-
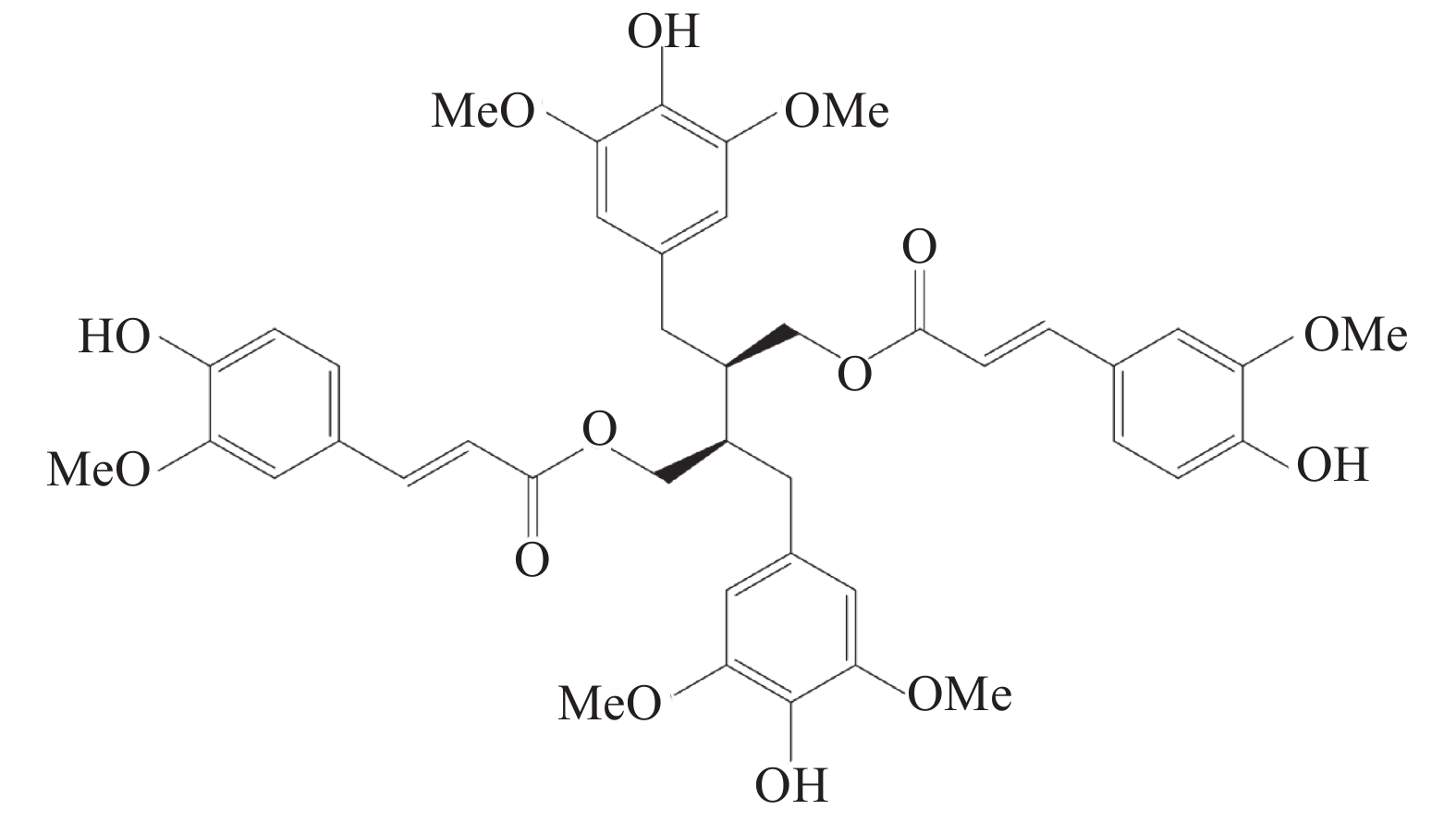
炎症泛指机体受到创伤或病原体感染等刺激后所产生的生理反应,主要表现为疼痛、红肿、发热等[1]。通常情况下,机体受到创伤等刺激后,所产生的炎症反应是一种生理性保护反应,有益于机体损伤的修复[2-3],但当机体内的抗炎系统过于活跃时,会引起自身免疫系统紊乱,对机体正常的细胞及组织进行攻击,如系统性红斑狼疮及类风湿性关节炎等免疫疾病[4]。目前,临床上常用的抗炎药物主要分为甾体类抗炎药(SAIDs)、非甾体类抗炎药(NSAIDs)及免疫调节药,但这几类药物多具有副作用[5-7],因此,新型抗炎药物的研发具有极高的科研前景和社会价值。豆豉姜是樟科植物山鸡椒Litsea cubeba(Lour.)Pers的根及根茎,被傣医学及中医作为广泛的民间方剂,多用于祛风散寒、息肝风、消肿、治风湿痹痛的治疗。前期课题组提取分离得到新型的二苄基丁烷型木脂素9,9’-O-二-(E)-阿魏酰基-内消旋-5,5’-二甲氧基开环异落叶松树脂酚(LCA)化学结构如图1所示,并发现其具有改善类弗氏完全佐剂大鼠关节炎模型中炎性症状的疗效[8],因此,课题组推测LCA是豆豉姜中具有抗炎活性的天然化合物,故采用小鼠耳肿胀和棉球肉芽肿模型及RAW264.7细胞实验,对其抗炎作用进行研究。
-
二甲苯致小鼠耳廓肿胀实验模型:5周龄SPF级雄性昆明小鼠,体重:(20±2)g;大鼠棉球肉芽肿实验:4周龄SPF级雄性SD大鼠,体重:(120±10)g。动物由海军军医大学实验动物中心提供,并饲养于海军军医大学药学院动物房,室温控制为24 ℃,12 h光照+12 h黑暗,水及食物充足供应。RAW264.7(海军军医大学药学院生药学教研室)。
-
LCA(海军军医大学药学系有机化学教研室合成及鉴定);吲哚美辛(海军军医大学附属长海医院);二甲苯、水合氯醛、羧甲基纤维素钠CMC-Na、对氨基苯磺酸、磷酸、N-α-萘乙二胺(中国医药集团);棉球(中国朝阳公司);ELISA试剂盒(中国联科公司);iNOS、COX-2、GAPDH抗体及二抗(CST,USA)。分析天平(MettlerToledo,USA);CO2恒温细胞培养箱(Sanyo,Japan);ELx 800酶标仪(Fisher,US);凝胶成像仪(Tanon-5200 multi,中国上海)。
-
小鼠耳廓肿胀模型建立:将60只5周龄健康雄性昆明鼠安置于动物实验中心适应1周,按体重随机分为4组,各10只,组别标记为:模型组;LCA组(20、40 mg/kg);吲哚美辛组(8 mg/kg)。给药以灌胃的形式每日一次,模型组则予相同体积的0.5% CMC-Na连续3 d灌胃,在末次给药1 h后,将二甲苯以0.05 ml/只剂量均匀涂布于小鼠右耳廓的双面,左耳不做处理,连续刺激45 min后处死小鼠,用打孔器分别于左右耳廓同一位置打下圆形耳片[9],称重,用于计算比较耳肿胀程度及耳肿胀抑制率(%)。
大鼠棉球肉芽肿模型建立:将20只4周龄健康雄性SD大鼠安置于动物实验中心适应1周,按体重随机分为4组:模型组;LCA组(20、40 mg/kg);吲哚美辛组(5 mg/kg)。用水合氯醛对大鼠进行麻醉,于左右两边腋窝下切开一小口,将2个灭菌棉球(20 mg)分别植入小鼠左右腋窝,缝合后第2天给药。且以灌胃的形式每日一次,模型组则予相同体积的0.5% CMC-Na连续7 d[10-11]。第8天处死大鼠,将腋下棉球及附着的肉芽组织剥离,称重,用于计算比较干湿重及抑制率。
-
耳肿胀度=右耳片重量-左耳片重量
耳肿胀抑制率(%)=(模型组平均耳肿胀度-给药组平均耳肿胀度)/模型组平均耳肿胀度×100%
平均棉球肉芽肿重量=(右侧棉球肉芽肿重量+左侧棉球肉芽肿重量)/2
棉球肉芽肿抑制率(%)=(模型组平均棉球肉芽肿重量-给药组平均棉球肉芽肿重量)/模型组平均棉球肉芽肿重量×100%
-
RAW264.7(小鼠单核巨噬细胞)用DMEM高糖完全培养基(10% DMEM+90% FBS+1%双抗)培养于37 ℃恒温细胞培养箱中。
-
将RAW264.7细胞以1×103个/孔接种于96孔板,完全培养基培养24 h,弃上清液,给予不同浓度的LCA培养液(3、1.5、0.75和0.375 μmol/L),并设置空白对照组,每组5个复孔,培养24 h,采用MTT法测定LCA对小鼠单核巨噬细胞的增殖作用。
-
将RAW264.7细胞以1×106个/孔接种于24孔板,24 h后弃上清液,脂多糖(LPS)组和LCA组中加入1 ml含2 μg/ml LPS的完全培养基,刺激2 h,于空白组和LPS组中加入1 ml完全培养基,给药组加入各浓度LCA(1.5、0.75和0.375 μmol/L)的完全培养基,作用24 h后,测NO含量:取100 μl上清液与100 μl Griess试剂(A液:对氨基苯磺酸0.1 g,加5%磷酸10ml;B液:N-α-萘乙二胺0.01 g,加10ml蒸馏水,等体积混合)混合避光显色30 min,用酶标仪于540 nm处测各孔吸光度(A)值;测TNF-α:取上清液,根据ELISA试剂盒说明书步骤测量上清液中炎症因子TNF-α的含量。
-
将RAW264.7细胞以1×106个/孔接种于6孔板,细胞贴壁后,处理组(LPS组、LCA组)中加入1ml含2 μg/ml LPS的完全培养基,刺激2 h,于空白组和LPS组中加入1 ml完全培养基,给药组加入各浓度LCA(1.5、0.75和0.375 μmol/L)的完全培养基,作用24 h后,细胞裂解提取蛋白并定量,蛋白变性后SDS-PAGE凝胶电泳分离后,转膜,封闭,依次用iNOS,COX-2及GAPDH于4 ℃孵育过夜,用洗膜液(TBST)清洗3次后,二抗室温孵育1 h,TBST洗涤后,用化学发光试剂盒于凝胶成像仪中显影拍片。
-
本实验指标均为定量数据,数据以(
$\bar{x}\pm s$ )形式表示。多组定量数据的比较,采用单因素方差分析,方差分析检验后两两比较采用Dunnett's t-test法。采用GraphPad Prism 5统计软件进行统计学分析。结果以P<0.05、P<0.01表示差异具有统计学意义。 -
如表1所示,与模型组对比,吲哚美辛(8 mg/kg)可抑制由二甲苯造成的小鼠耳廓肿胀(P<0.05),抑制率为29.80%;LCA(40 mg/kg)组显著抑制小鼠耳廓肿胀度(P<0.01),抑制率为37.41%,而LCA(20 mg/kg)组对小鼠耳廓肿胀度的抑制无统计学意义(P>0.05)。
表 1 LCA对二甲苯致小鼠耳廓肿胀的影响(n=10,
$\bar{ x}\pm {{s}}$ )组别 肿胀度(m/mg) 抑制率(%) 模型组 3.02±0.63 — LCA组(20 mg/kg) 2.52±0.29 16.56 LCA组(40 mg/kg) 1.89±0.54** 37.41 吲哚美辛组(8 mg/kg) 2.12±0.61* 29.80 *P<0.05,**P<0.01,与模型组比较。 -
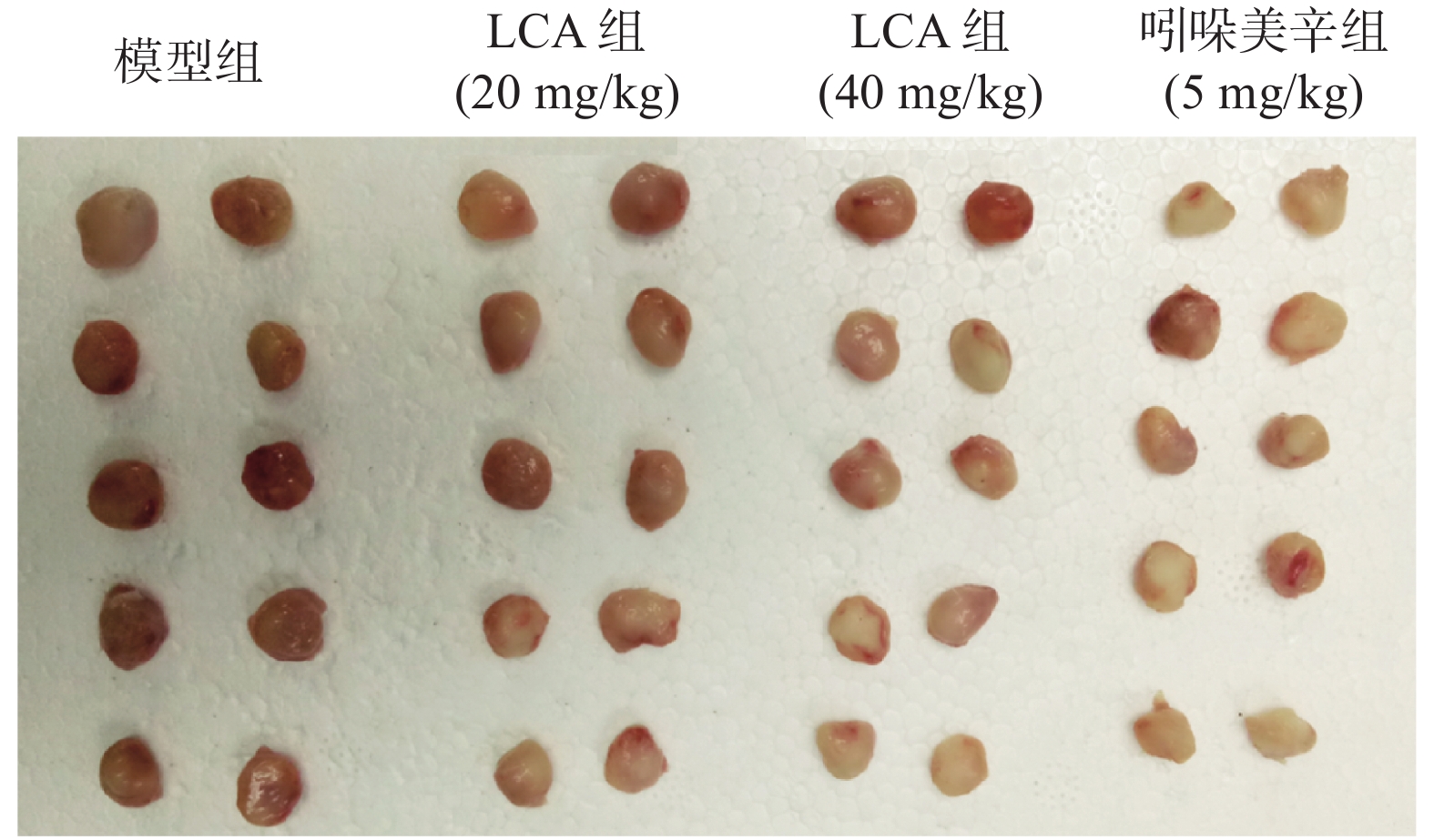
如图2,模型组大鼠棉球肉芽肿体积较大且颜色鲜红,吲哚美辛组的棉球肉芽肿体积小且颜色浅,而LCA组的棉球肉芽肿在色泽和大小上均有别于模型组。数据分析显示(表2、表3),与模型组相比,阳性药组吲哚美辛明显抑制大鼠的棉球肉芽肿(P<0.001),且干重、湿重的抑制率分别为48.73%和44.19%;LCA高剂量组也可明显减少棉球肉芽肿的干重及湿重(P<0.01),且抑制率达到23.89%和29.90%。
表 2 LCA对大鼠棉球肉芽肿湿重的影响(n=5,
$\bar{ x}\pm {{s}}$ )组别 棉球肉芽肿湿重(m/mg) 抑制率(%) 模型组 300.1±53.26 — LCA组(20 mg/kg) 256.0±36.36 14.70 LCA组(40 mg/kg) 228.4±51.16*** 23.89 吲哚美辛组(5 mg/kg) 167.5±15.74*** 44.19 ***P<0.001,与模型组比较。 表 3 LCA对大鼠棉球肉芽肿干重的影响(n=5,
$\bar{ x}\pm {{s}}$ )组别 棉球肉芽肿干重(m/mg) 抑制率(%) 模型组 48.1±10.05 — LCA组(20 mg/kg) 42.32±10.19 12.05 LCA组(40 mg/kg) 33.73±7.10** 29.90 吲哚美辛组(5 mg/kg) 24.67±7.39*** 48.73 **P<0.01,***P<0.001,与模型组比较。 -
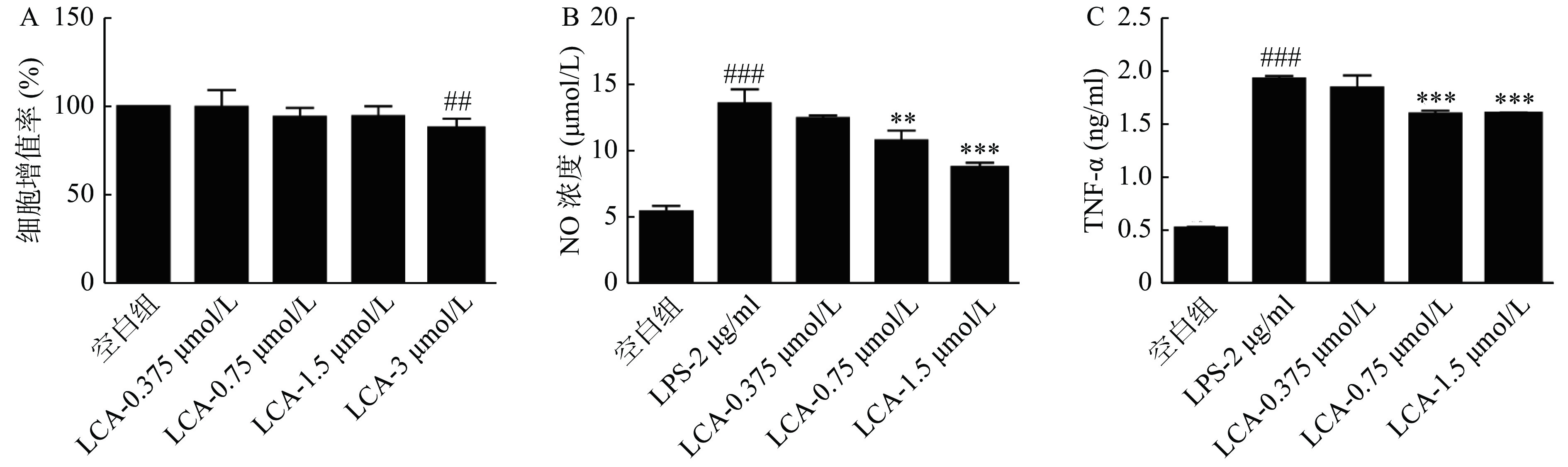
如图3所示,LCA的安全剂量不高于3 μmol/ml,且在0.75和1.5 μmol/ml浓度下显著降低LPS诱导的RAW264.7细胞分泌的NO和TNF-α浓度,差异均具有统计学意义(P<0.01)。
-
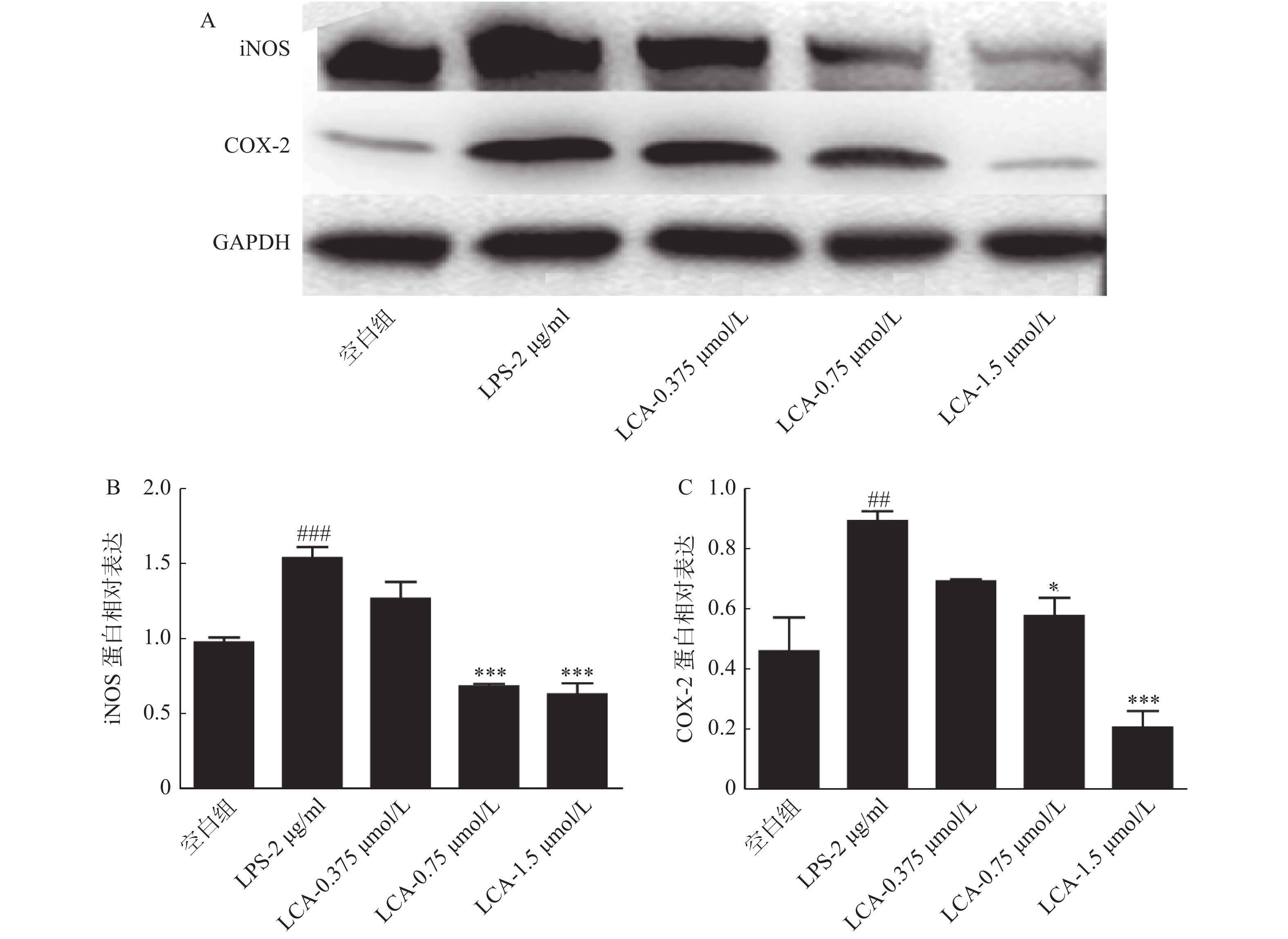
如图4所示,LCA在1.5 μmol/ml浓度下显著抑制了炎症相关蛋白iNOS、COX-2蛋白的表达(P<0.001)。
-
据报道[12],许多从中药中提取的活性天然化合物可以达到抗炎效果,包括黄芩苷、青藤碱和大黄素等,多为甘草次酸酰胺类衍生物。LCA不同于这些抗炎药物,它具有新颖的二苄基丁烷型结构,本研究从体内、体外两方面对它进行较为系统的抗炎药效评价。
二甲苯诱导的小鼠耳肿胀模型是评价药物体内抗炎活性的急性炎症模型之一[13],本实验模型组中二甲苯作为致炎剂刺激小鼠耳廓皮肤,促进血管舒张、增加血管通透性致使炎性物质渗出,耳片重量明显高于未经处理的耳片重量,LCA组能明显改善小鼠耳部的炎性渗出及肿胀程度,且高浓度组的抑制率高于阳性药吲哚美辛组(由于吲哚美辛高浓度有强烈的胃肠道反应,故根据临床用药剂量换算出实验动物的剂量,并参考相关文献确定用药量[13],即8 mg/kg),表明LCA对急性炎症有显著作用。在慢性炎症期,由于巨噬细胞和纤维母细胞等增生而形成肉芽组织,棉球肉芽肿实验可模拟炎症病理过程中炎性细胞浸润和肉芽组织的增生[14]。结果显示LCA降低棉球肉芽肿的湿重与干重,表明LCA可以控制慢性炎症过程中炎性渗出物的转移,同时抑制棉球外侧小血管和结缔组织的增生,证明LCA可以减少慢性炎症进程中肉芽组织的形成。结合上述两个动物实验可知LCA对急性炎症以及慢性炎症都具有一定的抑制作用。
炎症的普遍特征是病灶区域有大量炎症细胞的浸润,并释放大量炎症因子和介质,如IL-1、IL-6、TNF-α、前列腺素(PG)、组织胺和NO等。其中,NO可激活NF-κB信号通路,诱导促炎因子(IL-6、TNF-α等)的分泌,引起机体组织损伤以及血管扩张;TNF-α可刺激细胞免疫中T细胞产生炎症因子,进而诱导炎症的加重和扩大。iNOS可介导产生的大量NO并参与炎症反应;COX-2介导产生的前列腺素与慢性炎症的发展和持续有关,致使白细胞聚集和浸润,引起微血管内皮细胞的损伤。在本研究中,LPS刺激后,RAW264.7细胞分泌的NO和TNF-α含量,以及细胞内iNOS和COX-2蛋白水平明显增高,LCA浓度为1.5 μmol/ml时显著抑制激活状态下NO和TNF-α炎症介质的分泌,同时下调iNOS及COX-2蛋白的表达水平。由此进一步证实LCA具有抗炎作用。然而,LCA抗炎的具体作用机制尚不明确,有待今后进行更深层次的研究探讨。
豆豉姜活性成分9,9’-O-二-(E)-阿魏酰基-内消旋-5,5’-二甲氧基开环异落叶松树脂酚(LCA)抗炎作用研究
doi: 10.12206/j.issn.1006-0111.201912159
Anti-inflammatory study on 9,9'-O-di-(E)-feruloyl-meso-5,5'-dimethoxysecoisolariciresinol (LCA), an active ingredient in Litsea cubeba (Lour.) Pers
-
摘要:
目的 对傣药豆豉姜活性成分LCA在动物及细胞水平上的抗炎作用进行评价。 方法 采用耳肿胀和棉球肉芽肿模型进行LCA抗炎的体内药效学评价。采用脂多糖(LPS)诱导的小鼠单核巨噬细胞(RAW264.7),通过检测其一氧化氮(NO)、肿瘤坏死因子-α(TNF-α)的分泌量以及诱导型一氧化氮合酶(iNOS)、环氧合酶-2(COX-2)蛋白的表达水平,以评价LCA的体外抗炎活性。 结果 在小鼠耳肿胀的实验中,LCA组的耳肿胀度明显低于模型组,且高浓度组对小鼠耳肿胀的抑制率高于阳性药组;在大鼠棉球肉芽肿实验中,LCA高剂量组显著减轻了棉球肉芽肿的湿重与干重。此外,LCA显著减少LPS诱导的RAW264.7细胞中NO、TNF-α的分泌,降低iNOS、COX-2蛋白的表达。 结论 LCA具有显著的体内外抗炎作用,值得进一步深入研究。 -
关键词:
- LCA /
- 抗炎 /
- 耳肿胀 /
- 肉芽肿 /
- RAW264.7细胞
Abstract:Objective To evaluate the anti-inflammatory effects of LCA, which is the active component from Litsea cubeba (Lour.) Pers. Methods Pharmacodynamic evaluations of ear swelling and cotton ball granuloma models were used in animal experiments. In vitro experiment, lipopolysaccharide (LPS) stimulated monocyte macrophage RAW264.7 cells were used to evaluate the anti-inflammatory activity of LCA by detecting the secretions of nitric oxide (NO), tumor necrosis factor-α (TNF-α), the expressions of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) protein. Results In ear swelling experiment, the extent of ear swelling was significantly lower in the LCA treated group than the model group. The inhibition rate was greater in the high LCA concentration group than the positive drug group. In addition, LCA reduced the weight of cotton ball granuloma markedly. At the cell level, LCA significantly inhibited the secretions of NO, TNF-α and reduced the expressions of iNOS and COX-2 in LPS-stimulated RAW 264.7 cells. Conclusion LCA has significant anti-inflammatory effects in vitro and in vivo. The further studies are warranted for the development of anti-inflammatory drugs. -
Key words:
- LCA /
- anti-inflammatory /
- ear swelling /
- granuloma of cotton ball /
- RAW264.7 cell
-
炎症泛指机体受到创伤或病原体感染等刺激后所产生的生理反应,主要表现为疼痛、红肿、发热等[1]。通常情况下,机体受到创伤等刺激后,所产生的炎症反应是一种生理性保护反应,有益于机体损伤的修复[2-3],但当机体内的抗炎系统过于活跃时,会引起自身免疫系统紊乱,对机体正常的细胞及组织进行攻击,如系统性红斑狼疮及类风湿性关节炎等免疫疾病[4]。目前,临床上常用的抗炎药物主要分为甾体类抗炎药(SAIDs)、非甾体类抗炎药(NSAIDs)及免疫调节药,但这几类药物多具有副作用[5-7],因此,新型抗炎药物的研发具有极高的科研前景和社会价值。豆豉姜是樟科植物山鸡椒Litsea cubeba(Lour.)Pers的根及根茎,被傣医学及中医作为广泛的民间方剂,多用于祛风散寒、息肝风、消肿、治风湿痹痛的治疗。前期课题组提取分离得到新型的二苄基丁烷型木脂素9,9’-O-二-(E)-阿魏酰基-内消旋-5,5’-二甲氧基开环异落叶松树脂酚(LCA)化学结构如图1所示,并发现其具有改善类弗氏完全佐剂大鼠关节炎模型中炎性症状的疗效[8],因此,课题组推测LCA是豆豉姜中具有抗炎活性的天然化合物,故采用小鼠耳肿胀和棉球肉芽肿模型及RAW264.7细胞实验,对其抗炎作用进行研究。
1. 材料
1.1 实验动物
二甲苯致小鼠耳廓肿胀实验模型:5周龄SPF级雄性昆明小鼠,体重:(20±2)g;大鼠棉球肉芽肿实验:4周龄SPF级雄性SD大鼠,体重:(120±10)g。动物由海军军医大学实验动物中心提供,并饲养于海军军医大学药学院动物房,室温控制为24 ℃,12 h光照+12 h黑暗,水及食物充足供应。RAW264.7(海军军医大学药学院生药学教研室)。
1.2 试剂与器材
LCA(海军军医大学药学系有机化学教研室合成及鉴定);吲哚美辛(海军军医大学附属长海医院);二甲苯、水合氯醛、羧甲基纤维素钠CMC-Na、对氨基苯磺酸、磷酸、N-α-萘乙二胺(中国医药集团);棉球(中国朝阳公司);ELISA试剂盒(中国联科公司);iNOS、COX-2、GAPDH抗体及二抗(CST,USA)。分析天平(MettlerToledo,USA);CO2恒温细胞培养箱(Sanyo,Japan);ELx 800酶标仪(Fisher,US);凝胶成像仪(Tanon-5200 multi,中国上海)。
2. 方法
2.1 动物实验
2.1.1 动物模型建立及处理
小鼠耳廓肿胀模型建立:将60只5周龄健康雄性昆明鼠安置于动物实验中心适应1周,按体重随机分为4组,各10只,组别标记为:模型组;LCA组(20、40 mg/kg);吲哚美辛组(8 mg/kg)。给药以灌胃的形式每日一次,模型组则予相同体积的0.5% CMC-Na连续3 d灌胃,在末次给药1 h后,将二甲苯以0.05 ml/只剂量均匀涂布于小鼠右耳廓的双面,左耳不做处理,连续刺激45 min后处死小鼠,用打孔器分别于左右耳廓同一位置打下圆形耳片[9],称重,用于计算比较耳肿胀程度及耳肿胀抑制率(%)。
大鼠棉球肉芽肿模型建立:将20只4周龄健康雄性SD大鼠安置于动物实验中心适应1周,按体重随机分为4组:模型组;LCA组(20、40 mg/kg);吲哚美辛组(5 mg/kg)。用水合氯醛对大鼠进行麻醉,于左右两边腋窝下切开一小口,将2个灭菌棉球(20 mg)分别植入小鼠左右腋窝,缝合后第2天给药。且以灌胃的形式每日一次,模型组则予相同体积的0.5% CMC-Na连续7 d[10-11]。第8天处死大鼠,将腋下棉球及附着的肉芽组织剥离,称重,用于计算比较干湿重及抑制率。
2.1.2 动物指标测定
耳肿胀度=右耳片重量-左耳片重量
耳肿胀抑制率(%)=(模型组平均耳肿胀度-给药组平均耳肿胀度)/模型组平均耳肿胀度×100%
平均棉球肉芽肿重量=(右侧棉球肉芽肿重量+左侧棉球肉芽肿重量)/2
棉球肉芽肿抑制率(%)=(模型组平均棉球肉芽肿重量-给药组平均棉球肉芽肿重量)/模型组平均棉球肉芽肿重量×100%
2.2 RAW264.7细胞实验
2.2.1 细胞培养
RAW264.7(小鼠单核巨噬细胞)用DMEM高糖完全培养基(10% DMEM+90% FBS+1%双抗)培养于37 ℃恒温细胞培养箱中。
2.2.2 细胞增殖检测
将RAW264.7细胞以1×103个/孔接种于96孔板,完全培养基培养24 h,弃上清液,给予不同浓度的LCA培养液(3、1.5、0.75和0.375 μmol/L),并设置空白对照组,每组5个复孔,培养24 h,采用MTT法测定LCA对小鼠单核巨噬细胞的增殖作用。
2.2.3 炎症因子水平测定
将RAW264.7细胞以1×106个/孔接种于24孔板,24 h后弃上清液,脂多糖(LPS)组和LCA组中加入1 ml含2 μg/ml LPS的完全培养基,刺激2 h,于空白组和LPS组中加入1 ml完全培养基,给药组加入各浓度LCA(1.5、0.75和0.375 μmol/L)的完全培养基,作用24 h后,测NO含量:取100 μl上清液与100 μl Griess试剂(A液:对氨基苯磺酸0.1 g,加5%磷酸10ml;B液:N-α-萘乙二胺0.01 g,加10ml蒸馏水,等体积混合)混合避光显色30 min,用酶标仪于540 nm处测各孔吸光度(A)值;测TNF-α:取上清液,根据ELISA试剂盒说明书步骤测量上清液中炎症因子TNF-α的含量。
2.2.4 炎症相关蛋白表达检测
将RAW264.7细胞以1×106个/孔接种于6孔板,细胞贴壁后,处理组(LPS组、LCA组)中加入1ml含2 μg/ml LPS的完全培养基,刺激2 h,于空白组和LPS组中加入1 ml完全培养基,给药组加入各浓度LCA(1.5、0.75和0.375 μmol/L)的完全培养基,作用24 h后,细胞裂解提取蛋白并定量,蛋白变性后SDS-PAGE凝胶电泳分离后,转膜,封闭,依次用iNOS,COX-2及GAPDH于4 ℃孵育过夜,用洗膜液(TBST)清洗3次后,二抗室温孵育1 h,TBST洗涤后,用化学发光试剂盒于凝胶成像仪中显影拍片。
2.3 统计分析
本实验指标均为定量数据,数据以(
$\bar{x}\pm s$ )形式表示。多组定量数据的比较,采用单因素方差分析,方差分析检验后两两比较采用Dunnett's t-test法。采用GraphPad Prism 5统计软件进行统计学分析。结果以P<0.05、P<0.01表示差异具有统计学意义。3. 结果
3.1 LCA对小鼠耳肿胀的影响
如表1所示,与模型组对比,吲哚美辛(8 mg/kg)可抑制由二甲苯造成的小鼠耳廓肿胀(P<0.05),抑制率为29.80%;LCA(40 mg/kg)组显著抑制小鼠耳廓肿胀度(P<0.01),抑制率为37.41%,而LCA(20 mg/kg)组对小鼠耳廓肿胀度的抑制无统计学意义(P>0.05)。
表 1 LCA对二甲苯致小鼠耳廓肿胀的影响(n=10,$\bar{ x}\pm {{s}}$ )组别 肿胀度(m/mg) 抑制率(%) 模型组 3.02±0.63 — LCA组(20 mg/kg) 2.52±0.29 16.56 LCA组(40 mg/kg) 1.89±0.54** 37.41 吲哚美辛组(8 mg/kg) 2.12±0.61* 29.80 *P<0.05,**P<0.01,与模型组比较。 3.2 LCA对大鼠棉球肉芽肿的影响
如图2,模型组大鼠棉球肉芽肿体积较大且颜色鲜红,吲哚美辛组的棉球肉芽肿体积小且颜色浅,而LCA组的棉球肉芽肿在色泽和大小上均有别于模型组。数据分析显示(表2、表3),与模型组相比,阳性药组吲哚美辛明显抑制大鼠的棉球肉芽肿(P<0.001),且干重、湿重的抑制率分别为48.73%和44.19%;LCA高剂量组也可明显减少棉球肉芽肿的干重及湿重(P<0.01),且抑制率达到23.89%和29.90%。
表 2 LCA对大鼠棉球肉芽肿湿重的影响(n=5,$\bar{ x}\pm {{s}}$ )组别 棉球肉芽肿湿重(m/mg) 抑制率(%) 模型组 300.1±53.26 — LCA组(20 mg/kg) 256.0±36.36 14.70 LCA组(40 mg/kg) 228.4±51.16*** 23.89 吲哚美辛组(5 mg/kg) 167.5±15.74*** 44.19 ***P<0.001,与模型组比较。 表 3 LCA对大鼠棉球肉芽肿干重的影响(n=5,$\bar{ x}\pm {{s}}$ )组别 棉球肉芽肿干重(m/mg) 抑制率(%) 模型组 48.1±10.05 — LCA组(20 mg/kg) 42.32±10.19 12.05 LCA组(40 mg/kg) 33.73±7.10** 29.90 吲哚美辛组(5 mg/kg) 24.67±7.39*** 48.73 **P<0.01,***P<0.001,与模型组比较。 3.3 LCA对LPS刺激RAW264.7细胞炎症因子分泌的影响
如图3所示,LCA的安全剂量不高于3 μmol/ml,且在0.75和1.5 μmol/ml浓度下显著降低LPS诱导的RAW264.7细胞分泌的NO和TNF-α浓度,差异均具有统计学意义(P<0.01)。
3.4 LCA对炎症相关的蛋白表达的影响
如图4所示,LCA在1.5 μmol/ml浓度下显著抑制了炎症相关蛋白iNOS、COX-2蛋白的表达(P<0.001)。
4. 讨论
据报道[12],许多从中药中提取的活性天然化合物可以达到抗炎效果,包括黄芩苷、青藤碱和大黄素等,多为甘草次酸酰胺类衍生物。LCA不同于这些抗炎药物,它具有新颖的二苄基丁烷型结构,本研究从体内、体外两方面对它进行较为系统的抗炎药效评价。
二甲苯诱导的小鼠耳肿胀模型是评价药物体内抗炎活性的急性炎症模型之一[13],本实验模型组中二甲苯作为致炎剂刺激小鼠耳廓皮肤,促进血管舒张、增加血管通透性致使炎性物质渗出,耳片重量明显高于未经处理的耳片重量,LCA组能明显改善小鼠耳部的炎性渗出及肿胀程度,且高浓度组的抑制率高于阳性药吲哚美辛组(由于吲哚美辛高浓度有强烈的胃肠道反应,故根据临床用药剂量换算出实验动物的剂量,并参考相关文献确定用药量[13],即8 mg/kg),表明LCA对急性炎症有显著作用。在慢性炎症期,由于巨噬细胞和纤维母细胞等增生而形成肉芽组织,棉球肉芽肿实验可模拟炎症病理过程中炎性细胞浸润和肉芽组织的增生[14]。结果显示LCA降低棉球肉芽肿的湿重与干重,表明LCA可以控制慢性炎症过程中炎性渗出物的转移,同时抑制棉球外侧小血管和结缔组织的增生,证明LCA可以减少慢性炎症进程中肉芽组织的形成。结合上述两个动物实验可知LCA对急性炎症以及慢性炎症都具有一定的抑制作用。
炎症的普遍特征是病灶区域有大量炎症细胞的浸润,并释放大量炎症因子和介质,如IL-1、IL-6、TNF-α、前列腺素(PG)、组织胺和NO等。其中,NO可激活NF-κB信号通路,诱导促炎因子(IL-6、TNF-α等)的分泌,引起机体组织损伤以及血管扩张;TNF-α可刺激细胞免疫中T细胞产生炎症因子,进而诱导炎症的加重和扩大。iNOS可介导产生的大量NO并参与炎症反应;COX-2介导产生的前列腺素与慢性炎症的发展和持续有关,致使白细胞聚集和浸润,引起微血管内皮细胞的损伤。在本研究中,LPS刺激后,RAW264.7细胞分泌的NO和TNF-α含量,以及细胞内iNOS和COX-2蛋白水平明显增高,LCA浓度为1.5 μmol/ml时显著抑制激活状态下NO和TNF-α炎症介质的分泌,同时下调iNOS及COX-2蛋白的表达水平。由此进一步证实LCA具有抗炎作用。然而,LCA抗炎的具体作用机制尚不明确,有待今后进行更深层次的研究探讨。
-
表 1 LCA对二甲苯致小鼠耳廓肿胀的影响(n=10,
$\bar{ x}\pm {{s}}$ )组别 肿胀度(m/mg) 抑制率(%) 模型组 3.02±0.63 — LCA组(20 mg/kg) 2.52±0.29 16.56 LCA组(40 mg/kg) 1.89±0.54** 37.41 吲哚美辛组(8 mg/kg) 2.12±0.61* 29.80 *P<0.05,**P<0.01,与模型组比较。 表 2 LCA对大鼠棉球肉芽肿湿重的影响(n=5,
$\bar{ x}\pm {{s}}$ )组别 棉球肉芽肿湿重(m/mg) 抑制率(%) 模型组 300.1±53.26 — LCA组(20 mg/kg) 256.0±36.36 14.70 LCA组(40 mg/kg) 228.4±51.16*** 23.89 吲哚美辛组(5 mg/kg) 167.5±15.74*** 44.19 ***P<0.001,与模型组比较。 表 3 LCA对大鼠棉球肉芽肿干重的影响(n=5,
$\bar{ x}\pm {{s}}$ )组别 棉球肉芽肿干重(m/mg) 抑制率(%) 模型组 48.1±10.05 — LCA组(20 mg/kg) 42.32±10.19 12.05 LCA组(40 mg/kg) 33.73±7.10** 29.90 吲哚美辛组(5 mg/kg) 24.67±7.39*** 48.73 **P<0.01,***P<0.001,与模型组比较。 -
[1] ANTONISAMY P, DURAIPANDIYAN V, IGNACIMUTHU S, et al. Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha lam. in wistar rats[J]. Sijbs,2015,1(1):34. doi: 10.22205/sijbs/2015/v1/i1/100440 [2] CHEN M, FU Q T, SONG X, et al. Preparation of resveratrol dry suspension and its immunomodulatory and anti-inflammatory activity in mice[J]. Pharm Biol,2020,58(1):8-15. doi: 10.1080/13880209.2019.1699123 [3] ANTONISAMY P, AGASTIAN P, KANG C W, et al. Anti-inflammatory activity of Rhein isolated from the flowers of Cassia fistula L. and possible underlying mechanisms[J]. Saudi J Biol Sci,2019,26(1):96-104. doi: 10.1016/j.sjbs.2017.04.011 [4] 翁丽丽, 邱金文, 宋启印. 榆耳发酵液醇沉物抗炎作用[J]. 吉林中医药, 2007, 27(10):56. doi: 10.3969/j.issn.1003-5699.2007.10.047 [5] MEIRA N A, KLEIN L C Jr, ROCHA L W, et al. Anti-inflammatory and anti-hypersensitive effects of the crude extract, fractions and triterpenes obtained from Chrysophyllum cainito leaves in mice[J]. J Ethnopharmacol,2014,151(2):975-983. doi: 10.1016/j.jep.2013.12.014 [6] RATHI M A, MEENAKSHI P, GOPALAKRISHNAN V K. Hepatoprotective activity of ethanolic extract of Alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats[J]. Sijbs,2015,1(2):60. doi: 10.22205/sijbs/2015/v1/i2/100420 [7] WANG J W, CHEN S S, ZHANG Y M, et al. Anti-inflammatory and analgesic activity based on polymorphism of cedrol in mice[J]. Environ Toxicol Pharmacol,2019,68:13-18. doi: 10.1016/j.etap.2019.02.005 [8] LIN B, ZHANG H, ZHAO X X, et al. Inhibitory effects of the root extract of Litsea cubeba (lour.) pers. on adjuvant arthritis in rats[J]. J Ethnopharmacol,2013,147(2):327-334. doi: 10.1016/j.jep.2013.03.011 [9] BOOTS A W, WILMS L C, SWENNEN E L, et al. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers[J]. Nutrition,2008,24(7-8):703-710. doi: 10.1016/j.nut.2008.03.023 [10] 刘玉明, 刘慧民, 蒋定文, 等. 复方苦参酊剂对炎症小鼠和大鼠的抗炎作用研究[J]. 中国药师, 2019, 22(11):2108-2110. doi: 10.3969/j.issn.1008-049X.2019.11.039 [11] LI Z P, HU J F, SUN M N, et al. Anti-inflammatory effect of IMMLG5521, a coumarin derivative, on Sephadex-induced lung inflammation in rats[J]. Int Immunopharmacol,2012,14(2):145-149. doi: 10.1016/j.intimp.2012.06.004 [12] 张志锋, 吴尤娇, 陈丽, 等. 藏药材白花刺参总皂苷的抗炎作用及机制[J]. 中药药理与临床, 2018, 34(5):49-52. [13] LIU X B, YANG B X, ZHANG L, et al. An in vivo and in vitro assessment of the anti-inflammatory, antinociceptive, and immunomodulatory activities of Clematis terniflora DC. extract, participation of aurantiamide acetate[J]. J Ethnopharmacol,2015,169:287-294. doi: 10.1016/j.jep.2015.04.009 -





 下载:
下载:
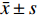
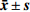
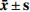
 下载:
下载:


