-
多形性胶质母细胞瘤(GBM)是恶性程度最高的脑肿瘤之一,呈强侵袭生长特点,患者中位生存期一般不超过15~17个月,5年生存率低于5%[1-3]。肿瘤最新机制研究聚焦高能量代谢特征,加州大学的研究人员揭示了癌症基因扩增和表观遗传重塑依赖于烟酰胺腺嘌呤二核苷酸(NAD+)途径,干预NAD+代谢成为癌症精准治疗的重要靶点[4]。肿瘤细胞通过生物合成上调来增加NAD+水平,对NAD+生物合成的限速酶——烟酰胺磷酸核糖转移酶(NAMPT)非常敏感[5]。Kim等[6]发现NAD+代谢在胶质瘤中过度活跃,GBM患者肿瘤细胞中NAMPT基因表达水平异常升高。PROTAC技术是一种化学诱导靶蛋白(POI)多聚泛素化,通过蛋白酶体通路降解POI的药物分子靶向新技术[7]。PROTAC分子由靶蛋白结合配体、连接链和E3泛素连接酶配体三部分组成,能特异性结合靶蛋白,同时招募E3泛素连接酶使POI多聚泛素化,最终通过蛋白酶体系统降解。并且标记POI促使蛋白降解后,可与POI解离在细胞内循环利用,起到亚化学计量催化的效果,减少药物剂量从而减少体内药物暴露量[8]。合作课题组合成了一种新型NAMPT酶抑制PROTAC分子NPT-B2,小鼠卵巢癌药效学研究表明NPT-B2具有比FK866更显著的抑制肿瘤效果。但是NPT-B2水溶性差,影响其适用性及吸收。纳米药物利用纳米尺度效应,对药物进行包载形成水分散的纳米粒子系统,具有较好的稳定性,降低药物毒性,并由载体特性具有缓控释能力。白蛋白(BSA)是一种生物安全性良好、可降解的纳米载体材料,已经被美国FDA收录可以用于人体[9]。此外,肿瘤组织对白蛋白具有摄取特性,白蛋白与肿瘤血管内皮细胞表面的Gp60受体结合,激活细胞内小窝蛋白-1,引起细胞膜内陷形成胞转小泡,将白蛋白载体从内皮细胞转运进入肿瘤组织间。同时肿瘤在生长过程中分泌的富含半胱氨酸的酸性蛋白功能类似于白蛋白受体,可吸引粘附白蛋白,促进其从组织间隙转运到肿瘤细胞内,聚集于肿瘤细胞内释放药物[10-11]。
本研究以牛血清白蛋白包载PROTAC药物NPT-B2,通过热驱动法[12]制备白蛋白纳米粒,提高其载药量和包封率,改善了PROTAC药物的水溶性不佳的问题,提高其适用性;并考察载药纳米粒对胶质瘤细胞增殖活性及NAMPT酶的抑制。
-
马尔文粒度电位仪(英国Malvern);透射电子显微镜(美国FEI);高效液相色谱仪(美国Thermo);垂直电泳仪组合套装(美国BIO-RAD);酶标仪(美国Thermo);凝胶成像系统(上海天能)。高糖DMEM培养基(中国源培);胰蛋白酶、青霉素-链霉素、DMSO(美国Sigma);CCK8试剂盒(中国索莱宝);蛋白酶抑制剂PMSF (100 mM)、RIPA裂解液、SDS-PAGE蛋白上样缓冲液、快速电泳液、快速转膜液、快速封闭液、PBS-Tween 20、BeyoColor™ Marker、特超敏ECL化学发光试剂盒、辣根过氧化物酶标记山羊抗兔IgG (H+L)、GAPDH兔多克隆抗体(中国碧云天);NAMPT兔单克隆抗体(英国Abcam);牛血清白蛋白(BSA)、十二烷基硫酸钠(SDS)、L-二硫苏糖醇(DTT)、2-(N-吗啉基)乙磺酸一水合物(MES)(中国源叶);PVDF膜(美国Millipore);NPT-B2由海军军医大学药学系药物化学教研室董国强课题组提供;人胶质瘤细胞U251(中科院上海细胞库)。
-
U251细胞培养。以10% FBS的高糖DMEM培养基培养细胞,当细胞达到80%汇合度时,进行传代培养或冻存。恒温CO2细胞培养箱条件均为37 ℃,5% CO2。
采用CCK8法考察NPT-B2对胶质瘤细胞的毒性作用。取对数生长期的U251细胞接种至96孔板。设置不同浓度含NPT-B2培养基作为实验组,不加药的培养基作为阴性对照组,PBS作为空白对照组(n=3)。孵育72 h后加入CCK8试剂孵育1 h,酶标仪检测吸光度值A,按以下公式计算细胞活力,绘制细胞活力曲线并计算IC50:细胞活力=(A实验组−A空白对照组)/(A阴性对照组−A空白对照组)×100%。
取对数生长期的U251细胞消化成单细胞悬液后铺12孔板。设置含不同浓度NPT-B2的培养基作为实验组,不加药培养基作为对照组(n=3)。孵育72 h后提取细胞蛋白,蛋白印迹法(Western Blot)检测细胞中NAMPT的降解。
Western Blot条件:以10%十二烷基硫酸钠-聚丙烯酰胺凝胶电泳分离蛋白样品。400 mA恒流20 min转移至PVDF膜上,转膜后置于快速封闭液中室温孵育封闭30 min,PBST洗膜后与NAMPT兔单克隆抗体(1∶1000)在4 ℃下孵育过夜,再次洗膜,蛋白条带转移至二抗,室温孵育1 h,曝光。
-
热驱动法制备包载NPT-B2的B2-BSA-NPs。将BSA分散于SDS/DTT溶液中,浓度为40 mg/ml,90 ℃反应2 h即得rBSA。取25 μl rBSA溶液,10 μl NPT-B2(10 μg/μl)加入定量MES(pH4.8)溶液中,37 ℃反应2 h得到B2-BSA-NPs。5000 g离心20 min取上清以去除游离药物。同法制备不加药物的空白白蛋白纳米粒(BSA-NPs)。
-
HPLC色谱条件:色谱柱:Agilent Pursuit XRs C18 USP L1(4.6×250 mm,5 μm);流动相:甲醇:水(81∶19);流速:1.0 ml/min;检测波长:227 nm;柱温:25 ℃;进样量:10 μl。
专属性测定:对照品:NPT-B2溶解于甲醇,过0.22 μm滤膜。B2-BSA-NPs纳米粒中药物提取方法:取1.25 ml B2-BSA-NPs溶液,加入适量SDS/DDT孵育10 min,加入甲醇超声10 min,16000 g离心15 min取上清,过0.22 μm滤膜。BSA-NPs:处理方法同B2-BSA-NPs。将处理后的各组溶液进行HPLC检测。
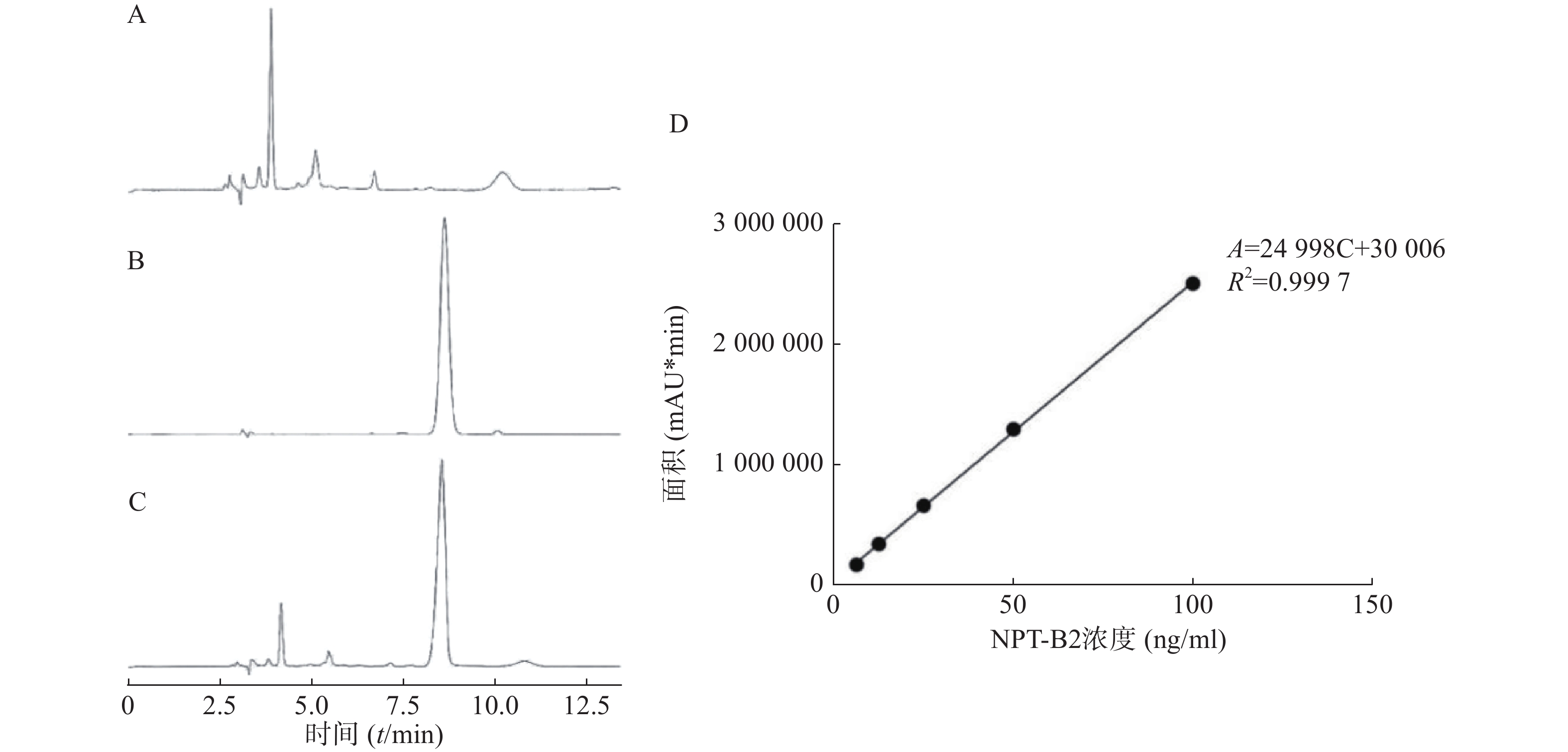
线性范围:精确称取NPT-B2以甲醇定容,配制浓度为100、50、25、12.5、6.25 ng/ml的对照品溶液,过0.22 μm滤膜后HPLC检测,以峰面积A与对照品浓度C进行线性回归制备标准曲线。
精密度测定:取低、中、高三个浓度的对照品溶液,一天内重复进样考察日内精密度,连续三天每天测定一次以考察日间精密度。
回收率测定:取低、中、高三个浓度对照品溶液,加入1.25 ml空白脂质体溶液,以甲醇定容后超声10 min,16000 g离心15 min,取上清进行HPLC检测,计算加样回收率。
-
药载比为1∶5、1∶10、1∶15、1∶20、1∶30时,分别制备B2-BSA-NPs,并测定其粒径、Zeta电位、包封率和载药量。其中包封率的计算公式为:包封率=M实际包载量/M投药量×100%。载药量的计算公式为:载药量=M实际包载量/M载药纳米粒×100%。
-
反应温度和时间分别为37 ℃ 1 h、37 ℃ 2 h、37 ℃ 3 h、70 ℃ 3 min、70 ℃ 5 min时,分别制备B2-BSA-NPs,并测定其粒径、Zeta电位、包封率和载药量。
-
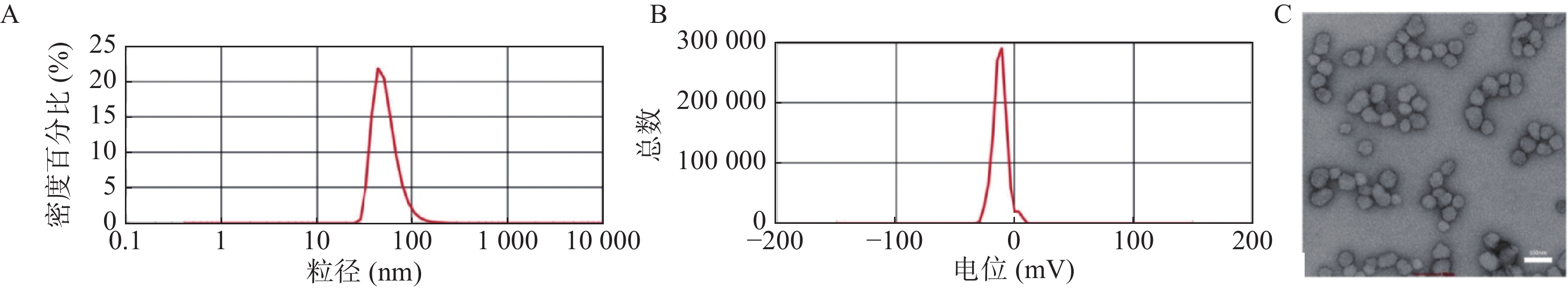
根据处方优化结果,选择反应条件为药载比=1∶10、反应温度37 ℃、反应时间2 h。制备B2-BSA-NPs。马尔文激光粒度仪测定粒径、Zeta电位。经醋酸铀负染后透射电镜观察纳米粒的外观形态,按1.5.1中方法测定其包封率和载药量。
-
采用CCK8法考察B2-BSA-NPs对胶质瘤细胞的增殖活性抑制。取对数生长期的U251细胞接种至96孔板。设置含不同浓度NPT-B2的B2-BSA-NPs所配制的培养基作为实验组,不加药的培养基作为阴性对照组,PBS作为空白对照组(n=3)。孵育72 h后加入CCK8试剂孵育1 h,酶标仪检测吸光度值A,按1.2中公式计算细胞活力,绘制细胞活力曲线并计算IC50。
取对数生长期的U251细胞消化成单细胞悬液后铺12孔板。设置含不同浓度NPT-B2的培养基作为实验组,不加药培养基作为对照组(n=3)。孵育72 h后提取细胞蛋白,按1.2中蛋白印迹法检测细胞NAMPT降解情况。
本研究统计学分析方法均采用GraphPad Prism 8(GraphPad Software, San Diego, CA)计算。两组间数据的比较采用Unpaired Student’s T-test,多组间的数据比较采用One-way ANOVA。P<0.05、P<0.01表示差异具有统计学意义。
-
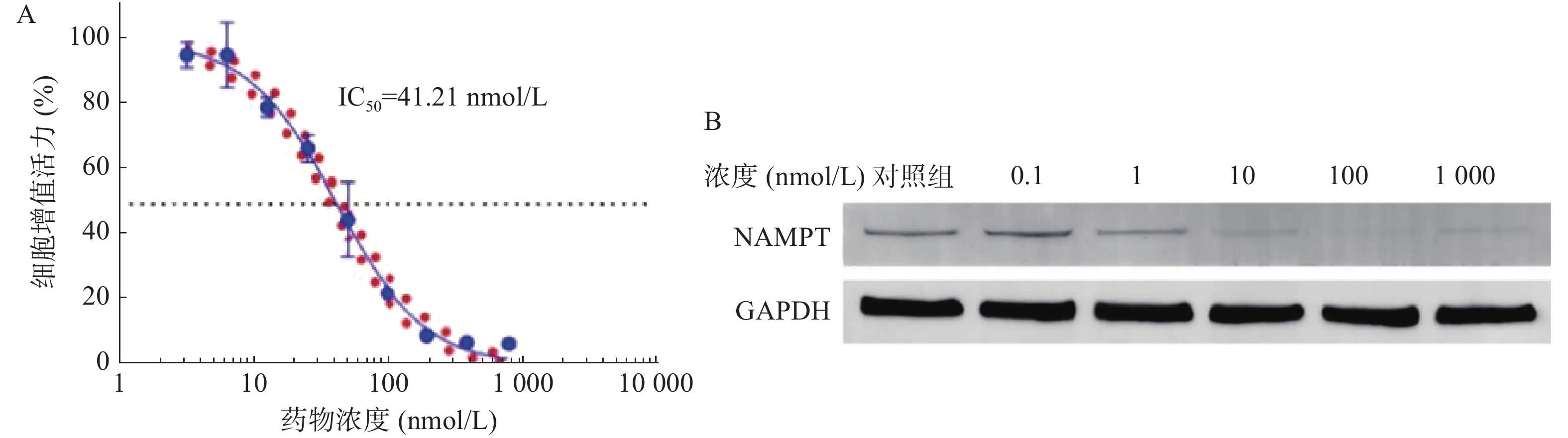
如图1A所示,NPT-B2对U251细胞的IC50值为61.16 nmol/L,表明NPT-B2对其有一定毒性作用。Western Blot结果如图1B所示,当NPT-B2给药浓度为100 nmol/L时,NPT-B2对U251细胞中NAMPT具有显著降解效果。
-
如图2所示,方法专属性良好,NPT-B2保留时间为8.5 min,载体对NPT-B2测定无干扰。6.25~100 ng/ml的浓度范围内,NPT-B2浓度(C, ng/ml)与曲线下面积(Area)呈良好线性关系,线性回归方程为A=24 998 C+30 006,R2=0.9997。
由表1可知,B2-BSA-NPs在低、中、高三个浓度都具有较好的精密度,日间精密度和日内精密度RSD值均小于5%。由表2可知,B2-BSA-NPs在低、中、高三个浓度的回收率在95%~115%范围内,且RSD值均小于5%,表明该方法稳定可靠,可用于样品中NPT-B2含量的测定。
表 1 NPT-B2的HPLC方法精密度(Mean±SD,n=3)
加样量
(ng/ml)日内精密度 日间精密度 测得量
(ng/ml)RSD
(%)测得量
(ng/ml)RSD
(%)12.5 11.78±0.04 0.34 11.78±0.02 0.20 50 50.47±0.14 0.28 51.29±0.70 1.36 80 82.18±0.11 0.14 82.91±1.00 1.22 表 2 NPT-B2的HPLC方法提取回收率(Mean±SD,n=3)
加样量
(ng/ml)测得量
(ng/ml)平均回收率
(%)RSD
(%)25 25.97±0.45 103.89±1.81 1.74 50 56.77±0.08 113.54±0.17 0.15 80 89.06±0.24 111.33±0.30 0.27 -
结果如表3所示,随着载体比例的降低,B2-BSA-NPs粒径逐渐增大,包封率逐渐减小,载药量先增大后减小。故选择药载比=1∶10作为最佳制备条件。
表 3 不同药载比对B2-BSA-NPs粒径、包封率、载药量的影响(Mean±SD,n=3)
药载比
(w/w)粒径
(nm)包封率
(%)载药量
(%)1∶30 33.18±0.60 99.53±0.75 3.21±0.02 1∶20 55.48±5.29 98.06±1.69 4.67±0.08 1∶15 77.67±1.42 96.75±1.31 6.05±0.08 1∶10 86.86±1.50 94.41±1.28 8.58±0.12 1∶5 109.40±0.71 29.13±1.75 4.86±0.29 -
结果如表4所示,反应温度为37 ℃时,随着反应时间的延长,B2-BSA-NPs的粒径逐渐减小,包封率逐渐增大,载药量逐渐增大;反应温度为70 ℃时,随着反应时间的延长,B2-BSA-NPs的粒径逐渐增大,包封率逐渐增大,载药量逐渐增大。反应温度为37 ℃,反应时长2 h和3 h时,B2-BSA-NPs的粒径、包封率、载药量无显著性差异(P>0.05)。选择温度为37 ℃,反应时长为2 h作为最佳制备条件。
表 4 不同反应温度和时间对B2-BSA-NPs粒径、包封率、载药量的影响(Mean±SD,n=3)
反应温度和时间 粒径
(nm)包封率
(%)载药量
(%)37 ℃, 1 h 86.15±2.84 73.25±1.83 6.66±0.17 37 ℃, 2 h 54.92±1.61 94.74±1.25 8.61±0.11 37 ℃, 3 h 52.15±2.69 96.85±0.57 8.78±0.05 70 ℃, 3 min 54.29±0.68 47.33±2.31 4.30±0.21 70 ℃, 5 min 76.04±1.12 51.52±1.82 5.23±0.17 -
选择最优处方后制备B2-BSA-NPs并对其进行表征。如图3所示,B2-BSA-NPs的粒径、Zeta电位分别为55.48±5.30 nm,−12.9±1.21 mV,透射电镜照片显示B2-BSA-NPs为类圆形粒子,粒径约为50 nm。纳米粒包封率为94.74±1.25%,载药量为8.61±0.11%。
-
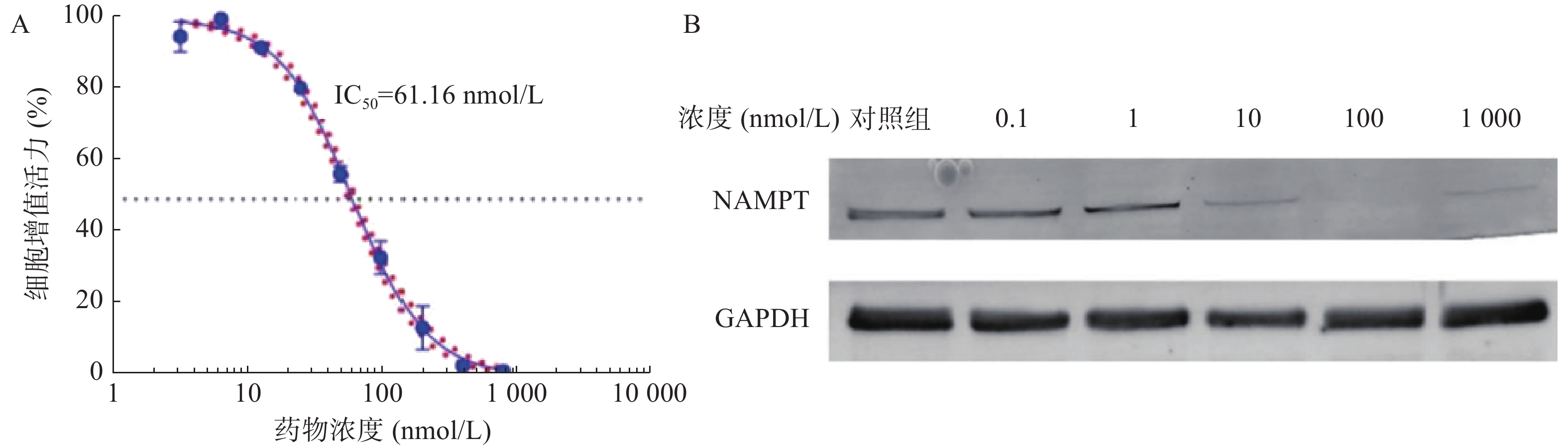
如图4A所示,B2-BSA-NPs对U251细胞的IC50值为41.21 nmol/L,显著优于NPT-B2,表明B2-BSA-NPs对U251细胞有更强的毒性作用。如图4B所示,当B2-BSA-NPs给药浓度为100 nmol/L时,对U251中NAMPT具有非常显著降解效果。
-
多形性胶质母细胞瘤是恶性程度最高的脑肿瘤之一,具有强浸润生长及术后高复发的特点,因此术后的放化疗策略尤为重要,而首选药物替莫唑胺对GBM产生广泛的耐药性,有效率约40%,因此改善GBM化疗效果的新靶点和新方法的研究具有重要意义。肿瘤具有快速增殖和更高的能量代谢特征,干预NAD+代谢是癌症精准治疗的重要靶点,NAD+生物合成的限速酶-烟酰胺磷酸核糖转移酶(NAMPT)的抑制是干扰NAD+能量代谢的关键步骤,是有效的抗肿瘤策略。目前进入临床Ⅱ期试验的NAMPT抑制剂有FK866、GMX1778、GMX1777等,但具有细胞靶向毒性如血小板减少、视网膜和心脏损害等缺点[13]。具有高度选择性和效能的靶向蛋白降解技术得到关注,PROTAC分子由靶蛋白结合配体、连接链和E3泛素连接酶配体三部分组成,能特异性结合靶蛋白,同时招募E3泛素连接酶使POI多聚泛素化,最终通过蛋白酶体系统降解。本研究针对新型合成的PROTAC分子NPT-B2,考察其抑制胶质瘤细胞增殖活性以及肿瘤细胞中NAMPT的降解特性。为了改善药物水溶性差的问题,结合白蛋白在肿瘤细胞中特异性的摄取机制,以牛血清白蛋白包载NPT-B2制备纳米粒。制备白蛋白纳米粒的方法有溶解挥发、喷雾干燥、乳化、Nab-技术、pH凝聚等[14]。本研究通过还原BSA将分子内二硫键打开,形成分子间的二硫键网络,可将疏水药物或者分子量较大的药物通过疏水作用及氢键作用稳定在BSA结构中,优化处方提高载药量和包封率。载药纳米粒粒径约55.48 nm,较小的粒径是纳米粒在肿瘤组织中深层渗透的主要影响因素之一,同时纳米载体改善了PROTAC药物的水溶性差、适用性差的缺陷,本研究可作为后续载药纳米粒用于胶质瘤治疗研究的基础。
Construction of albumin nanoparticles loading PROTAC and its inhibition effect on NAD+ in glioma cells
-
摘要:
目的 制备载蛋白降解靶向嵌合体(PROTAC)分子的白蛋白纳米粒并优化处方,考察纳米粒对胶质瘤细胞增殖活性及烟酰胺腺嘌呤二核苷酸(NAD+)代谢的抑制。 方法 以热驱动法制备载NPT-B2的白蛋白纳米粒并表征,建立NPT-B2的高效液相色谱(HPLC)检测方法,以CCK8法和蛋白免疫印迹法分别考察NPT-B2和白蛋白纳米粒(B2-BSA-NPs)对胶质瘤细胞的增值活性抑制作用,及肿瘤细胞限速酶-烟酰胺磷酸核糖转移酶(NAMPT)的降解。 结果 HPLC方法线性良好,精密度、回收率符合测定要求。纳米粒粒径为55.48 nm、电位−12.9 mV,包封率94.74%,载药量8.61%。NPT-B2对胶质瘤细胞的IC50为61.16 nmol/L,同时具有NAMPT降解作用。B2-BSA-NPs对胶质瘤细胞的IC50为41.21 nmol/L,对NAMPT具有非常显著降解效果。 结论 构建了载PROTAC分子的白蛋白纳米粒,优化处方提高药物包封率,改善PROTAC分子水溶性差的问题,纳米粒对胶质瘤细胞增殖活性及NAD+能量代谢有显著抑制作用。 Abstract:Objective To prepare and optimize the formulation of Albumin nanoparticles loading PROTAC molecule and observe the inhibition effect of nanoparticles on the proliferation and NAD+ metabolism of glioma cells. Methods Albumin nanoparticles loading NPT-B2 were prepared and characterized with a thermal driving method, and the prescription was optimized. An HPLC method was established to determine the content of NPT-B2. The proliferation inhibition of NPT-B2 and B2-BSA-NPs on U251 cells were investigated by the CCK8 method, and the degradation effects of NPT-B2 and B2-BSA-NPs on NAMPT in glioma cells were investigated by western blotting. Results The HPLC method was stable, with good linearity, precision, and recovery rate. The nanoparticles had a particle size of about 55.48 nm, a potential of about −12.9 mV, an encapsulation rate of about 94.74%, and a drug loading amount of about 8.61%. The IC50 of NPT-B2 on glioma cells was 61.16 nmol/L, which had a degradation effect on NAMPT. The IC50 of B2-BSA-NPs on glioma was 41.21 nmol/L, which had a very significant degradation effect on NAMPT. Conclusion Albumin nanoparticles loading PROTAC molecules were constructed. The prescription was optimized to improve the drug encapsulation rate, and the low water solubility of PROTAC molecule was improved, which had a significant inhibitory effect on the proliferation and NAD+ energy metabolism of glioma cells. -
Key words:
- Glioma /
- Nanoparticle /
- Albumin /
- PROTAC /
- HPLC
-
表 1 NPT-B2的HPLC方法精密度(Mean±SD,n=3)
加样量
(ng/ml)日内精密度 日间精密度 测得量
(ng/ml)RSD
(%)测得量
(ng/ml)RSD
(%)12.5 11.78±0.04 0.34 11.78±0.02 0.20 50 50.47±0.14 0.28 51.29±0.70 1.36 80 82.18±0.11 0.14 82.91±1.00 1.22 表 2 NPT-B2的HPLC方法提取回收率(Mean±SD,n=3)
加样量
(ng/ml)测得量
(ng/ml)平均回收率
(%)RSD
(%)25 25.97±0.45 103.89±1.81 1.74 50 56.77±0.08 113.54±0.17 0.15 80 89.06±0.24 111.33±0.30 0.27 表 3 不同药载比对B2-BSA-NPs粒径、包封率、载药量的影响(Mean±SD,n=3)
药载比
(w/w)粒径
(nm)包封率
(%)载药量
(%)1∶30 33.18±0.60 99.53±0.75 3.21±0.02 1∶20 55.48±5.29 98.06±1.69 4.67±0.08 1∶15 77.67±1.42 96.75±1.31 6.05±0.08 1∶10 86.86±1.50 94.41±1.28 8.58±0.12 1∶5 109.40±0.71 29.13±1.75 4.86±0.29 表 4 不同反应温度和时间对B2-BSA-NPs粒径、包封率、载药量的影响(Mean±SD,n=3)
反应温度和时间 粒径
(nm)包封率
(%)载药量
(%)37 ℃, 1 h 86.15±2.84 73.25±1.83 6.66±0.17 37 ℃, 2 h 54.92±1.61 94.74±1.25 8.61±0.11 37 ℃, 3 h 52.15±2.69 96.85±0.57 8.78±0.05 70 ℃, 3 min 54.29±0.68 47.33±2.31 4.30±0.21 70 ℃, 5 min 76.04±1.12 51.52±1.82 5.23±0.17 -
[1] CUDDAPAH V A, ROBEL S, WATKINS S, et al. A neurocentric perspective on glioma invasion[J]. Nat Rev Neurosci, 2014, 15(7):455-465. doi: 10.1038/nrn3765 [2] GRILL J, OWENS C. Central nervous system tumors[J]. Handb Clin Neurol, 2013, 112:931-958. [3] PRELAJ A, REBUZZI S E, CAFFARENA G, et al. Therapeutic approach in glioblastoma multiforme with primitive neuroectodermal tumor components: case report and review of the literature[J]. Oncol Lett, 2018, 15(5):6641-6647. [4] CHOWDHRY S, ZANCA C, RAJKUMAR U, et al. NAD metabolic dependency in cancer is shaped by gene amplification and enhancer remodelling[J]. Nature, 2019, 569(7757):570-575. doi: 10.1038/s41586-019-1150-2 [5] SCHOLZ N, KURIAN K M, SIEBZEHNRUBL F A, et al. Targeting the ubiquitin system in glioblastoma[J]. Front Oncol, 2020, 10:574011. doi: 10.3389/fonc.2020.574011 [6] GUJAR A D, LE S, MAO D D, et al. An NAD+-dependent transcriptional program governs self-renewal and radiation resistance in glioblastoma[J]. Proc Natl Acad Sci U S A, 2016, 113(51):E8247-E8256. [7] ZENG S X, HUANGW H, ZHENG X L, et al. Proteolysis targeting chimera (PROTAC) in drug discovery paradigm: Recent progress and future challenges[J]. Eur J Med Chem, 2021, 210:112981. doi: 10.1016/j.ejmech.2020.112981 [8] BRICELJ A, STEINEBACH C, KUCHTA R, et al. E3 ligase ligands in successful PROTACs: an overview of syntheses and linker attachment points[J]. Front Chem, 2021, 9:707317. doi: 10.3389/fchem.2021.707317 [9] SILVA N I O, SALVADOR E A, FRANCOI R, et al. Bovine serum albumin nanoparticles induce histopathological changes and inflammatory cell recruitment in the skin of treated mice[J]. Biomed Pharmacother, 2018, 107:1311-1317. doi: 10.1016/j.biopha.2018.08.106 [10] HOANG B, ERNSTING M J, ROY A, et al. Docetaxel-carboxymethylcellulose nanoparticles target cells via a SPARC and albumin dependent mechanism[J]. Biomaterials, 2015, 59:66-76. doi: 10.1016/j.biomaterials.2015.04.032 [11] MOCAN L, MATEA C, TABARAN F A, et al. Photothermal treatment of liver cancer with albumin-conjugated gold nanoparticles initiates Golgi Apparatus-ER dysfunction and caspase-3 apoptotic pathway activation by selective targeting of Gp60 receptor[J]. Int J Nanomedicine, 2015, 10:5435-5445. [12] LIU Y C, HAN Y, FANG T, et al. Turning weakness into strength: albumin nanoparticle-redirected amphotericin B biodistribution for reducing nephrotoxicity and enhancing antifungal activity[J]. J Control Release, 2020, 324:657-668. doi: 10.1016/j.jconrel.2020.05.026 [13] GALLI U, COLOMBO G, TRAVELLI C, et al. Recent advances in NAMPT inhibitors: a novel immunotherapic strategy[J]. Front Pharmacol, 2020, 11:656. doi: 10.3389/fphar.2020.00656 [14] MENG R, ZHU H M, WANG Z W, et al. Preparation of drug-loaded albumin nanoparticles and its application in cancer therapy[J]. J Nanomater, 2022, 2022:1-12. -





 下载:
下载: