-
脓毒症是因感染反应失调而导致的危及生命的器官功能障碍,其发病率和死亡率很高。根据柳叶刀杂志最新报道,2017年全球记录的脓毒症病例为4890万例,与脓毒症相关的死亡为1100万例,约占全球所有死亡病例的20%[1]。发达国家如美国每年脓毒症病例约为170万例,与脓毒症相关的死亡约27万例[2]。在低收入及中等收入的国家,脓毒症更是重症监护病房(ICU)患者的主要死亡原因,死亡率高达80%[3]。世界卫生组织已经认识到脓毒症对全球健康的重大威胁,并加强了对脓毒症的预防、诊断和治疗[4]。尽管如此,脓毒症的死亡率依然很高,主要原因之一是目前并没有诊断脓毒症的金标准,临床缺乏早期诊断和病情预测的手段。传统的标准微生物培养方法非常耗时,且有相当比例的假阴性结果。降钙素原(PCT)是唯一写进临床指南的脓毒症标志物,可用于指导抗生素的使用,但并不具有独立诊断的能力。C反应蛋白(CRP)作为传统的炎性指标,其诊断特异性较差。为了给临床提供准确及时的诊断及预后标准,脓毒症标志物一直是研究热点。本文对近年来关注度较高的、新的脓毒症候选生物标志物进行了综述,主要包括急性期蛋白、可溶性受体、非编码RNA和其他候选标志物。
-
PTX-3作为正五聚蛋白(pentraxins,PTX)长五肽亚家族的一员,由脂多糖(LPS)等微生物成分或炎性细胞因子刺激分泌,可以参与髓系细胞对病原体的识别,通过产生抗微生物微环境发挥抵抗作用[5]。
PTX-3的产生不依赖肝脏,在反映快速炎症过程方面优于传统的生物标志物。多项研究已经证明其较好的诊断价值[6-8],认为其诊断能力并不弱于PCT和CRP 。
PTX-3还被认为可以作为脓毒症的预后标志物,既是28 d死亡率的独立相关因素[9],也可以预测脓毒症休克[10]。除此之外,Hu等[11]的研究认为PTX-3(AUC=0.78)在预测28 d死亡率方面优于PCT和乳酸,并提出将PTX-3、PCT和乳酸作为一组标志物能更好的预测死亡率(AUC=0.90,95%CI 0.83~0.94),这也为多种生物标志物的联合应用指明了方向。
-
肾上腺髓质素(ADM)来源于更大的前体肽(Pro-ADM),MR-proADM是其活性形式。LPS、细胞因子和缺氧刺激导致的感染会促进ADM的合成和分泌。在脓毒症过程中,ADM既可以通过cAMP介导血管扩张作用,也具有稳定内皮屏障和抗菌抗炎的作用。在脓毒症休克时ADM水平可上升20~30倍。脓毒症患者初诊ADM浓度升高与血管活性药物需求增加、器官衰竭恶化和死亡率增加有关。值得注意的是,内源性ADM水平在高血压、缺血、内分泌和代谢紊乱等情况下也会增加。
有研究表明,在贯序器官衰竭评估(SOFA)评分阴性的情况下,MR-proADM同样具有重要的诊断意义,且与器官衰竭和28 d死亡率显著相关[12-13]。值得注意的是,Buendgens等[14]发现了ADM在长达26个月的随访中仍具有死亡率预测价值。
Daga等[15]发现女性脓毒症患者血清ADM水平升高更为明显,且女性患者的死亡率更低,认为主要原因是ADM发挥了作为神经激素的正面保护作用。而后Ajith[16]对此文章发表了评论,虽然肯定了初诊ADM水平对血管活性药物需求、恶化的器官功能障碍和死亡率的预测能力,但ADM与脓毒症的利害关系尚需继续研究。有趣的是Daga等[17]也对此评论做出了回复,进一步解释并坚持其原有看法。
-
CD14是单核细胞和巨噬细胞等免疫细胞表面的LPS受体,Presepsin是机体感染后产生的可溶性CD14亚型。多项研究已经表明Presepsin具有诊断脓毒症的能力[18],但关于Presepsin对预后的预测能力尚有争议。有研究称Presepsin与免疫功能低下的脓毒症患者的预后相关,而PCT与不良预后无明显相关性,究其原因,可能是生物标志物产生机制的不同,PCT是由LPS和某些细胞因子诱导的,而Presepsin的产生则并不依赖LPS和细胞因子,这也解释了一些研究者发现Presepsin在小鼠盲肠结扎穿孔(CLP)模型中升高,而在LPS模型中无明显变化[19]。有趣的是,Kaplan等[20]提出Presepsin与白蛋白的比值具有更好的脓毒症预后的预测能力。在预测脓毒症并发症方面,除了脓毒症休克, Presepsin还可预测脓毒症急性肾损伤、急性呼吸窘迫综合征、弥散性血管内凝血[21]。另外,Presepsin是革兰阴性菌细胞壁中LPS的受体之一,全血与细菌共培养结果表明革兰阴性菌诱导的Presepsin水平高于革兰阳性菌,说明Presepsin具有鉴别细菌感染种类的潜能[22]。
-
髓样细胞触发受体-1(TREM-1)表达于髓系细胞表面,在细菌和真菌感染时表达显著增加,具有炎症放大器的作用。sTREM-1作为TREM-1的可溶性形式,可以代表TREM-1的激活情况,被认为是脓毒症的诊断和预测的候选生物标志物。
多项研究认为sTREM-1具有诊断脓毒症的作用,但诊断能力仍有争议[23-24]。荟萃分析显示sTREM-1对脓毒症的诊断能力中等,需要更多的大规模研究来进一步评估sTREM-1的诊断准确性[25]。除此之外,sTREM-1还被发现表达于内皮细胞和血小板,在非感染性炎症中以及心血管手术后甚至非病理状态下也会升高[26]。因此,血浆sTREM-1作为脓毒症诊断标志物仍需继续研究。
sTREM-1作为脓毒症的预后标志物似乎更有希望,Gibot等[27]认为sTREM-1是与预后相关的独立因素。多项研究描述了类似的结果,并且Zhang等[28]认为血清sTREM-1浓度比CRP和PCT更准确地反映脓毒症的严重程度,对脓毒症预后的动态评估更敏感。Charles等[29]的一篇高质量研究也提供了有力证据,认为与PCT和CD64相比,sTREM-1是更为理想的预后标志物。
-
尿激酶型纤溶酶原激活物受体(uPAR)是尿激酶型纤溶酶原激活物的膜结合型受体。SuPAR是其可溶性形式,广泛存在于体液中。当身体处于炎症或其他疾病状态时,激活的免疫细胞尤其是中性粒细胞使SuPAR水平显著上调,其浓度与免疫系统活动呈正相关[30]。
在鉴别脓毒症和全身炎症反应综合征(SIRS)方面,SuPAR表现出了比PCT更好的鉴别能力(AUC 0.89 vs 0.82),但在预测脓毒症患者死亡率方面PCT更具优势[31]。
SuPAR的预后价值研究更为广泛。希腊的脓毒症研究小组在2020年报道,SuPAR<4 ng/ml被认为可以安全出院,SuPAR>6 ng/ml是不良结果的警示信号,SuPAR>12 ng/ml的患者28 d死亡率在17%~50%[32]。一项法国学者[33]的前瞻性、多中心的国际研究表明,SuPAR联合可溶性血管内皮生长因子受体-2(sVEGFR2)对早期病情恶化有较好的预测能力(AUC=0.70)。Pregernig等[34]的一项纳入44项研究的荟萃分析肯定了SuPAR预测死亡率的作用,得出临界值为5.2 ng/ml (95% CI 4.5~6.0,P<0.01)。2020年的一篇综述,系统地回顾分析了SuPAR的脓毒症诊断和预后价值,共纳入30项研究的6906名患者,认为SuPAR具有诊断脓毒症(AUC=0.83)、预测死亡率(AUC=0.78)以及鉴别脓毒症与SIRS的作用(AUC=0.81)。与以往Meta分析确定的PCT有效性相比,SuPAR具有相似的临床指导价值,但SuPAR表现出更高的特异性,有助于弥补PCT的不足[30]。
-
miRNA是由20到24个核苷酸组成的非编码RNA,可与靶mRNA结合减少蛋白质表达甚至导致转录沉默。在应激条件下,miRNAs可通过对多个靶标的协同效应来改变细胞反应,精细调节基因表达的模式[35]。近年来陆续有学者发现了miRNAs的脓毒症诊断和预测作用。
在诊断方面,miR-16a作为新生儿败血症的诊断标志物,可抑制IL-6和TNF-α等促炎因子的mRNA表达,并促进IL-10等抗炎因子mRNA表达[36]。miR-328也表现出了较好的诊断潜能(AUC=0.926),抑制miR-328的表达水平可以改善脓毒症患者的心功能障碍和心脏炎症[37]。Hermann等[38]的研究表明,miR-193a-5p和miR-542-3p可用于鉴别非感染性疾病和感染性疾病(社区获得性肺炎或脓毒症),miR-1246的表达水平随着疾病严重程度的增加而发生显著变化,从而鉴别健康人群、社区获得性肺炎和脓毒症。
在预测方面,循环miR-10a水平可以区分脓毒症和感染,并预测28 d的死亡率。脓毒症患者miR-223的表达与APACHE II评分(P<0.001,r=0.526)和SOFA评分(P<0.001,r=0.390)呈正相关。脓毒症患者外周血单个核细胞miR-10a水平降低,且与病情严重程度呈负相关[39]。另外,在28 d内死亡的患者中,miR-223的表达高于28 d的幸存者[40]。
此外,miRNAs在预测脓毒症并发急性肾损伤方面具有独特作用。脓毒症患者其血、尿miR-22-3p水平都会降低,可预测急性肾损伤和28 d生存率[41]。血清 miR-21-3p联合实验室指标对脓毒症并发急性肾损伤具有较高的预测价值(AUC=0.962) [42]。
Chen等[43]综述了miR-155与脓毒症的相关性,对比多项研究,肯定了循环miR-155作为诊断标志物和预后标志物的作用,但入院后48 h循环miR-155明显下降,表明miR-155的上调仅存在于脓毒症早期。需要注意的是,Link等[44]提出了不同意见,在对miR-26b-5p、miR-122-5p、miR-143-3p、miR-146a-5p、miR-193-3p、miR-223-3p研究之后,并没有发现独立的miRNAs能够明确区分脓毒症和非脓毒症ICU患者,认为miRNAs的诊断能力并不优于现行评分系统。
-
lncRNA是一类超过200个核苷酸的RNA,通过调控靶基因的表达广泛参与细胞的增殖、分化和凋亡[42]。有结果显示脓毒症患者的血浆中多种lncRNA表达增加至28~70倍,单纯的LPS诱导也有相同的结果[45]。
lncRNA肺腺癌转移相关转录因子1( MALAT1)已被证明通过抑制NF-кB活性来调节LPS刺激的促炎细胞因子TNF-α和IL-6的表达[46]。一项对120名脓毒症患者进行的前瞻性队列研究显示,lncRNA MALAT1具有诊断脓毒症的价值(AUC=0.910),对脓毒症休克和死亡率也有较好的预测能力[47]。 lncRNA锌指蛋白反义链1(zinc finger antisense 1,ZFAS1)被认为与类风湿性关节炎和急性心肌梗死等炎症性疾病有关,Xu等[48]发现lncRNA ZFAS1对脓毒症也有较好的诊断(AUC=0.814)和预测死亡率(AUC=0.628)的能力。
其余lncRNA 多在预后方面起预测作用。lncRNA富含丰富的转录本1 (nuclear-enriched abundant trans-cript 1,NEAT1)是核体类斑点结构的重要组成部分,调节IL-8等抗病毒基因的表达,在先天免疫反应中发挥重要作用。脓毒症患者中lncRNA NEAT1水平的升高与APACHE II和SOFA评分呈正相关,并与不良预后相关[49-50]。lnc母系表达基因3 (maternally expressed gene 3 ,MEG3)的水平也与炎症反应和器官损伤程度正相关,通过作为多种miRNAs的分子海绵即长效竞争性抑制剂发挥作用,如miR-21。近期一项纳入219名脓毒症患者和219名健康对照者的研究中,对入院后24 h内采集的血浆样本中lnc-MEG3和miR-21含量进行分析,发现lnc-MEG3(AUC=0.887)和lnc-MEG3/miR-21比值(AUC=0.934)对预测脓毒症风险升高有较好的预测价值,而miR-21(AUC=0.801)对脓毒症风险降低有较好的预测价值[51]。与之相反,脓毒症患者lncRNA牛磺酸上调基因1 (taurine up-regulated 1,TUG1)的表达与急性生理与慢性健康评分(APACHE II)和序贯器官衰竭评分(SOFA)呈负相关,28 d死亡组的lncRNA TUG1表达低于28 d存活组[40]。
-
钙保护素是一种异源二聚体钙结合蛋白,高表达于髓系细胞的胞浆中。近期的两项研究[52-53]都认为钙保护素对脓毒症有较好的诊断效能。有趣的是,Wirtz等[54]提出初诊钙保护素高水平可能与死亡风险相关,而入院后钙保护素水平的升高则与长期良好结局相关。
-
nCD64又称高亲和力Fcγ受体I,在中性粒细胞中静息状态下水平很低,在感染过程中被炎性因子上调。近期一篇纳入133名患者的研究[55]表明,nCD64对诊断新生儿脓毒症的敏感度为94.7%,特异度为93.6%,AUC值为0.925。Hashem等[56]关于诊断作用也得出了类似的结论(AUC=0.922)。我国学者2020年的一项研究[57]同样表明nCD64在诊断和预测死亡率方面均优于PCT。
-
肠道是所有人体部位中微生物分布最密集和异质性最强的部位,肠道微生物群被认为是糖尿病、肝硬化、癌症和动脉粥样硬化等疾病发生发展的生物标志物。脓毒症会损害肠道微生物群的完整性,肠道微生物群也会影响脓毒症和器官衰竭的进程。根据El MEHS等[58]的报道,肠道微生物群检测可在发病前1天预测迟发性脓毒症的发生(AUC=0.78)。Agudelo等[59]发现脓毒症患者的肠道微生物群特征为副杆菌属、梭杆菌属和嗜血杆菌属等炎症相关微生物的增加,认为其可以预测脓毒症的发展,尤其是肠球菌种类的丰富度可能是脓毒症潜在的预后生物标志物。遗憾的是,近期的一项纳入150名患者的前瞻性队列研究[60]表明微生物多样性降低与死亡率的增加无关。而且饮食、抗生素和其他治疗手段都会引起肠道微生物群的变化,肠道微生物群作为脓毒症的生物标志物前景并不乐观。
-
血管生成素(Angpt)是血管生成生长因子家族中的一员,由血管内皮细胞在炎症等应激条件下分泌。Angpt-1发挥稳定血管内皮和抗炎的正面作用,而Angpt-2对Angpt-1产生竞争性拮抗作用[61]。Angpt-2可能具有预测脓毒症休克的能力(AUC=0.631)[10]。Angpt-2/Angpt-1的比值也具有预测作用,据韩国的一项报道[62],Angpt-2/1预测28 d死亡率的能力与SOFA评分无显著差异(AUC 0.736 vs 0.745 )。
-
本文综述的急性期蛋白、可溶性受体、非编码RNA和其他的标志物的作用及效能如表1所示。早期诊断和正确治疗是降低脓毒症病死率的关键。但由于脓毒症病理生理机制复杂,个体差异较大,体征和症状无特异性,早期诊断极为困难。目前被研究的诊断标志物主要参与先天免疫反应的初始发病机制,预后标志物通常与脓毒症引起的器官功能障碍有关。这些候选标志物在脓毒症发病机制中的作用以及最佳联合使用策略,都需要进一步的研究,以供将来的临床使用。
表 1 脓毒症生物标志物作用及效能比较
分类 标志物 作用 效能 参考文献 AUC 临界值 敏感性(%) 特异性(%) 急性期蛋白 PTX-3 诊断新生儿脓毒症 0.875 ? 100 94.3 [6] 诊断新生儿脓毒症 0.995 5.6 μg/L 98.3 96.7 [7] 预测28天死亡率 0.69 ? ? ? [9] 预测脓毒症休克 0.798 15877 pg/ml 50 100 [10] 预测28天死亡率 0.78 49.9 ng/ml 83.3 64.2 [11] ADM 诊断脓毒症 0.85 1.5 pg/ml 83 76.47 [12] 预测器官衰竭 0.69 75 pg/ml ? ? [13] 诊断脓毒症 0.731 ? ? ? [14] 预测总死亡率 0.655 1.4 nmol/L 81.1 39.8 可溶性受体 Presepsin 诊断脓毒症 0.792 380 pg/ml 83.5 62.2 [18] 预测30天死亡率 0.683 556 pg/ml 73.1 59.6 诊断免疫功能低下患者的脓毒症 0.87 1248 pg/ml 66 100 [19] sTREM-1 诊断脓毒症 0.97 60 ng/ml 96 89 [25] 诊断脓毒症 0.868 108.9 pg/ml 83 81 [26] 预测脓毒症死亡率 0.74 180 pg/ml 86 70 [29] 预测脓毒症休克 0.823 222.5 pg/ml 59.5 93.3 [30] 预测脓毒症死亡率 0.64 954.4 pg/ml 54.5 78 [31] SuPAR 诊断脓毒症 0.89 5.58 pg/ml 96 72.2 [33] 预测脓毒症病情恶化 0.66 ? 90 20 [35] 预测脓毒症死亡率 ? 5.2 ng/ml ? ? [36] 诊断脓毒症 0.83 7.5 pg/ml 76 78 [32] 预测死亡率 0.78 9.6 pg/ml 74 70 鉴别脓毒症与CIRS 0.81 7.5 pg/ml 67 82 MicroRNA miRNA-16a 诊断新生儿脓毒症 0.968 3.164 88 98 [36] miR-328 诊断脓毒症 0.926 0.305 87.6 86.36 [37] miR-10a 诊断脓毒症 0.804 0.18 65 85.7 [39] 预测28天死亡率 0.699 ? ? ? miR-223 诊断脓毒症 0.924 ? ? ? [40] 预测28天死亡率 0.711 ? ? ? miR-21-3p 预测脓毒症并发急性肾损伤 0.962 ? 97 91.4 [42] lncRNA lncRNA MALAT1 诊断脓毒症 0.91 1.895 83.33 85 [47] 预测脓毒症休克 0.836 3.665 70.37 92.42 预测脓毒症死亡率 0.886 3.62 81.82 89.47 lncRNA ZFAS1 诊断脓毒症 0.814 ? 92.1 63.5 [48] 预测脓毒症死亡率 0.628 ? 92.1 35.5 lncRNA NEAT1 诊断脓毒症 0.785 ? ? ? [49] 预测28天死亡率 0.726 ? ? ? lnc-MEG3 诊断脓毒症 0.887 ? ? ? [51] 预测28天死亡率 0.704 ? ? ? lncRNA TUG1 诊断脓毒症 0.846 ? ? ? [40] 预测28天死亡率 0.705 ? ? ? 其他 Calprotectin 诊断脓毒症 0.79 1.3 ng/l 81 56 [52] 诊断脓毒症 0.91 ? ? ? [53] nCD64 诊断新生儿脓毒症 0.925 41.60% 94.7 93.6 [55] 诊断脓毒症 0.879 8 MFI 75 89.4 [56] 预测28天死亡率 0.85 5.45 MFI 93.3 65.3 诊断脓毒症 0.922 43% 85.6 93 [57] 肠道微生物群 诊断迟发性脓毒症 0.78 ? ? ? [58] Angiopoietin 2 预测脓毒症休克 0.631 9047 pg/ml 42.31 88.24 [61] Angiopoietin 2/1 预测28天死亡率 0.736 3.2 69.8 70.6 [62]
Recent advances in biomarkers of sepsis
-
摘要: 脓毒症可以导致危及生命的器官功能障碍,是危重患者死亡的主要原因之一。脓毒症的早期诊断与正确治疗是降低病死率的关键,但目前尚无诊断的金标准。理想的脓毒症生物标志物应该具有早期诊断和预测不良预后的能力,且具有较好的敏感性和特异性。脓毒症的候选生物标志物众多,本文重点综述了急性期蛋白、可溶性受体、非编码RNA和其他近期关注度较高的候选标志物的最新进展。Abstract: Sepsis can cause life-threatening organ dysfunction and is one of the leading causes of death in critically ill patients. Early diagnosis and correct treatment of sepsis are the key to reducing the fatality, however, there is no golden standard for diagnosis at present. The ideal sepsis biomarker can be used for early diagnosis and predicting poor prognosis with good sensitivity and specificity. There are many candidate biomarkers for sepsis. This article reviews the latest developments on acute phase proteins, soluble receptors, non-coding RNAs and other candidate biomarkers of sepsis that attracted more recent attention.
-
中药挥发性成分(VOCs)是指中药中一类具有芳香气并易挥发的成分,其化学组成复杂,主要包括挥发油类以及其他分子量较小、易挥发的化合物,例如萜类、脂肪族、芳香族化合物等。VOCs具有发汗解表、芳香开窍、镇咳祛痰、理气、驱风、抑菌、镇痛、杀虫等多种功效。作为中药学研究的热点之一,高效率、高标准的检测药材中VOCs十分关键。因此,利用现代化方法来实现对中草药挥发性成分的细致分析,意义巨大。
VOCs常用气相色谱-质谱联用(GC-MS)方法进行分析,虽分离能力强,但样品需要预处理且分析时间长。气相色谱-离子迁移谱(GC-IMS)法结合了GC突出的分离能力和IMS快速响应、高灵敏度的特点,具有样品准备简便、高灵敏度、高分辨率等显著优势,结合化学计量学分析中药材VOCs所呈现图谱,可实现对药材VOCs无损、快速区分[1]。
1. 气相色谱–离子迁移谱联用技术原理
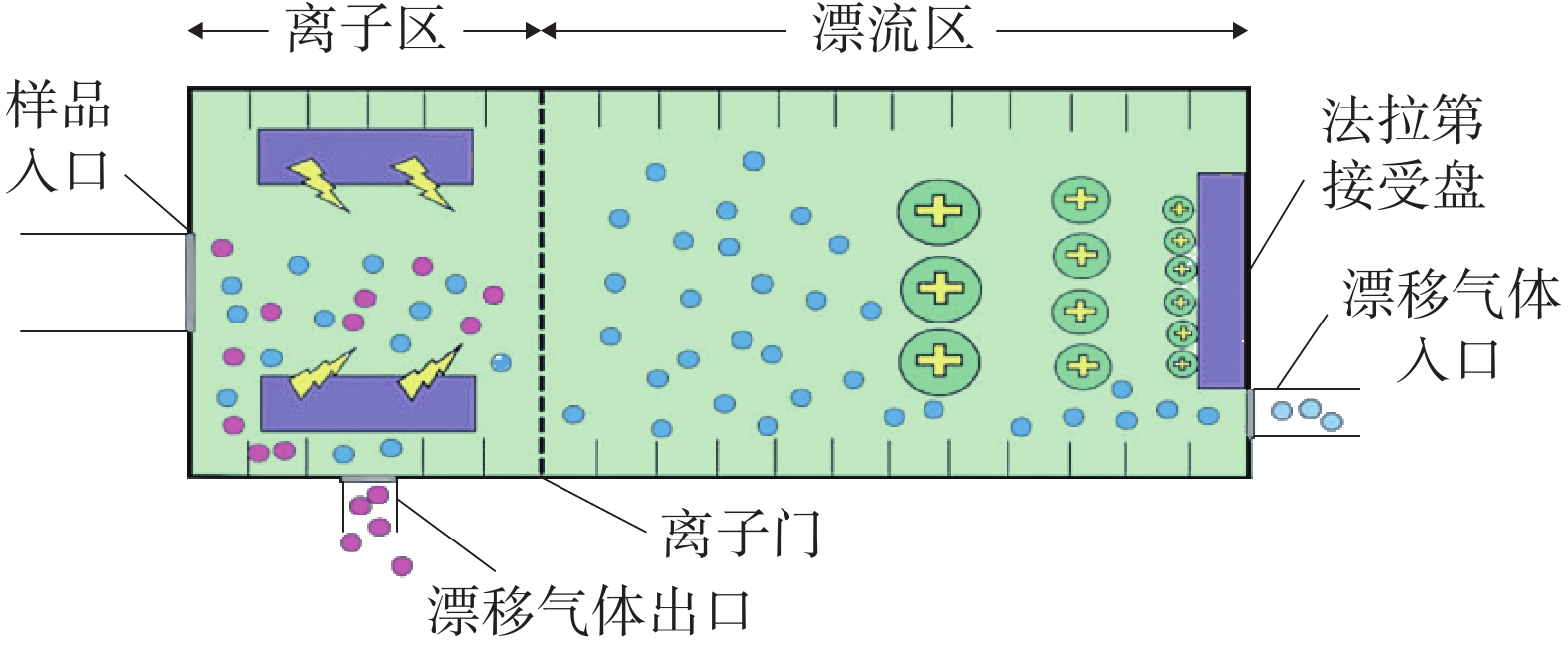
GC-IMS 技术的基本原理[2]是通过将样品混合物引进气相色谱仪进行分离,样品分子和载气分子在离子源放射性物质的作用下发生一系列反应形成产物离子,这些产物离子在不同的电场驱动下通过离子门进入迁移区,与逆向而来的中性迁移气体分子发生碰撞而损失能量。产物离子在电场中的迁移速率不同,到达检测器上的时间不同,从而使样品差异化分离(如图1),最后可得到一个包含有离子迁移时间(X轴),气相色谱保留时间(Y轴),离子强度(Z轴)的三维谱图(如图2)。
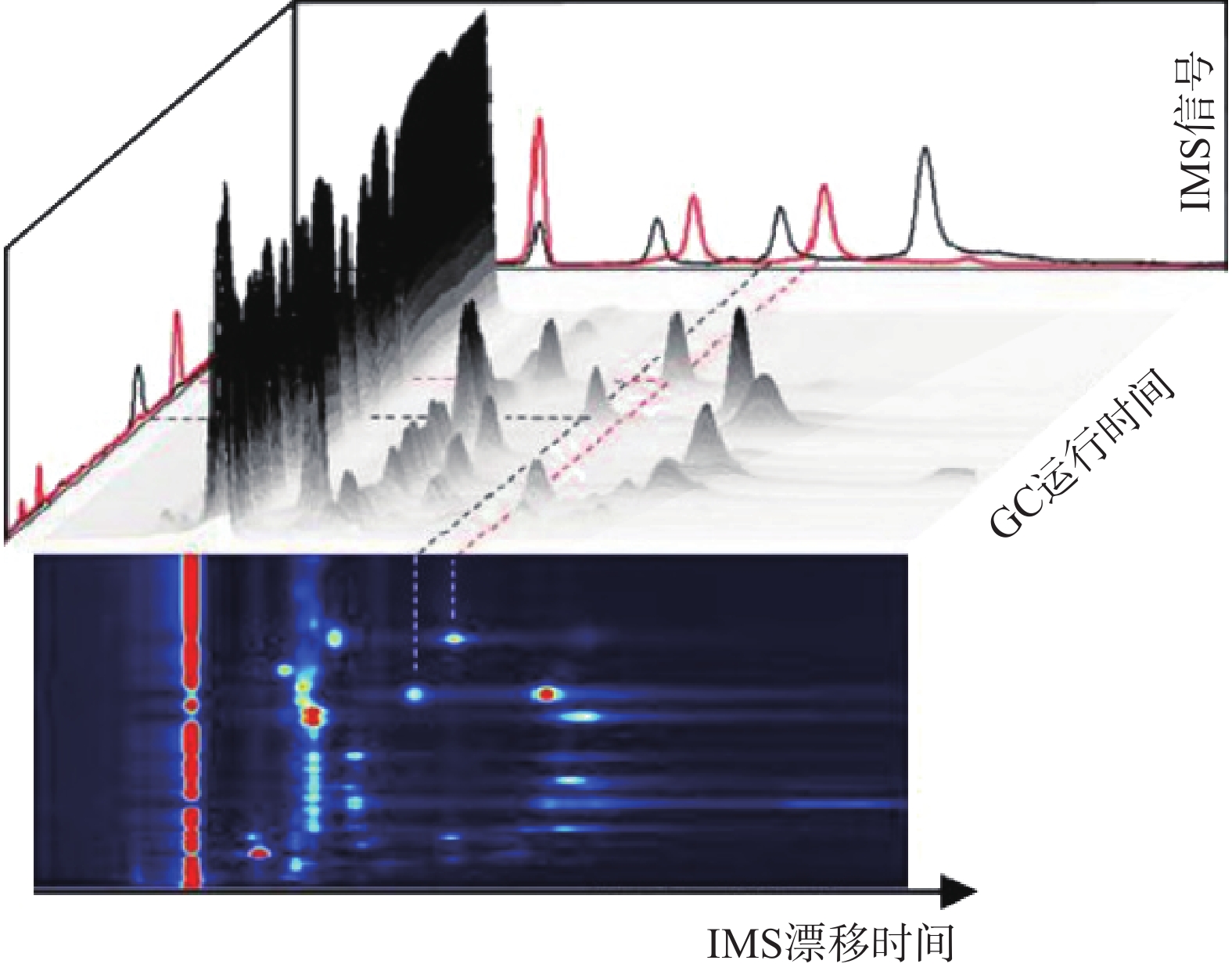 图 2 气相色谱-离子迁移谱联用技术结果三维谱图[3]
图 2 气相色谱-离子迁移谱联用技术结果三维谱图[3]顶空-气相色谱-离子迁移谱(HS-GC-IMS)是应用于检测药材中VOCs最为广泛的方式之一 [3]。其原理是首先将样品中的VOCs通过热孵育和振摇使之从药材中逸出,随后抽取顶空气体进行分析,避免复杂基质干扰,再使之进入气相色谱(GC)、离子迁移谱(IMS)中,得到相应谱图。HS-GC-IMS无需样品预处理,在分析复杂样品或需要快速检测场景方面更具优势。
自从1972年第一张经过GC分离的IMS谱图出现[2],早期主要用于军事领域(如化学战剂检测)和毒品筛查的先进技术逐渐面向市场,应用于研究(如图3),引进国内后广泛使用于中药研究。现阶段,通过GC-IMS捕捉到中药材中的微量VOCs,将其应用于区分中药的不同种类和产地、监控中药炮制过程以及中药复方组分分析等[4],为中药材的产地及真伪鉴别提供可靠的依据,同时也为质量控制和药效研究等方面提供数据支持。
2. 在中药鉴定中的应用
2.1 同属不同种的中药鉴别
同属不同种之间的中药材亲缘关系比较接近,在VOCs种类差异上不显著,利用GC-IMS技术善于捕捉挥发性化合物种类微小差异的特点,可对样品进行品种鉴别。
陈皮中主要含有三萜类、挥发油类等几百种成分,有平喘止咳、调节血管等药理作用[5-6],刘主洁等[7]和Lv等[8]采用HS-GC-IMS对陈皮和广陈皮样品中VOCs进行研究,分析二者的特征信号,以邻氨基苯甲酸二甲酯为广陈皮最明显的特征标记物,柠檬烯、癸醛等也可作为广陈皮的特征信号,利用这些特征信号可对陈皮和广陈皮进行区分。山莓是覆盆子的混淆品之一,为保证用药安全有效,有必要对两者进行区分[9],严爱娟等[10]采用GC-IMS技术检测山莓和覆盆子VOCs,明确鉴定出覆盆子特征成分较山莓多,其中癸醛、1-辛烯-3-醇等在覆盆子样品中的含量更高,苯甲醛、2-丁酮等在山莓样品中的含量更高,以此来实现山莓和覆盆子的区分。彭旭阳等[11]采用GC-IMS分析新疆和田地区“梭梭”和“红柳”肉苁蓉这两种不同寄主肉苁蓉挥发性物质之间的不同,找到了二者主要差异物质为苯甲醛、庚醛等。
2.2 中药不同产地鉴别
道地药材是指生长在特定的自然生态环境中,经过一系列技术培育和加工而成,且被公认为比其他地方生产的同种药材的质量和治疗效果好的药材[12]。但随着产地的迁移、品种的引入,在外观性状上对道地药材和其他产地的中药进行简单鉴别已经不能满足需求。
王振洲等[13]采用GC-IMS对来自不同产区的人参VOCs进行检测,发现2,5-二甲基吡嗪和2,6-二甲基吡嗪等VOCs在吉林集安四年生人参中含量较高,而吉林敦化四年生人参中含量相对较低,由此鉴别四年生吉林集安和敦化的人参。西洋参同属于五加科人参属,成功引种进入我国后,辽宁、吉林和山东是其主产区[14],王燕等[15]使用GC-IMS技术对美国、加拿大、山东等五个不同产地的西洋参进行研究,共鉴定出53种VOCs,其中美国西洋参中2-庚酮等成分的含量较高,而加拿大产α-蒎烯等成分的含量显著高于其它产区,中国产芳樟醇等成分的含量较高,其中吉林产地的含量是辽宁、山东产地的2.60和3.60倍,通过这些显著差异可对西洋参进行产地溯源和鉴别。
李曼祎等[16]使用GC-IMS技术对新疆、宁夏、内蒙古、青海四个主要产区的枸杞进行化学成分测定,分析出了四个地区含量差异较大的16种物质,发现内蒙古枸杞区别于另外三个产地特有的物质为正丁醇,同时筛选出了叶醇等五种标记性物质对枸杞产地进行区分。Li[17]等分别采用HS-SPME-GC-MS和HS-GC-IMS检测河北、河南、江苏、浙江、安徽以及山东6个不同地区的五味子,结果显示安徽的五味子萜类物质含量较高,同时该研究也证明了HS-GC-IMS对样品的分类效果优于HS-SPME-GC-MS。山东为瓜萎的道地产区[18],河北也是瓜萎的重要产区,不同产地的瓜萎可能在品质及所含成分上有所差别,从而对药效也会产生一定的影响[19-20]。张敏敏等[19]采用GC-IMS在瓜萎皮样品中共鉴定出醛类和醇类等88种VOCs,分析发现山东瓜蒌皮中2-庚酮、正壬醛等物质的含量低于河北瓜蒌皮,但1-己醇、糠醇等含量更高的结论,通过这些差异基本实现了两地区瓜萎皮的区分。Li等[21]采用HS-GC-IMS联合PCA建立松茸特征图谱,对分别来自云南和四川的松茸样品以及它们的菌盖和菌柄进行研究,发现虽然指纹图谱相似度较高,但各自也有其特征性挥发物:苯乙醛和糠醇等仅在云南产松茸的菌柄中被发现,戊烷仅在四川产松茸的菌柄中被发现,戊醛仅在云南产松茸的菌盖中检出,甲基吡嗪仅在四川产松茸的菌盖中检出,通过特征性挥发物的不同,可对分别产自云南和四川的松茸进行区分。
2.3 中药复方中主成分的鉴别
中药复方成分众多且复杂,确保其质量符合标准、疗效可靠、使用安全是关键,采用HPLC特征图谱进行分析是目前公认的良好方法之一[22]。GC-IMS检测灵敏度高、分离效果好,是对HPLC表征中药复方质量分析的有益补充。
Yuan等[23]设置了对照组、慢性不可预测轻度应激(CUMS)组和CUMS+百合鸡子黄汤组,采用HS-GC-IMS等方法对百合鸡子黄汤治疗CUMS大鼠粪便中挥发性化合物含量进行研究,鉴定出了11个生物标志物,找出了对照组大鼠粪便样品中甲硫醚含量较高,而CUMS组则较低,同时百合子鸡汤组抑郁表现出保护性干预作用;Yin等[24]采用HS-GC-IMS对开心散中的挥发性化合物进行分析,鉴别出β-细辛酮等十种VOCs可作为开心散的质量标记物,进一步为开心散质量控制及药效机制等的相关研究奠定基础;李巍等[25]利用HS-GC-IMS对清感秋饮中的VOCs进行定性定量分析,共鉴定出120种VOCs,其中,紫苏属酮、β-石竹烯等可能为其主要药效成分。
3. 在中药加工炮制中的应用
中药的加工炮制是提高临床疗效的重要手段,也是保证临床用药安全的重要措施[26]。应用不同的炮制方法可能会引起中药中的化学成分发生含量加减、成分转化与破坏等变化,采用GC-IMS技术对中药加工炮制过程中的VOCs进行动态监控,对于炮制工艺的规范、制定更优炮制方案等具有指导意义。
干燥是中药材和饮片加工制备过程中的重要且关键的环节之一,而中药中的VOCs易受干燥工艺的影响,对于富含VOCs的中药,准确控制干燥工艺有利于减少有效成分的损失。陈树鹏等[27]采用GC-IMS等技术确定烘干样品的整体香气属性优于晒干样品,这可能是由于烘干工艺对环境温度的调整使得烘干过程更有利于果香、柑橘香以及甜香香气保留。其中苯乙酸乙酯、乙酸乙酯等 12 种物质为晒干主要成分,2-甲基-1-丁醇、(E)-2-己烯-1-醇等为烘干主要成分;Wang等[24]在8S-GC-IMS技术的辅助下,了解了柑橘皮干燥以及不同条件下挥发性成分情况,研究柑橘皮各成分在不同干燥温度下的缺失,其中,70 ℃下干燥会导致2,2-苯基-1-苦基肼基和铁还原抗氧化能力显著降低。Yu等[29]通过GC-MS比较总离子色谱图中的峰面积与苯乙酸乙酯的峰面积,将VOCs的含量进行半定量再通过GC-IMS呈现图谱对结果进行比较,确定VOCs的身份,从而证实晒干有利于两个品种的网纹柑橘中萜醇类化合物的保存,热风干燥有利于脂肪族醛和倍半萜的保存,而冷冻干燥是保存酯类和酚类物质的最佳方法;Zhou等[30]对肉苁蓉进行酒制增效后粉碎、超微粉碎、醇提、水提等处理,采用HS-GC-IMS方法检测其VOCs并建立指纹图谱,发现增效处理的肉苁蓉VOCs的种类和含量有所减少,分析原因可能为各种化学物质之间在处理过程中会发生化学变化,而新鲜肉苁蓉则保存了更多种类的VOCs,超微粉碎处理和水提处理后的肉苁蓉挥发性化合物主要以醛类为主。除此之外,将其它中药基于GC-IMS技术在不同炮制方法中的应用汇总于表1。
表 1 GC-IMS技术在炮制研究方面的应用作者 药材及炮制方法 采用方式 实例 高以丹等 [31] 柴达木枸杞
冷冻干燥、自然阴干、热风烘干GC-IMS 从枸杞样品中鉴定出反-2-壬烯醛、2,4-庚二烯醛等52种VOCs,表明冷冻干燥法比自然阴干、热风烘干以及微波干燥更好,能够有效保留枸杞中的VOCs,使枸杞保持较高的品质。 时海燕等 [32] 六神曲
生品、炒品、焦品HS-GC-IMS 从六神曲生品、炒品和焦品中鉴别出60种化合物通过比较种类和差异,得出炒神曲比焦神曲健胃消食的效果更好。 林秀敏等 [33] 当归
酒洗、酒炙、酒浸GC-IMS 2-十一烯醛、丙酮等为酒洗与酒浸当归的主要差异性物质,2-十一烯醛、丙酮等为酒洗与酒炙当归的主要差异性物质,2-十一烯醛、辛酸乙酯等为酒浸与酒炙当归的主要差异性物质。 武旭等 [34] 胆南星
发酵炮制GC-IMS 发酵炮制有助于胆南星矫味矫臭 王雨晨等 [35] 太子参
常温晾干、晒干、热风干燥、
真空冷冻干燥GC-IMS 40 ℃热风干燥可以有效保留太子参样品中的VOCs,与晒干、晾干样品无差异,但真空冷冻干燥对太子参挥发性成分的影响较大,会造成挥发性成分以及风味的损失 焦焕然等 [36] 侧柏叶
常温晾干、晒干,热风干燥、
变温干燥GC-IMS 40 ℃和60 ℃热风干燥能够较好地保留瓜蒌样品中的核苷类和黄酮类成分 4. 与电子鼻联用
国内外也有许多采用GC-IMS与电子鼻联用对中药挥发性成分进行研究。电子鼻是一种通过模拟人嗅觉系统对检测物质进行品质评价的感官仪器,其原理是通过传感器阵列对气味分子进行检测和响应,将产生的信号经过预处理后送入模式识别系统,通过指纹图谱对挥发性成分或是气体进行定量或定性分析[37]。两种技术的联用为实验的结果研究提供了更高的准确度。
Feng等 [38]采用GC-IMS、GC-MS对不同地理标志的八种花椒的VOCs进行测定,证明了两种方法均可用于对不同花椒的分类,但相较之下GC-IMS操作时间更短,且有能够检测到含量很低物质,结果表明红花椒比青花椒能够释放出更多的萜烯、酯类和更少的醇类,同时该研究还与电子鼻联用表征花椒中的香气物质,W1W、W2W和W5S传感器对花椒样品VOCs的响应更强,说明花椒产品中可能含有更高丰度的萜烯、有机硫化物和氮氧化物。陈小爱等 [39]利用GC-MS、GC-IMS和电子鼻技术联用,分析老香黄在发酵期间的VOCs变化,GC-MS共鉴别出包括醇类等八个种类的46种VOCs,GC-IMS则检测出包括杂环类等九个类别的38种VOCs,同时电子鼻PCA有效区分了不同发酵时间的样品,发现发酵6个月后老香黄挥发性组分开始发生较大的变化,其中柠檬烯等14种是发酵期间含量较高且相对稳定的成分,发酵过程中产生的庚醛、糠醛等是构成老香黄特有气味的特征性成分。王世丽等 [40]通过电子鼻辨识南北柴胡气味特征物质与GC-IMS检测其挥发性成分,发现南北柴胡中短链烷烃、醛类等物质差异较大,癸醛、异戊烯醛等可作为南柴胡的特征物质,2-甲基丙酸、3-甲基丁醇可作为北柴胡的特征物质,此外乙酸、乙酸甲酯等成分在北柴胡中显著高于南柴胡。
5. 总结和展望
GC-IMS在中药研究中的应用前景非常广阔,不仅可以对同属不同种、不同产地来源、不同采收期以及不同贮存时间的中药VOCs进行分析鉴别,还可以帮助分析炮制前后中药VOCs含量变化以及在复方中寻找质量标志物,为药物质量控制与药效研究提供帮助。另外,随着技术的不断进步和中药现代化需求的增加,GC-IMS可以与特征图谱相结合,构建特征指纹图谱;也可以与电子鼻等其他分析手段融合,发挥出新的效果,让其所能提供的信息更加全面。但该项技术作为新兴科技仍需解决许多问题,比如应探索融合数据库的体系架构[41]。目前,GC-IMS通常使用的数据库为NIST出版的标准质谱图,对于中药VOCs的专业数据库搭建还不全面,部分VOCs需要自行判断建立文档保存入库,对实验进程造成不便。由于中药挥发性成分复杂,GC-IMS可能因峰重叠导致部分成分无法准确定性,例如分析复方丹参片时,GC-IMS仅能明确鉴定其中60%的化合物,需GC-MS辅助验证。而且GC-IMS对象单一,无法检测多糖、生物碱等非挥发性成分,难以全面评价中药质量。
总的来说,GC-IMS技术为中药研究提供了一种新的科学工具,有利于推动中药科学研究深入,也为中药产业的发展走向国际化和标准化提供支持。
-
表 1 脓毒症生物标志物作用及效能比较
分类 标志物 作用 效能 参考文献 AUC 临界值 敏感性(%) 特异性(%) 急性期蛋白 PTX-3 诊断新生儿脓毒症 0.875 ? 100 94.3 [6] 诊断新生儿脓毒症 0.995 5.6 μg/L 98.3 96.7 [7] 预测28天死亡率 0.69 ? ? ? [9] 预测脓毒症休克 0.798 15877 pg/ml 50 100 [10] 预测28天死亡率 0.78 49.9 ng/ml 83.3 64.2 [11] ADM 诊断脓毒症 0.85 1.5 pg/ml 83 76.47 [12] 预测器官衰竭 0.69 75 pg/ml ? ? [13] 诊断脓毒症 0.731 ? ? ? [14] 预测总死亡率 0.655 1.4 nmol/L 81.1 39.8 可溶性受体 Presepsin 诊断脓毒症 0.792 380 pg/ml 83.5 62.2 [18] 预测30天死亡率 0.683 556 pg/ml 73.1 59.6 诊断免疫功能低下患者的脓毒症 0.87 1248 pg/ml 66 100 [19] sTREM-1 诊断脓毒症 0.97 60 ng/ml 96 89 [25] 诊断脓毒症 0.868 108.9 pg/ml 83 81 [26] 预测脓毒症死亡率 0.74 180 pg/ml 86 70 [29] 预测脓毒症休克 0.823 222.5 pg/ml 59.5 93.3 [30] 预测脓毒症死亡率 0.64 954.4 pg/ml 54.5 78 [31] SuPAR 诊断脓毒症 0.89 5.58 pg/ml 96 72.2 [33] 预测脓毒症病情恶化 0.66 ? 90 20 [35] 预测脓毒症死亡率 ? 5.2 ng/ml ? ? [36] 诊断脓毒症 0.83 7.5 pg/ml 76 78 [32] 预测死亡率 0.78 9.6 pg/ml 74 70 鉴别脓毒症与CIRS 0.81 7.5 pg/ml 67 82 MicroRNA miRNA-16a 诊断新生儿脓毒症 0.968 3.164 88 98 [36] miR-328 诊断脓毒症 0.926 0.305 87.6 86.36 [37] miR-10a 诊断脓毒症 0.804 0.18 65 85.7 [39] 预测28天死亡率 0.699 ? ? ? miR-223 诊断脓毒症 0.924 ? ? ? [40] 预测28天死亡率 0.711 ? ? ? miR-21-3p 预测脓毒症并发急性肾损伤 0.962 ? 97 91.4 [42] lncRNA lncRNA MALAT1 诊断脓毒症 0.91 1.895 83.33 85 [47] 预测脓毒症休克 0.836 3.665 70.37 92.42 预测脓毒症死亡率 0.886 3.62 81.82 89.47 lncRNA ZFAS1 诊断脓毒症 0.814 ? 92.1 63.5 [48] 预测脓毒症死亡率 0.628 ? 92.1 35.5 lncRNA NEAT1 诊断脓毒症 0.785 ? ? ? [49] 预测28天死亡率 0.726 ? ? ? lnc-MEG3 诊断脓毒症 0.887 ? ? ? [51] 预测28天死亡率 0.704 ? ? ? lncRNA TUG1 诊断脓毒症 0.846 ? ? ? [40] 预测28天死亡率 0.705 ? ? ? 其他 Calprotectin 诊断脓毒症 0.79 1.3 ng/l 81 56 [52] 诊断脓毒症 0.91 ? ? ? [53] nCD64 诊断新生儿脓毒症 0.925 41.60% 94.7 93.6 [55] 诊断脓毒症 0.879 8 MFI 75 89.4 [56] 预测28天死亡率 0.85 5.45 MFI 93.3 65.3 诊断脓毒症 0.922 43% 85.6 93 [57] 肠道微生物群 诊断迟发性脓毒症 0.78 ? ? ? [58] Angiopoietin 2 预测脓毒症休克 0.631 9047 pg/ml 42.31 88.24 [61] Angiopoietin 2/1 预测28天死亡率 0.736 3.2 69.8 70.6 [62] -
[1] RUDD K E, JOHNSON S C, AGESA K M, et al. Global, regional, and national Sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study[J]. Lancet,2020,395(10219):200-211. doi: 10.1016/S0140-6736(19)32989-7 [2] Center for Disease Control and Prevention(CDC). Sepsis 2021 [J/OL].https://www.cdc.gov/sepsis/. [3] SCHULTZ M J, DUNSER M W, DONDORP A M, et al. Current challenges in the management of Sepsis in ICUs in resource-poor settings and suggestions for the future[J]. Intensive Care Med,2019,43(5):612-624. [4] World Health Organization (WHO). Sepsis 2018 [J/OL]. https://www.who.int/health-topics/sepsis. [5] PORTE R, DAVOUDIAN S, ASGARI F, et al. The long pentraxin PTX3 as a humoral innate immunity functional player and biomarker of infections and Sepsis[J]. Front Immunol,2019,10:794. doi: 10.3389/fimmu.2019.00794 [6] Sabry A, Ibrahim M, Khashana A, et al. Assessment of pentraxin 3 in a systemic inflammatory response occurring with neonatal bacterial infection [J/OL]. J Neonatal Perinatal Med, 2021 Jan 28. https://content.iospress.com/ articles/journal-of-neonatal-perinatal-medicine/npm200550. [7] FAHMEY S S, MOSTAFA N. Pentraxin 3 as a novel diagnostic marker in neonatal Sepsis[J]. J Neonatal Perinatal Med,2019,12(4):437-442. [8] HAMED S, BEHNES M, PAULY D, et al. Pentraxin-3 predicts short- and mid-term mortality in patients with Sepsis and septic shock during intensive care treatment[J]. Clin Lab,2018,64(6):999-1011. [9] HANSEN C B, BAYARRI-OLMOS R, KRISTENSEN M K, et al. Complement related pattern recognition molecules as markers of short-term mortality in intensive care patients[J]. J Infect,2020,80(4):378-387. doi: 10.1016/j.jinf.2020.01.010 [10] TIAN R, WANG X L, PAN T T, et al. Plasma PTX3, MCP1 and Ang2 are early biomarkers to evaluate the severity of Sepsis and septic shock[J]. Scand J Immunol,2019,90(6):e12823. [11] HU C, ZHOU Y, LIU C, et al. Pentraxin-3, procalcitonin and lactate as prognostic markers in patients with Sepsis and septic shock[J]. Oncotarget,2018,9(4):5125-5136. doi: 10.18632/oncotarget.23701 [12] SPOTO S, NOBILE E, CARNÀ E P R, et al. Best diagnostic accuracy of Sepsis combining SIRS criteria or qSOFA score with Procalcitonin and Mid-Regional pro-Adrenomedullin outside ICU[J]. Sci Rep,2020,10(1):16605. doi: 10.1038/s41598-020-73676-y [13] LEMASLE L, BLET A, GEVEN C, et al. Bioactive adrenomedullin, organ support therapies, and survival in the critically ill: results from the French and European outcome registry in ICU study[J]. Crit Care Med,2020,48(1):49-55. [14] BUENDGENS L, YAGMUR E, GINSBERG A, et al. Midregional proadrenomedullin (MRproADM) serum levels in critically ill patients are associated with short-term and overall mortality during a two-year follow-up[J]. Mediat Inflamm,2020,2020:7184803. [15] DAGA M K, MAWARI G, KUMAR L, et al. Adrenomedullin and its possible role in improved survival in female patients with Sepsis: a study in south east Asian region[J]. Indian J Crit Care Med,2020,24(12):1180-1184. doi: 10.5005/jp-journals-10071-23672 [16] AJITH KUMAR A K. Adrenomedullin in Sepsis: finally, a friend or an enemy? Indian J Crit Care Med,2020,24(12):1151-1153. doi: 10.5005/jp-journals-10071-23669 [17] LEE W L. Reply to mehmood: adrenomedullin: a double-edged sword in septic shock and heart failure therapeutics? Am J Respir Crit Care Med,2020,201(9):1165. [18] RUANGSOMBOON O, PANJAIKAEW P, MONSOMBOON A, et al. Diagnostic and prognostic utility of presepsin for Sepsis in very elderly patients in the emergency department[J]. Clin Chim Acta,2020,510:723-732. doi: 10.1016/j.cca.2020.09.014 [19] LEE J, KIM S, KIM K H, et al. The association between dynamic changes in serum presepsin levels and mortality in immunocompromised patients with Sepsis: a prospective cohort study[J]. Diagnostics (Basel),2021,11(1):60. doi: 10.3390/diagnostics11010060 [20] KAPLAN M, DUZENLI T, TANOGLU A, et al. Presepsin: albumin ratio and C-reactive protein: albumin ratio as novel Sepsis-based prognostic scores: a retrospective study[J]. Wien Klin Wochenschr,2020,132(7-8):182-187. doi: 10.1007/s00508-020-01618-9 [21] SHIMOYAMA Y, UMEGAKI O, KADONO N, et al. Presepsin values predict septic acute kidney injury, acute respiratory distress syndrome, disseminated intravascular coagulation, and shock[J]. Shock,2021,55(4):501-506. doi: 10.1097/SHK.0000000000001664 [22] KOIZUMI Y, SAKANASHI D, MOHRI T, et al. Can presepsin uniformly respond to various pathogens? -an in vitro assay of new Sepsis marker[J]. BMC Immunol,2020,21(1):33. doi: 10.1186/s12865-020-00362-z [23] GIBOT S, KOLOPP-SARDA M N, BÉNÉ M C, et al. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected Sepsis[J]. Ann Intern Med,2004,141(1):9-15. doi: 10.7326/0003-4819-141-1-200407060-00009 [24] SU L X, HAN B C, LIU C T, et al. Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among Sepsis patients with new fever in intensive care units: a prospective cohort study[J]. BMC Infect Dis,2012,12:157. doi: 10.1186/1471-2334-12-157 [25] PONTRELLI G, DE CRESCENZO F, BUZZETTI R, et al. Diagnostic value of soluble triggering receptor expressed on myeloid cells in paediatric Sepsis: a systematic review[J]. Ital J Pediatr,2016,42:44. doi: 10.1186/s13052-016-0242-y [26] JOLLY L, CARRASCO K, DERIVE M, et al. Targeted endothelial gene deletion of triggering receptor expressed on myeloid cells-1 protects mice during septic shock[J]. Cardiovasc Res,2018,114(6):907-918. doi: 10.1093/cvr/cvy018 [27] GIBOT S, CRAVOISY A, KOLOPP-SARDA M N, et al. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during Sepsis[J]. Crit Care Med,2005,33(4):792-796. doi: 10.1097/01.CCM.0000159089.16462.4A [28] ZHANG J, SHE D Y, FENG D, et al. Dynamic changes of serum soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) reflect Sepsis severity and can predict prognosis: a prospective study[J]. BMC Infect Dis,2011,11:53. doi: 10.1186/1471-2334-11-53 [29] CHARLES P E, NOEL R, MASSIN F, et al. Significance of soluble triggering receptor expressed on myeloid cells-1 elevation in patients admitted to the intensive care unit with Sepsis[J]. BMC Infect Dis,2016,16(1):559. doi: 10.1186/s12879-016-1893-4 [30] HUANG Q R, XIONG H Y, YAN P J, et al. The diagnostic and prognostic value of suPAR in patients with Sepsis: a systematic review and meta-analysis[J]. Shock,2020,53(4):416-425. doi: 10.1097/SHK.0000000000001434 [31] SHARMA A, RAY S, MAMIDIPALLI R, et al. A comparative study of the diagnostic and prognostic utility of soluble urokinase-type plasminogen activator receptor and procalcitonin in patients with Sepsis and systemic inflammation response syndrome[J]. Indian J Crit Care Med,2020,24(4):245-251. doi: 10.5005/jp-journals-10071-23385 [32] VELISSARIS D, DIMOPOULOS G, PARISSIS J, et al. Prognostic role of soluble urokinase plasminogen activator receptor at the emergency department: a position paper by the Hellenic Sepsis study group[J]. Infect Dis Ther,2020,9(3):407-416. doi: 10.1007/s40121-020-00301-w [33] LAFON T, CAZALIS M A, VALLEJO C, et al. Prognostic performance of endothelial biomarkers to early predict clinical deterioration of patients with suspected bacterial infection and Sepsis admitted to the emergency department[J]. Ann Intensive Care,2020,10(1):113. doi: 10.1186/s13613-020-00729-w [34] PREGERNIG A, MÜLLER M, HELD U, et al. Prediction of mortality in adult patients with Sepsis using six biomarkers: a systematic review and meta-analysis[J]. Ann Intensive Care,2019,9(1):125. doi: 10.1186/s13613-019-0600-1 [35] WINTER J, JUNG S, KELLER S, et al. Many roads to maturity: microRNA biogenesis pathways and their regulation[J]. Nat Cell Biol,2009,11(3):228-234. doi: 10.1038/ncb0309-228 [36] EL-HEFNAWY S M, MOSTAFA R G, EL ZAYAT R S, et al. Biochemical and molecular study on serum miRNA-16a and miRNA- 451 as neonatal Sepsis biomarkers[J]. Biochem Biophys Rep,2021,25:100915. [37] SUN B, LUAN C Y, GUO L S, et al. Low expression of microRNA-328 can predict Sepsis and alleviate Sepsis-induced cardiac dysfunction and inflammatory response[J]. Braz J Med Biol Res,2020,53(8):e9501. doi: 10.1590/1414-431x20209501 [38] HERMANN S, BRANDES F, KIRCHNER B, et al. Diagnostic potential of circulating cell-free microRNAs for community-acquired pneumonia and pneumonia-related Sepsis[J]. J Cell Mol Med,2020,24(20):12054-12064. doi: 10.1111/jcmm.15837 [39] ZHENG G, QIU G, GE M, et al. miR-10a in peripheral blood mononuclear cells is a biomarker for Sepsis and has anti-inflammatory function[J]. Mediators Inflamm,2020,2020:4370983. [40] LI N, WU S S, YU L. The associations of long non-coding RNA taurine upregulated gene 1 and microRNA-223 with general disease severity and mortality risk in Sepsis patients[J]. Medicine (Baltimore),2020,99(50):e23444. doi: 10.1097/MD.0000000000023444 [41] ZHANG H, CHE L, WANG Y, et al. Deregulated microRNA-22-3p in patients with sepsis-induced acute kidney injury serves as a new biomarker to predict disease occurrence and 28-day survival outcomes[J]. Int Urol Nephrol,2021,53:2107-2116. doi: 10.1007/s11255-021-02784-z [42] WU S Y, ZHANG H, WU W, et al. Value of serum miR-21-3p in predicting acute kidney injury in children with Sepsis[J]. Zhongguo Dang Dai Er Ke Za Zhi, 2020, 22(3): 269-273. C: \Users\sxchen\Downloads\Chinese_https: \www.ncbi.nlm.nih.gov\pubmed\32204765\. [43] CHEN M, WANG F, XIA H, et al. MicroRNA-155: regulation of immune cells in Sepsis[J]. Mediators Inflamm,2021,2021:8874854. [44] LINK F, KROHN K, BURGDORFF A M, et al. Sepsis diagnostics: intensive care scoring systems superior to MicroRNA biomarker testing[J]. Diagnostics,2020,10(9):701. doi: 10.3390/diagnostics10090701 [45] HASHEMIAN S M, POURHANIFEH M H, FADAEI S, et al. Non-coding RNAs and exosomes: their role in the pathogenesis of Sepsis[J]. Mol Ther Nucleic Acids,2020,21:51-74. doi: 10.1016/j.omtn.2020.05.012 [46] ZHAO G, SU Z Y, SONG D, et al. The long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced inflammatory response through its interaction with NF-κB[J]. FEBS Lett,2016,590(17):2884-2895. doi: 10.1002/1873-3468.12315 [47] CHEN J J, HE Y F, ZHOU L L, et al. Long non-coding RNA MALAT1 serves as an independent predictive biomarker for the diagnosis, severity and prognosis of patients with Sepsis[J]. Mol Med Report,2020,21(3):1365-1373. [48] XU Y H, SHAO B B. Circulating long noncoding RNA ZNFX1 antisense RNA negatively correlates with disease risk, severity, inflammatory markers, and predicts poor prognosis in Sepsis patients[J]. Medicine (Baltimore),2019,98(9):e14558. doi: 10.1097/MD.0000000000014558 [49] HE F, ZHANG C, HUANG Q. Long noncoding RNA nuclear enriched abundant transcript 1/miRNA-124 axis correlates with increased disease risk, elevated inflammation, deteriorative disease condition, and predicts decreased survival of Sepsis[J]. Medicine (Baltimore),2019,98(32):e16470. doi: 10.1097/MD.0000000000016470 [50] HUANG Q H, HUANG C Y, LUO Y, et al. Circulating lncRNA NEAT1 correlates with increased risk, elevated severity and unfavorable prognosis in Sepsis patients[J]. Am J Emerg Med,2018,36(9):1659-1663. doi: 10.1016/j.ajem.2018.06.008 [51] NA L, DING H J, XING E H, et al. Lnc-MEG3 Acts as a potential biomarker for predicting increased disease risk, systemic inflammation, disease severity, and poor prognosis of Sepsis via interacting with miR-21[J]. J Clin Lab Anal,2020,34(4):e23123. [52] LARSSON A, TYDÉN J, JOHANSSON J, et al. Calprotectin is superior to procalcitonin as a Sepsis marker and predictor of 30-day mortality in intensive care patients[J]. Scand J Clin Lab Invest,2020,80(2):156-161. doi: 10.1080/00365513.2019.1703216 [53] BARTÁKOVÁ E, ŠTEFAN M, STRÁNÍKOVÁ A, et al. Calprotectin and calgranulin C serum levels in bacterial Sepsis[J]. Diagn Microbiol Infect Dis,2019,93(3):219-226. doi: 10.1016/j.diagmicrobio.2018.10.006 [54] WIRTZ T H, BUENDGENS L, WEISKIRCHEN R, et al. Association of serum calprotectin concentrations with mortality in critically ill and septic patients[J]. Diagnostics,2020,10(11):990. doi: 10.3390/diagnostics10110990 [55] HASHEM H E, ABDEL HALIM R M, EL MASRY S A, et al. The utility of neutrophil CD64 and presepsin as diagnostic, prognostic, and monitoring biomarkers in neonatal Sepsis[J]. Int J Microbiol,2020,2020:8814892. [56] HASHEM H E, EL MASRY S A, MOKHTAR A M, et al. Valuable role of neutrophil CD64 and highly sensitive CRP biomarkers for diagnostic, monitoring, and prognostic evaluations of Sepsis patients in neonatal ICUs[J]. Biomed Res Int,2020,2020:6214363. [57] YIN W P, LI J B, ZHENG X F, et al. Effect of neutrophil CD64 for diagnosing Sepsis in emergency department[J]. World J Emerg Med,2020,11(2):79-86. doi: 10.5847/wjem.j.1920-8642.2020.02.003 [58] EL MANOUNI EL HASSANI S, NIEMARKT H J, BERKHOUT D J C, et al. Profound pathogen-specific alterations in intestinal microbiota composition precede late-onset Sepsis in preterm infants: a longitudinal, multicenter, case-control study[J]. Clin Infect Dis,2021,73(1):e224-e232. doi: 10.1093/cid/ciaa1635 [59] AGUDELO-OCHOA G M, VALDÉS-DUQUE B E, GIRALDO-GIRALDO N A, et al. Gut microbiota profiles in critically ill patients, potential biomarkers and risk variables for Sepsis[J]. Gut Microbes,2020,12(1):1707610. doi: 10.1080/19490976.2019.1707610 [60] YIN L, WAN Y D, PAN X T, et al. Association between gut bacterial diversity and mortality in septic shock patients: a cohort study[J]. Med Sci Monit,2019,25:7376-7382. doi: 10.12659/MSM.916808 [61] LELIGDOWICZ A, RICHARD-GREENBLATT M, WRIGHT J, et al. Endothelial activation: the ang/Tie axis in Sepsis[J]. Front Immunol,2018,9:838. doi: 10.3389/fimmu.2018.00838 [62] SEOL C H, YONG S H, SHIN J H, et al. The ratio of plasma angiopoietin-2 to angiopoietin-1 as a prognostic biomarker in patients with Sepsis[J]. Cytokine,2020,129:155029. doi: 10.1016/j.cyto.2020.155029 -





 下载:
下载:
 下载:
下载: 

