(DIC)和1–羟基苯并三氮唑(HOBT)为缩合剂,9–芴基甲氧基羰基(Fmoc)保护的氨基酸,按照序列依次缩合,以三氟乙醇(TFE)作为切割试剂,获得全保护直链肽。以六氟磷酸苯并三唑–1–基–氧基三吡咯烷基磷(PyBOP)和1–羟基苯并三氮唑(HOBT)为环合试剂,全保护直链肽在二氯甲烷(DCM)溶液中环合,以三氟乙酸(TFA)为脱保护试剂,获得天然环肽auyuittuqamide A。用高效液相制备色谱进行纯化,采用HR-Q-TOF-MS, 500MHz 1H-NMR进行表征分析。 结果 获得纯度大于95%的天然环肽auyuittuqamide A,总收率5.48%。 结论 此法合成步骤简单,产率较高,首次建立天然环肽auyuittuqamide A的全合成方法,为auyuittuqamide A的进一步研究奠定基础。 -
关键词:
- auyuittuqamide A /
- 多肽固相合成 /
- 环肽
Abstract:Objective To synthesize the natural cyclopeptide auyuittuqamide A by Fmoc-based solid phase linear synthesis and liquid phase cyclization. Methods Using 2-chlorotriphenylmethyl chloride (CTC) resin as the solid support, 1,3-diisopropylcarbodiimide (DIC) and 1-hydroxybenzotriazole (HOBt) as the condensing agents, 9-fluorenylmethoxycarbonyl (Fmoc) to protect amino acids were assembled in sequence, and then the linear peptide bearing the protected groups was obtained in presence of trifluoroethanol (TFE) cutting reagent. The protected linear peptide was cyclized with the aid of benzotriazole hexafluorophosphate (PyBOP) and 1-hydroxybenzotriazole (HOBt) in dichloromethane (DCM) solution, followed by trifluoroacetic acid (TFA) deprotection to obtain the cyclic peptide, auyuittuqamide A that was purified by preparative HPLC and characterized by HR-MS and 500MHz 1H-NMR. Results The purity of auyuittuqamide A was more than 95% and the total yield was 5.48%. Conclusion This method has simple synthesis steps and high yield. It is the first to establish a fully synthesis method for the natural cyclic peptide auyuittuqamide A, which lays the foundation for further research of auyuittuqamide A. -
Key words:
- auyuittuqamide A /
- solid phase peptide synthesis /
- cyclopeptide
-
环肽主要源于植物、真菌,以及海洋天然产物和绝大多数生物有机体中[1]。环肽是具有特殊的生物活性和较强的药理活性的一类化合物[2-3]。而且大多数环肽结构比直链肽更加稳定,且脂溶性高、穿膜性强、体内半衰期长等优点[4-5],从而使环肽在抗肿瘤、抗病毒、天然领域中起着重要的作用[6-9]。
Auyuittuqamide A是从极地海洋天然产物微孢子菌属(RKAG186)中分离得到的一种环肽化合物,它是由10个氨基酸残基组成。Russell等[10]从Sesquicillium microsporum中分离得到四个环十肽auyuittuqamide A-D。该类肽有一定的天然活性和对人体低毒性[11-12]。auyuittuqamide A经过核磁共振光谱法和串联质谱法证明了这类化合物的结构。用Marfey法[13]确定了氨基酸的绝对结构。然而,天然产物中分离提取的auyuittuqamide A的含量很低,很难推进构效关系和下一步的研究与应用。因此,采用固相合成法合成环十肽具有成本低、时间短、操作简单等优点[14-16]。
本文用2–氯三苯甲基氯(CTC)树脂为固相载体,通过HOBT/DIC为缩合体系,依次缩合氨基酸,完成全保护直链肽的合成。再以PyBOP/HOBT/DIPEA为缩合体系,在DCM溶液中液相环合[17-19],随后利用TFA进行保护基的脱除,进而得到环十肽auyuittuqamide A(图1)。
1. 实验部分
1.1 试剂和仪器
Fmoc-L–亮氨酸(Fmoc-Leu-OH)、Fmoc–甘氨酸(Fmoc-Gly-OH)、Fmoc-L–缬氨酸(Fmoc-Val-OH)、Fmoc-O–叔丁基–L–丝氨酸(Fmoc-Ser(tBu)-OH)、Fmoc-L–异亮氨酸(Fmoc-Ile-OH)、N-(9–芴甲氧羰酰基)-N–甲基–L–苯丙氨酸(Fmoc-N-Me-L-Phe-OH)、Fmoc-N–甲基–L–缬氨酸(Fmoc-N-Me-L-Val-OH)、N-Fmoc-N–甲基–O–叔丁基–L–苏氨酸(Fmoc-N-Me-Thr(tBu)-OH)[希施生物科技(上海)有限公司];2–氯三苯甲基氯(CTC)树脂(上海吉尔生化有限公司);N,N–二异丙基乙胺(DIPEA)、六氟磷酸苯并三唑–1–基–氧基三吡咯烷基磷(PyBOP)、1–羟基苯并三氮唑(HOBT)、1,3–二异丙基碳二亚胺(DIC)、三氟乙醇(TFE)、三氟乙酸(TFA)购自北京百灵威科技有限公司;N,N–二甲基甲酰胺(DMF)、二氯甲烷(DCM)(国药集团化学试剂有限公司);乙腈为色谱纯。
CHA-S气浴恒温振荡器(江苏金坛国胜实验仪器厂);SK7200BT超声波清洗器(上海科导超声仪器有限公司);LD5-2A低速离心机(北京京立离心机有限公司);低温恒温反应浴(槽)、SHZ-D(Ⅲ)循环水式真空泵(上海东玺制冷仪器设备有限公司);WSZ-50A轨道式振荡器(上海-恒科技有限公司);Waters2695/E2695高效液相色谱仪(美国沃特世公司);LC-1型反向制备液相色谱仪(北京创新恒通科技有限公司);6538 UHD Accurate Mass Q-TOF LC/MS质谱仪(美国安捷伦公司)。
1.2 实验方法
按图2路线,固相合成天然环肽auyuittuqamide A。
1.2.1 直链肽固相合成
用称量纸称取2–氯三苯甲基氯(CTC)树脂1 g(载样量为0.45 mmol/g)于多肽固相合成反应管中,加入DCM(10 ml)和DMF(10 ml)溶胀树脂,20 min后抽干。用DMF和DCM冲洗5遍,加Fmoc-Gly-OH(600 mg,2 mmol)和DIPEA(666 μl)的DMF溶液入反应管中,反应管固定于CHA-S气浴恒温振荡器中常温震荡4 h。再用DCM和DMF各洗涤5遍,抽干之后再加入20%哌啶的DMF溶液(10 ml),重复2遍,每一遍10 min,从而脱去氨基酸上的Fmoc保护基,再依次使用DMF和DCM各洗涤5遍。之后将已经配置好的Fmoc–氨基酸–OH(2 mmol,5倍当量),HOBT(2 mmol,5倍当量),DIC(2 mmol,5倍当量)的DMF溶液加入到反应管中,反应管固定于CHA-S气浴恒温振荡器中常温震荡2 h,反应完成之后,再使用DMF和DCM冲洗5遍。重复上述步骤,依次偶联氨基酸Fmoc-N-Me-L-Val-OH、Fmoc-Val-OH、Fmoc-Ser(tBu)-OH、Fmoc-Ile-OH、Fmoc-Gly-OH、Fmoc-N-Me-L-Phe-OH、Fmoc-Val-OH、Fmoc-N-Me-Thr(tBu)-OH、Fmoc-Leu-OH。
1.2.2 直链肽的切割与纯化
所有氨基酸完成偶联之后,获得直链肽–树脂复合物,使用无水乙醚将其挥干,加入TFE/DCM(1∶4,V/V)混合溶液10 ml,放置在轨道式振荡器,常温震荡4 h。过滤并收集滤液,滤液用旋转蒸发仪蒸干,获得直链肽粗品,粗品用制备型RP-HPLC进行纯化,再使用冻干机将其干燥,获得纯品直链肽。
1.2.3 直链肽的环合
在0 ℃的条件下将直链肽溶于50 ml的DCM溶液缓慢滴加入HOBT(3 eq)、PyBOP(5 eq)、DIPEA(10 eq)溶于DCM 50 ml中,滴加完成之后,于常温搅拌反应过夜。反应结束后,使用旋转蒸发仪蒸干反应溶剂,获得目标粗品,再将粗品用制备型RP-HPLC纯化,再使用冻干机将其干燥,获得纯品环肽。
1.2.4 环肽侧链保护基的脱除与纯化
先配置脱除侧链保护基溶液12 ml(TFA/DCM=1/3),将溶液加入到环肽冻干后的纯品中,固定于轨道式振荡器,常温震荡反应4 h。
1.2.5 纯化条件
色谱条件:Ryoung C18色谱柱(20mm×250 mm,10 μm);流动相A:水+0.1%TFA,流动相B:乙腈+0.1%TFA,梯度洗脱:(0~5 min, 40% B;5~60 min,40%~70% B);流速:20ml/min; 紫外检测波长214 nm。
2. 结果与讨论
2.1 Auyuittuqamide A直链肽的HPLC和高分辨质谱表征
本步是将直链肽从树脂上切割,得到油状物粗品360 mg,再将粗品进行纯化,得到纯品100 mg,收率为:[实际值100mg/(理论值1144×0.45 mmol)]×100%=19.42%,HPLC图谱如图3所示,HR-Q-TOF-MS质谱图中对应的[M+H]+峰1145.7444,[M+Na]+峰1167.7266显示的分子量与直链肽auyuittuqamide A相吻合。
本步是将直链肽纯品进行环合,得到油状物粗品,再将粗品进行纯化,得到纯品30 mg,此步收率为:[实际值30mg/(理论值1126×0.45 mmol)]×100%=5.92%,HPLC图谱如图4所示,HR-Q-TOF-MS质谱图中对应的[M+H]+峰1127.7346,[M+Na]+峰1149.7159显示的分子量与auyuittuqamide A直链肽环合后分子量相吻合。
本步是将环合后纯品脱除侧链保护基,得到油状物粗品,再用乙腈和水溶解,最后冻干得到纯品25 mg,收率为:[实际值25mg/(理论值1014×0.45mmol)]×100%=5.48%,HPLC图谱如图5所示,HR-Q-TOF-MS质谱图中对应的[M+H]+峰1015.6115,[M+Na]+峰1037.5937显示的分子量与环肽auyuittuqamide A分子量相吻合。
2.2 Auyuittuqamide A的核磁共振氢谱表征
本研究通过500MHz核磁共振氢谱对环肽进一步表征,确认与文献中auyuittuqamide A的核磁共振氢谱相符。1H-NMR (500MHz, d-DMSO) δ: 8.91 (d, J = 9.85 Hz, 1H), 8.70 (d, J = 9.5Hz, 1H), 8.55 (d, J = 6.95 Hz, 1H), 7.75 (t, J = 5.5 Hz, 1H), 7.47−7.43 (m, 3H), 7.27−7.17 (m, 5H), 5.01 (d, J = 9.75 Hz, 1H), 4.88 (m, 1H), 4.59−4.52 (m, 2H), 4.45 (t, J = 9.15 Hz, 1H), 4.39 (t, J = 8.8 Hz, 1H), 3.32 (dd, J = 4.3 Hz, 1H), 3.88−3.82 (m, 2H), 3.78 (t, J = 6.5 Hz, 2H), 3.74 (d, J = 4.45 Hz, 1H), 3.33−3.27 (m, 4H), 3.22 (s, 3H), 3.17−3.13 (m, 4H), 2.98−2.93 (m, 1H), 2.48−2.45 (m, 7H), 1.97 (m, 1H), 1.87−1.82 (m, 2H), 1.64−1.60 (m, 2H), 1.20−1.13 (m, 3H), 1.01 (d, J = 6.5 Hz, 3H), 0.86 (d, J = 5.75 Hz, 3H), 0.82−0.78 (m, 17H), 0.76−0.72 (m, 5H), 0.70 (d, J = 6.45 Hz, 3H)。
3. 讨论
本法采用先固相合成直链肽,再液相进行环合获得目标产物auyuittuqamide A。并且采用先环合,再脱除侧链保护基,从而有效地避免了侧链裸露的羟基对环合时的影响[20]。使用高效液相色谱进行纯化,得到纯品纯度大于95%,收率为5.48%的目标产物auyuittuqamide A。本研究首次完成了对auyuittuqamide A的全合成,本法优点:更为省时,方法简单,易于操作,且经济实用。缺点:产率还有待提高,方法需进一步优化。总的来说,本方法为该类环肽化合物的全合成提供了参考。
-
[1] CASCALES L, CRAIK D J. Naturally occurring circular proteins: distribution, biosynthesis and evolution[J]. Org Biomol Chem,2010,8(22):5035-5047. doi: 10.1039/c0ob00139b [2] PIFFERI C, BERTHET N, RENAUDET O. Cyclopeptide scaffolds in carbohydrate-based synthetic vaccines[J]. Biomater Sci,2017,5(5):953-965. doi: 10.1039/C7BM00072C [3] ANDAVAN G S, LEMMENS-GRUBER R. Cyclodepsipeptides from marine sponges: natural agents for drug research[J]. Mar Drugs,2010,8(3):810-834. doi: 10.3390/md8030810 [4] LI Q J, MA L, YI D R, et al. Novel cyclo-peptides inhibit Ebola pseudotyped virus entry by targeting primed GP protein[J]. Antiviral Res,2018,155:1-11. doi: 10.1016/j.antiviral.2018.04.020 [5] VASCO A V, BRODE M, MÉNDEZ Y, et al. Synthesis of lactam-bridged and lipidated cyclo-peptides as promising anti-phytopathogenic agents[J]. Molecules,2020,25(4):E811. doi: 10.3390/molecules25040811 [6] ESMAEILI M A, ABAGHERI-MAHABADI N, HASHEMPOUR H, et al. Viola plant cyclotide vigno 5 induces mitochondria-mediated apoptosis via cytochrome C release and caspases activation in cervical cancer cells[J]. Fitoterapia,2016,109:162-168. doi: 10.1016/j.fitote.2015.12.021 [7] KANG K B, MING G, KIM G J, et al. Jubanines F-J, cyclopeptide alkaloids from the roots of Ziziphus jujuba[J]. Phytochemistry,2015,119:90-95. doi: 10.1016/j.phytochem.2015.09.001 [8] THEVENARD J, RAMONT L, DEVY J, et al. The YSNSG cyclopeptide derived from tumstatin inhibits tumor angiogenesis by down-regulating endothelial cell migration[J]. Int J Cancer,2010,126(5):1055-1066. [9] ZHOU X, HUANG H B, CHEN Y C, et al. Marthiapeptide A, an anti-infective and cytotoxic polythiazole cyclopeptide from a 60 L scale fermentation of the deep sea-derived Marinactinospora thermotolerans SCSIO 00652[J]. J Nat Prod,2012,75(12):2251-2255. doi: 10.1021/np300554f [10] GRUNWALD A L, CARTMELL C, KERR R G. auyuittuqamide A-D, cyclic decapeptides from Sesquicillium microsporum RKAG 186 isolated from Frobisher bay sediment[J]. J Nat Prod,2021,84(1):56-60. doi: 10.1021/acs.jnatprod.0c00966 [11] MAESTÁ I, NITECKI R, DESMARAIS C C F, et al. Effectiveness and toxicity of second-line actinomycin D in patients with methotrexate-resistant postmolar low-risk gestational trophoblastic neoplasia[J]. Gynecol Oncol,2020,157(2):372-378. doi: 10.1016/j.ygyno.2020.02.001 [12] ZORZI A, DEYLE K, HEINIS C. Cyclic peptide therapeutics: past, present and future[J]. Curr Opin Chem Biol,2017,38:24-29. doi: 10.1016/j.cbpa.2017.02.006 [13] SETHI S, MARTENS J, BHUSHAN R. Assessment and application of Marfey's reagent and analogs in enantioseparation: a decade's perspective[J]. Biomed Chromatogr,2021,35(1):e4990. doi: 10.1002/bmc.4990 [14] AGOURIDAS V, DIEMER V, MELNYK O. Strategies and open questions in solid-phase protein chemical synthesis[J]. Curr Opin Chem Biol,2020,58:1-9. [15] GIESLER R J, ERICKSON P W, KAY M S. Enhancing native chemical ligation for challenging chemical protein syntheses[J]. Curr Opin Chem Biol,2020,58:37-44. doi: 10.1016/j.cbpa.2020.04.003 [16] LAPS S, SATISH G, BRIK A. Harnessing the power of transition metals in solid-phase peptide synthesis and key steps in the (semi)synthesis of proteins[J]. Chem Soc Rev,2021,50(4):2367-2387. doi: 10.1039/D0CS01156H [17] GODOI K R R, BASSO R C, MING C C, et al. Crystallization, microstructure and polymorphic properties of soybean oil organogels in a hybrid structuring system[J]. Food Res Int,2020,137:109460. doi: 10.1016/j.foodres.2020.109460 [18] HENZEL S, BECKER S, HENNEN D, et al. Highly strained nanoscale bicyclophane monolayers entering the third dimension: a combined synthetic and scanning tunneling microscopy investigation[J]. Chempluschem,2021,86(6):797. doi: 10.1002/cplu.202100099 [19] LUTZ C, SIMON W, WERNER-SIMON S, et al. Total synthesis of α- and β-amanitin[J]. Angew Chem Int Ed Engl,2020,59(28):11390-11393. doi: 10.1002/anie.201914935 [20] AHARONI N, MAMANE H, BIRAN D, et al. Gene expression in Pseudomonas aeruginosa exposed to hydroxyl-radicals[J]. Chemosphere,2018,199:243-250. doi: 10.1016/j.chemosphere.2018.02.012 -






 下载:
下载:
 下载:
下载:


