-
全球每年由念珠菌引发的侵袭性真菌感染病例约有70万例[1],特别是在重症监护室中,患者由于免疫力低下,更易受到真菌感染所带来的危害。据报道,成年人感染念珠菌血症的死亡率高达40%~70%[2]。与此同时,白念珠菌(Candida albicans)作为临床最常见的机会致病性念珠菌,又是全球范围内侵袭性念珠菌的最主要病原体[1]。因此,针对白念珠菌感染的药物治疗研究,一直是人们关注的热点问题。目前,临床上广泛应用于治疗白念珠菌感染的抗真菌药物主要有唑类(氟康唑、伊曲康唑)、多烯类(两性霉素B)、棘白菌素类(卡泊芬净)和5-氟胞嘧啶。然而,随着临床上抗真菌药物的滥用,耐药菌株的报道也越来越多,严重影响了常规抗真菌药的疗效。更加不幸的是,近年来研究发现,即便是严格使用经体外药敏试验提示敏感的抗真菌药物进行治疗,仍有部分患者表现出迁延不愈和复发的症状,这种临床疗效与菌株低耐药性不一致的现象可能与白念珠菌药物耐受菌株的增加有关[3]。
耐受性是指药物敏感菌株在最低抑菌浓度(MIC)以上的高浓度药物中的生长能力,其特征是能够在高剂量抗真菌药物中存活而MIC不变。相反,耐药性则通常是由遗传突变引起的,其特征是测试菌株的 MIC升高[4]。临床菌株的抗真菌药物敏感性实验是在24 h读取的,但耐受菌株生长缓慢,在培养24~48 h后才会显现出高于MIC下生长的能力,因此,在临床检测中,真菌对药物的耐受性在很大程度上被忽视[3, 5]。然而,随着持续性念珠菌死亡率的增加,人们对菌株耐受性问题的关注也日益增多[6]。已有多项研究表明,临床分离株中有20%~60%的菌株表现出对药物的耐受性,进一步解释了临床菌株耐受性和感染治疗失败的关联[3, 5]。由此可见,白念珠菌的耐受问题给目前的临床治疗带来了更加严峻的挑战,积极应对真菌耐受性问题,开发相应的药物和治疗策略迫在眉睫。
小檗碱(BBR)又称黄连素,是一种分离自植物黄连根茎的天然季苄基异喹啉生物碱[7]。BBR临床上广泛运用于治疗胃肠炎,细菌性痢疾等肠道疾病,它的抗菌谱较广,在体外对多种革兰阳性及阴性细菌均具有抑菌作用,且具有安全性高,不良反应少的特点。据报道,BBR在其浓度为10~50 mg/L时具有一定的抗真菌作用[8]。课题组前期研究显示,BBR与氟康唑(FLC)联合使用具有显著的协同抗耐药真菌作用,通过本课题的进一步研究发现,BBR与FLC联合使用对FLC耐受白念珠菌也具有良好的抑制作用,为抗耐受真菌药物的研发提供了一定的实验基础。
-
白念珠菌实验株SC5314来自美国Georgetown大学。白念珠菌(C. albicans)临床株Y0109、9821、7879、7654、9296、9196、7781由海军军医大学第一附属医院及第二附属医院提供。
-
BBR(CAS号: 2086-83-1,货号:B875003-100 mg,纯度≥99%,购自Macklin公司); FLC(CAS号: 86386-73-4,货号:F830935-5g,纯度:98%,购自Macklin公司);二甲基亚砜(DMSO,购自国药集团化学试剂有限公司);酵母浸膏(购自BD公司);蛋白胨(购自BD公司);RPMI Medium 1640(购自Gibco公司);吗啉基丙磺酸(MOPS,购自Amresco公司);NaOH(购自Amresco公司)。
-
生物安全柜(BSC-1004II A2,苏州安泰空气技术有限公司);小型离心机(Hitachi CT15RE);生物显微镜(LW100T,北京测维光电技术有限公司);涡旋仪(Thermo vortex maxi mix Ⅱ);霉菌培养箱(MJ-150-Ⅰ,上海一恒科学仪器有限公司);酶标仪(Thermo Multiskan FC,赛默飞世尔上海仪器有限公司)。
-
YPD液体培养基:酵母浸膏10.0 g,蛋白胨20.0 g,加入800 ml超纯水,充分搅拌溶解后加入D-葡萄糖20.0 g,溶解后,继续加入超纯水定容至1 000 ml,经高压蒸汽灭菌(121℃,15 min)后,自然冷却至室温,密封放置于4℃冰箱保存[9]。YPD固体培养基:在YPD液体培养基成分的基础上加入20.0 g琼脂,高压蒸汽灭菌后,趁热倒入90 mm平皿中,晾干备用。
PBS缓冲溶液:精密称取NaCl 8.0 g,Na2HPO4·12H2O 3.57 g,KCl 0.2 g,KH2PO4 0.24 g,通过三蒸水充分溶解,并定容至1 000 ml,高温高压灭菌(121℃,15 min)后置于4℃冰箱内保存[9]。
RPMI
1640 培养基:RPMI Medium1640 10.0 g,NaHCO3 2.0 g,吗啉基丙磺酸34.5 g,加超纯水充分溶解后,定容至1 000 ml,加入NaOH适量,调pH值至7.0,经0.22 μm微孔滤膜过滤除菌,放置于冰箱4℃保存备用[9]。 -
菌株于YPD与甘油1∶1混合的溶液中保存,冻存于−80℃冰箱中。菌株活化时,吸取10 μl菌液加入装有1 ml 新鲜YPD培养液的15 ml玻璃摇菌管中,在30℃ 气浴恒温振荡培养箱中震荡(200 r/min)培养24 h,之后再次从菌悬液中吸取10 μl加入到1 ml新鲜YPD培养液中,继续以30℃震荡培养16 h后即活化完成,此时真菌处于指数生长的后期,可用于后续实验。
将活化完成的待测菌株转移至1.5 ml离心管中,以
3000 rpm离心1 min,吸弃上清液,随后使用1 ml PBS缓冲液反复吹打洗涤菌株,次离心弃上清液,如此反复3次后再用1 ml PBS缓冲液重悬菌液。取10 μl真菌原液稀释后使用细胞计数板于生物显微镜下计数,计算出真菌原液浓度,用RPMI 1640培养液稀释配制成实验所需菌液浓度。 -
MIC依据美国临床和实验室标准协会(CLSI)出版的微量液基稀释法实验手册(M27-A3)所规定的实验方法与试验标准来测定。具体方法如下:首先将处于指数生长末期的待测菌株用RPMI 1640培养基调整菌浓度至1×103 CFU/ml(菌落形成单位)。取一块96孔板,在第1列加入RPMI 1640培养基100 μl作为空白对照,在第2列加入菌液200 μl,第3~12列各加入菌液100 μl;随后于第2列各孔中加入提前配置好的FLC药液,吹打混匀后,吸出100 μl含药菌液加入到后一列各孔中,如此以2倍倍比稀释至第11列,第12列作为只含菌液的生长对照;并且保证每列至少做3个复孔,以减少实验误差。最后,将96孔板放置于30℃恒温箱中静置培养。培养24、48 h后,用酶标仪测定各孔在630 nm波长处的A值,其中A630刚好小于生长对照孔50%和80%所对应的药物浓度即为该菌FLC的MIC50和MIC80。
-
将活化好的待测菌株使用PBS缓冲液洗净重悬,并用PBS缓冲液将其稀释成浓度为1×106 CFU/ml的菌悬液,吸取100 μl菌液均匀涂布于YPD固体培养基中(考察协同作用时,培养基中应根据需求加入相应浓度的BBR)。随后,在培养基中每隔一定距离放置共7片灭菌滤纸片,在纸片上滴加等体积(5 μl)提前配制好的FLC药液,使滤纸片的载药量依次为0、0.78、1.56、3.125、6.25、12.5、25 μg,待药液吸收完全后将其倒置于30℃恒温培养箱中静置培养72 h,每24 h取出观察真菌生长状况并拍照记录。在筛选耐受菌时,在培养基正中间放入1片灭菌滤纸片,在纸片上滴加50 mg/ml的FLC药液5 μl,使纸片的载药量为250 μg,放置于30℃恒温培养箱中静置培养48 h,每24 h取出观察真菌生长状况并拍照记录[10]。
-
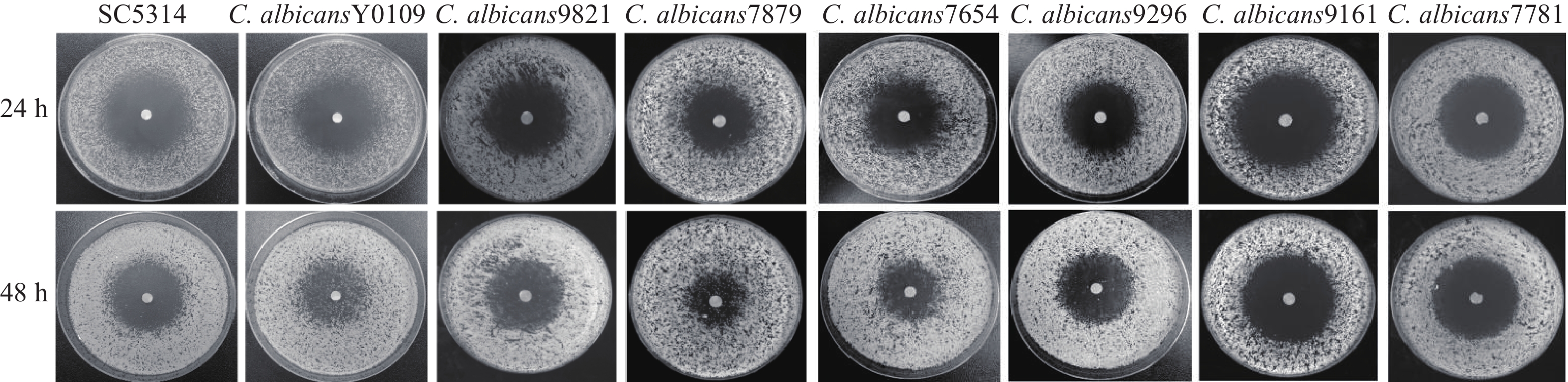
实验选取了8株白念珠菌(含7株临床株)测定其对FLC的MIC值(表1)。8株白念珠菌的MIC值均小于0.5 μg/ml,该结果表明,这8株白念珠菌皆为FLC敏感菌株。
表 1 测定8株白念珠菌对FLC的MIC值
菌株 MIC50(μg/ml) MIC80(μg/ml) SC5314 0.125 0.250 Y0109 0.250 0.500 9821 0.125 0.125 7879 0.125 0.125 7654 0.125 0.125 9296 0.250 0.250 9161 0.250 0.250 7781 0.125 0.125 -
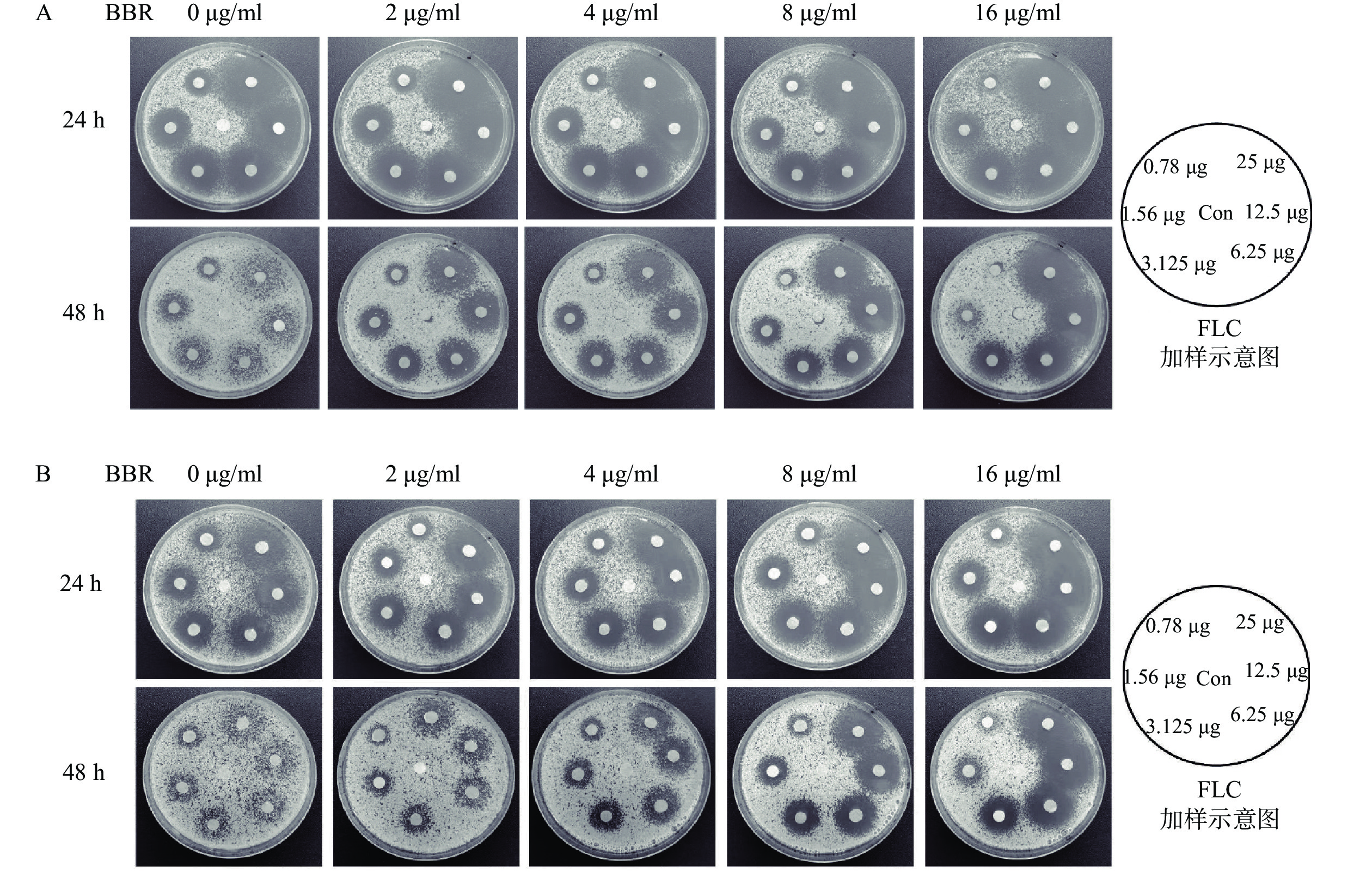
利用琼脂平皿纸片扩散实验,考察上述8株敏感型白念珠菌对FLC的耐受性。结果显示,在单用FLC的情况下,恒温培养24 h后,8株菌株均可观察到明显的抑菌圈,而培养48 h后,在菌株 Y0109、9821、7879、7654、9296的抑菌圈中,可以观察到明显的菌落生长,而其余菌株的抑菌圈内无菌落生长,该结果表明,菌株Y0109、9821、7879、7654、9296对FLC产生了耐受现象,可将其归类为FLC耐受菌株(图1)。
-
从考察菌株耐受性的抑菌圈实验中可以看出,5株氟康唑耐受菌株根据耐受强度可大致分为2类:高度耐受菌株Y0109、7879、7654;低度耐受菌株9821、9296。课题组从这2类中分别选择了Y0109及9821作为研究对象,利用琼脂平皿纸片扩散实验考察BBR与FLC联用是否具有抗FLC耐受白念珠菌作用。结果显示,耐受菌Y0109和9821在培养48 h后,与不含BBR的对照培养皿相比,含不同浓度(2~16 μg/ml)BBR平皿上的FLC载药滤纸片周围形成了明显的抑菌圈,抑菌圈内的菌落数随着BBR浓度的升高而逐渐减少,抑菌圈随BBR浓度的增加而逐渐清晰,随FLC载药量的增大而增大,显示出剂量依赖关系(图2)。上述结果表明,BBR与FLC联合用药具有良好的抗FLC耐受白念珠菌的作用。
-
白念珠菌对抗真菌药物的耐药问题一直是临床抗真菌感染研究的热点,而针对其耐受相关问题的研究却往往被人们忽视。多项研究表明,耐受是耐药的重要前提和基础,耐药突变更有可能源自于真菌的耐受亚群[3, 11-12]。因此,研究白念珠菌对抗真菌药物的耐受机制和相应的治疗策略具有重大的临床意义。
BBR作为一味历史悠久的抗菌药,在我国作为OTC药物用于防治腹泻性疾病已超过60年,其疗效和安全性均得到了普遍认可。本课题组前期研究发现,BBR与FLC在体外具有较好的协同抗FLC耐药白念珠菌的作用,这种协同作用与菌株对FLC的抗性有关,且两药的协同作用取决于BBR的浓度,而不是FLC的浓度[13]。进一步研究发现,FLC能够对细胞膜造成损伤,从而促进BBR对白念珠菌的侵袭,随后,BBR通过引起白念珠菌的细胞周期阻滞和DNA损伤发挥抗菌作用[14]。
目前,越来越多的研究表明,耐受和耐药的产生机制不同,而压力应答调控被认为是白念珠菌产生药物耐受的最重要方式之一。压力应答调控是真菌的一种应激策略,这一机制的产生对药物的靶点没有直接的影响[15]。它主要通过包括热休克蛋白90(Hsp 90)、蛋白激酶C、钙调磷酸酶以及雷帕霉素靶蛋白(TOR)等在内的关键因子与其相应的Ca2+-钙调磷酸酶、PKC-MAPK、Rim途径和HOG信号通路来调节白念珠菌对压力环境的应答,从而对唑类、多烯类和棘白菌素类等抗真菌药物产生耐受作用[16]。此外,白念珠菌还可以通过压力应答调控来影响其对药物的摄取和外排,从而间接地促进白念珠菌对药物的耐受能力[3]。在本研究中,我们发现BBR与FLC的联合用药对于FLC耐受白念珠菌具有很好的疗效,其作用机制是否与抑制白念珠菌的压力应答调控有关有待进一步探究。
联合用药一直以来都是临床抗真菌治疗的重要发展方向,该治疗策略不仅可以克服真菌的耐受与耐药难题,还可以增强原有药物的抗真菌作用,极大缓解新药研发的压力。本课题组研究发现,BBR与FLC联用不仅具有显著的协同抗FLC耐药白念珠菌作用,对FLC耐受白念珠菌也具有较强的抑制作用,为寻找新的抗耐药和耐受白念珠菌药物和治疗方法提供了新思路,值得我们进一步深入探索。
Study on the effect of berberine combined with fluconazole on fluconazole-tolerant Candida albcians strains
-
摘要:
目的 研究小檗碱(BBR)与氟康唑(FLC)联合用药的体外抗FLC耐受白念珠菌作用。 方法 利用微量液基稀释法测定FLC单用对8株白念珠菌的最低抑菌浓度(MIC)以确定其对FLC的敏感性;通过琼脂平皿纸片扩散实验从FLC敏感菌株中筛选出FLC耐受菌株;利用琼脂平皿纸片扩散实验考察BBR与FLC联合用药对FLC耐受白念珠菌的作用。 结果 选取的8株白念珠菌皆为FLC敏感菌株,其MIC50值均<0.5 μg/ml;菌株Y0109、9821、7879、7654、9296在恒温培养48 h后抑菌圈内出现菌落生长,表现出对FLC的耐受现象;菌株Y0109与9821在BBR与FLC联用时,恒温培养48 h后抑菌圈内的菌落数随BBR浓度的升高而逐渐减少,抑菌圈随BBR浓度的升高而逐渐清晰,随FLC载药量的增大而增大,显示出剂量依赖关系。 结论 BBR与FLC联合用药具有良好的抗FLC耐受白念珠菌效果。 Abstract:Objective To investigate the combined effect of berberine (BBR) and fluconazole (FLC) on FLC-tolerant Candida albicans in vitro. Methods The sensitivity of 8 strains of Candida albicans to FLC was assessed by determining their minimal inhibitory concentration (MIC) using broth microdilution method. FLC-tolerant strains were screened from FLC-sensitive strains by disk diffusion assay. The effect of BBR combined with FLC on FLC-tolerant Candida albicans was investigated by disk diffusion assay. Results All eight strains of Candida albicans exhibited sensitivity to FLC, with minimal inhibitory concentration (MIC50) values below 0.5 μg/ml. Strains Y0109, 9821, 7879, 7654, and 9296 displayed colony growth in the inhibition zone after 48 h of constant temperature incubation, indicating FLC tolerance. When strains Y0109 and 9821 were subjected to a combination of BBR and FLC, the number of colonies within the inhibition zone decreased progressively with the increase of BBR concentration following a 48 h constant temperature culture. The inhibition zone became clear with the increasing of BBR concentration and increased with the increase of FLC loading, which showed a dose-dependent relationship. Conclusion The BBR combined with FLC demonstrated efficacy against FLC-tolerant strains. -
Key words:
- Candida albicans /
- berberine /
- fluconazole /
- drug combination /
- drug tolerance
-
放线菌以其能产生结构新颖且有良好生物活性的先导化合物而备受关注[1],一直被认为是天然药物的重要生产者,其主要结构类型包括聚酮、生物碱、多肽和萜烯类化合物等,同时涵盖了多种多样的生物活性如抗菌、抗寄生虫、免疫调节、抗炎、抗癌等[2-4],这突显了放线菌具有不可预估的药物开发潜力。
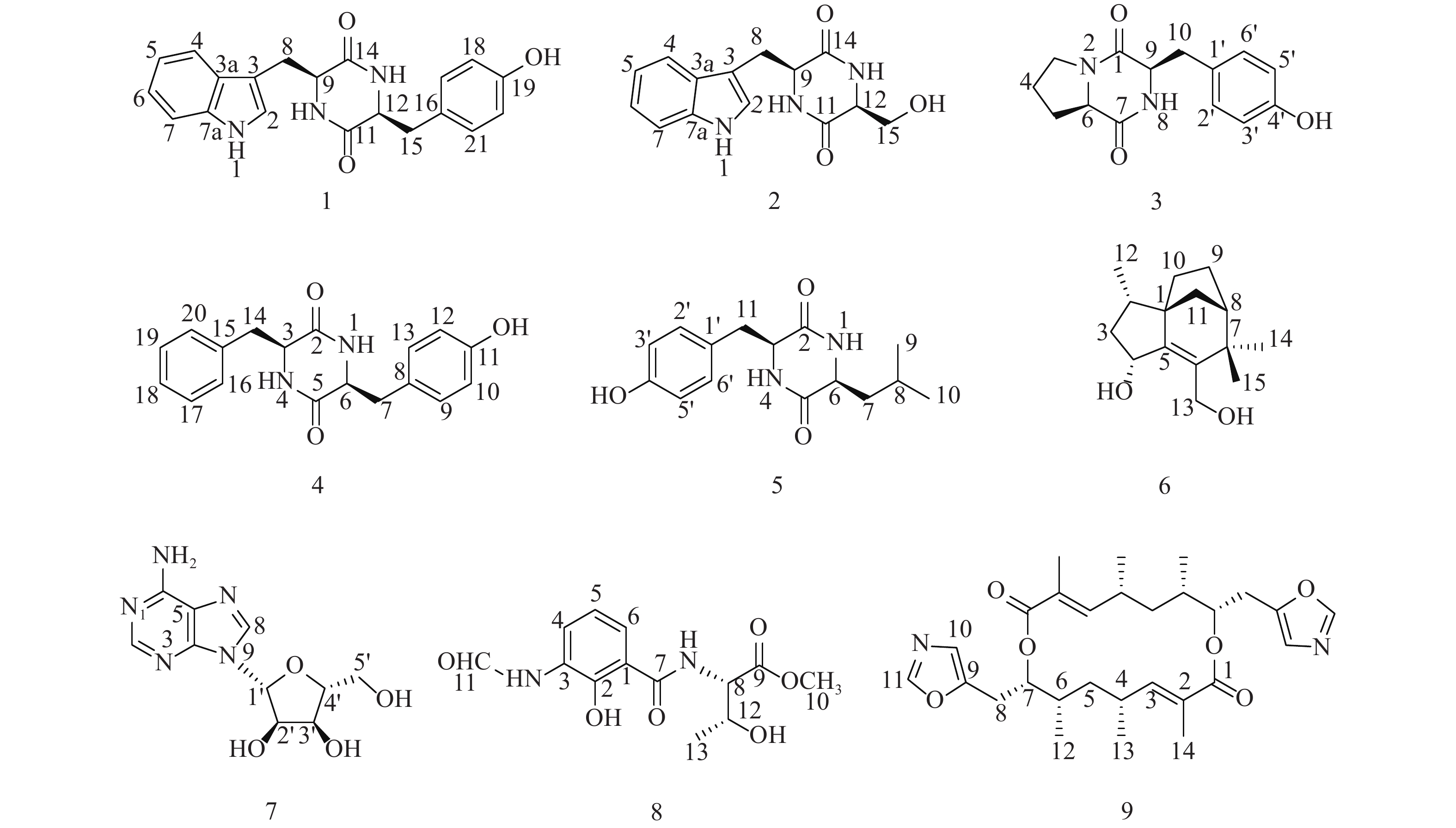
随着研究的深入,陆地和普通环境中的资源日趋枯竭,很多微生物及其次生代谢产物被重复开发和提取分离,发现新活性分子的几率愈来愈低,开发创新药物的难度越来越大[5-6];而极地极端的生态环境造就的微生物具有产生更为特别的化学骨架和活性次生代谢产物的能力,是新型药源分子的重要来源。本文以采自北极楚克奇海域海绵共附生放线菌Streptomyces sp. LHW11-07为研究对象,从其发酵浸膏中分离鉴定了9个单体化合物1~9(图1),其中化合物1和2为该属内首次分离得到。
1. 材料和方法
1.1 实验仪器与试剂
AMX-600型核磁共振仪(德国Bruker公司);Xevo G2-XS Q-TOF液质联用仪、1525/2996, 2998型高效液相色谱仪(美国Waters公司);半制备型HPLC色谱柱(Atlantis Prep T3,美国Waters公司;YMC C18,日本YMC公司);中压柱色谱仪(法国Interchim公司);恒温振荡培养箱(上海一恒科学仪器有限公司);N-1000型旋转蒸发仪(上海爱郎仪器有限公司);反相ODS硅胶和Sephadex LH-20柱色谱填料(Pharmacia公司);正相硅胶(200-300目)和TLC薄层板(烟台江友硅胶开发有限公司);分析级试剂(上海化学试剂公司);色谱级试剂(德国Merck公司);氘代试剂(美国剑桥同位素实验室公司)。
1.2 菌株的来源及鉴定
菌株分离于北极海域来源的海绵样本,经16S rRNA基因序列鉴定为Streptomyces sp.,编号为LHW11-07,菌种保存于上海交通大学医学院附属仁济医院药学部海洋药物研究中心。
1.3 菌株的大发酵
培养基为ISP2:葡萄糖(4 g/L)、酵母提取物(4 g/L)、麦芽糖提取物(10 g/L)以及海盐(25 g/L),加水溶解后调节pH为7.2~7.4,分装后高压灭菌20 min (121 ℃),冷却备用。
挑取Streptomyces sp. LHW11-07单菌落至1级种子培养基里(100 ml ISP2培养基至250 ml三角瓶),置于30 ℃,220 r/min的恒温摇床培养3 d,得1级种子液;将1级种子液按5%接种量接到2级种子培养基里(150 ml ISP2培养基至500 ml三角瓶),置于30 ℃,220 r/min的恒温摇床培养3 d,得2级种子液;将2级种子液按5%接种量接到大发酵培养基里(700 ml ISP2培养基至2 L三角瓶),置于30 ℃,220 r/min的恒温摇床培养7 d,共得到发酵液50 L。
1.4 发酵产物的提取与分离
菌株培养7 d后,用等体积的乙酸乙酯萃取3次,合并乙酸乙酯提取液,减压浓缩得粗浸膏9.8 g。粗浸膏先经凝胶柱分离,二氯甲烷:甲醇(1∶1)的混合溶剂进行洗脱,得到组份Fr.1~Fr.7。
组份Fr.4经正相中压柱色谱分离(二氯甲烷:甲醇100:0~0:100),得到组份Fr.4a~Fr.4j。组份Fr.4d再经反相中压柱色谱分离(10%~100%乙腈水),得到组份Fr.4d1~Fr.4d8,Fr.4d2和Fr.4d3用反相半制备HPLC纯化(35%甲醇水,YMC C18),分别得到化合物1 (12 mg, tR=26 min)和2 (47.6 mg, tR=30 min);Fr.4d6用反相半制备HPLC纯化(49%甲醇水,YMC C18),得到化合物3 (8.4 mg, tR=23 min)和4 (2.0 mg, tR=30 min);组份Fr.4g再经凝胶柱纯化,洗脱剂为正己烷:二氯甲烷:甲醇(4∶5∶1),得到组份Fr.4g1~Fr.4g11,其中Fr.4g3用反相半制备HPLC纯化(25%乙腈水,YMC C18),得到化合物5 (1.2 mg, tR=18 min)。
组份Fr.7经反相中压柱色谱分离(10%~100%乙腈水),得到组份Fr.7A~Fr.7D。组份Fr.7A用反相半制备HPLC纯化(20%乙腈水,YMC C18),得到化合物6 (18 mg, tR=19 min)和7 (3.4 mg, tR=25 min);组份Fr.7B经正相中压柱分离(石油醚:丙酮100:0~0:100),得到组份Fr.7B1~Fr.7B9,Fr.7B4用反相半制备HPLC纯化(88%乙腈水,Atlantis Prep T3),得到化合物8 (12 mg, tR=23 min)和9 (8.4 mg, tR=26 min)。
2. 结构鉴定
化合物1:淡黄色固体,ESI-MS显示准分子离子峰m/z 372 [M+Na]+。1H-NMR (600 MHz, DMSO)显示在低场区有吲哚环的特征信号δH 7.00 (1H, s, H-2),7.49 (1H, d, J=8.0 Hz, H-4),7.02 (1H, t, J=7.6 Hz, H-5),7.05 (1H, t, J=7.6 Hz, H-6),7.32 (1H, d, J=8.0 Hz, H-7);4个活泼氢质子信号δH 10.89 (1H, d, J=2.6 Hz, NH-1),7.83 (1H, d, J=3.0 Hz, NH-10),7.62 (1H, d, J=3.0 Hz, NH-13),9.20 (1H, s, OH-19);4个芳香质子信号δH 6.53 (2H, d, J=8.4 Hz, H-17, H-21),6.59 (2H, d, J=8.4 Hz, H-18, H-20),提示分子中有1个对位二取代的苯环;在高场区有两组亚甲基质子信号δH 2.80 (1H, dd, J=14.5, 4.5 Hz, H-8),2.43 (1H, ov, H-8),δH 1.83 (1H, dd, J=13.4, 6.9 Hz, H-15),2.47 (1H, ov, H-15),两个次甲基质子信号δH 4.01 (1H, m, H-9),3.95 (1H, m, H-12)。13C-NMR (150 MHz, DMSO)结合DEPT谱表明其有20个碳信号,2个酮羰基碳δC 166.7,166.2,14个芳香碳,2个亚甲基碳δC 30.0,40.0和2个次甲基碳δC 55.9,55.2。对其碳信号进行归属:δC 118.7 (C-2)、108.9 (C-3)、127.5 (C-3a)、118.4 (C-4)、120.8 (C-5)、124.3 (C-6)、111.3 (C-7)、136.0 (C-7a)、30.0 (C-8)、55.9 (C-9)、166.7 (C-11)、55.2 (C-12)、166.2 (C-14)、40.1 (C-15)、126.4 (C-16)、130.7 (C-17, C-21)、114.9 (C-18, C-20)、156.0 (C-19)。以上数据与文献[7]对比基本一致,故确定为cyclo-(L-Tyr-L-Trp)。
化合物2:淡黄色固体,ESI-MS显示准分子离子峰m/z 274 [M+H]+。1H-NMR (600 MHz, DMSO)发现其与化合物1一样有吲哚环的特征信号δH 7.09 (1H, s, H-2),7.52 (1H, d, J = 7.9 Hz, H-4),6.99 (1H, t, J = 7.8 Hz, H-5),7.02 (1H, t, J = 7.8 Hz, H-6),7.30 (1H, d, J = 7.8 Hz, H-7);3个活泼氨基质子信号δH 10.87 (1H, s, NH-1),δH 8.30 (1H, m, NH-10),7.85 (1H, d, J = 2.9 Hz, NH-13);两组亚甲基质子信号δH 3.21 (1H, m, H-8),3.13 (1H, m, H-8),δH 3.65 (1H, m, H-15),3.05 (1H, m, H-15),两个次甲基质子信号δH 4.87 (1H, m, H-9),4.00 (1H, m, H-12)。13C-NMR (150 MHz, DMSO)结合DEPT谱表明其有14个碳信号,2个酮羰基碳δC 167.2,165.7,8个芳香碳,2个亚甲基碳δC 63.0,30.3和2个次甲基碳δC 57.3,55.5。对其碳信号进行归属:δC 127.6 (C-2)、111.2 (C-3)、136.0 (C-3a)、118.6 (C-4)、120.8 (C-5)、124.0 (C-6)、118.3 (C-7)、109.0 (C-7a)、30.3 (C-8)、57.3 (C-9)、167.2 (C-11)、55.5 (C-12)、165.7 (C-14)、63.0 (C-15)。以上数据与文献[8]对比基本一致,故确定为cyclo-(L-Trp-L-Ser)。
化合物3:白色固体,ESI-MS显示准分子离子峰m/z 261 [M+H]+。1H-NMR (600 MHz, DMSO)提示有2个活泼氢质子信号δH 7.87 (1H, s, NH-8),9.22 (1H, s, OH-4’);1组对位二取代的苯环芳香质子信号δH 7.04 (2H, d, J = 8.2 Hz, H-2’, H-6’),6.63 (2H, d, J = 8.2 Hz, H-3’, H-5’);2个次甲基质子信号δH 4.24 (1H, t, J = 8.2 Hz, H-6),4.03 (1H, dd, J = 9.9, 2.9 Hz, H-9);4组亚甲基质子信号δH 3.42 (1H, m, H-3),3.24 (1H, m, H-3),1.73 (2H, m, H2-4),2.00 (1H, m, H-5),1.41 (1H, m, H-5),2.93 (2H, m, H2-10)。13C-NMR (150 MHz, DMSO)结合DEPT谱表明其有14个碳信号,2个酮羰基碳δC 168.9,165.1,6个芳香碳,2个次甲基碳δC 58.4,56.0以及4个亚甲基碳δC 44.6,34.7,27.8,21.9。对其碳信号进行归属:δC 165.1 (C-1)、44.6 (C-3)、21.9 (C-4)、27.8 (C-5)、58.4 (C-6)、168.9 (C-7)、56.0 (C-9)、34.7 (C-10)、127.0 (C-1’)、130.8 (C-2’, C-6’)、114.8 (C-3’, C-5’)、155.9 (C-4’)。以上数据与文献[9]对比基本一致,故确定为cyclo-(D-Tyr-D-Pro)。
化合物4:白色固体,ESI-MS显示准分子离子峰m/z 311 [M+H]+。1H-NMR (600 MHz, DMSO)显示3个活泼氢质子信号δH 9.30 (1H, s, OH-11),7.84 (2H, t, J = 2.9 Hz, NH-1, NH-4);9个芳香区质子信号:4个归为1组对位二取代苯环δH 6.85 (2H, d, J = 8.5 Hz, H-9, H-13),6.65 (2H, t, J = 8.5 Hz, H-10, H-12),5个归为1组单取代苯环δH 7.20 (1H, t, J = 7.6 Hz, H-18),7.04 (2H, d, J = 6.9 Hz, H-16, H-20),7.28 (2H, t, J = 7.6 Hz, H-17, H-19);2组亚甲基质子信号δH 2.58 (1H, dd, J = 13.6, 5.0 Hz, H-7),2.20 (1H, d, J = 6.5 Hz, H-7),2.19 (2H, dd, J = 13.6, 6.5 Hz, H2-14),2个次甲基质子信号δH 3.95 (1H, m, H-3),3.90 (1H, m, H-6)。13C-NMR (150 MHz, DMSO)结合DEPT谱显示其有18个碳信号,2个酮羰基碳δC 166.2,166.2,12个芳香碳,2个亚甲基碳δC 40.1,38.5和2个次甲基碳δC 55.7,55.4。对其碳信号进行归属:δC 166.2 (C-2)、55.7 (C-3)、166.3 (C-5)、55.4 (C-6)、40.1 (C-7)、126.5 (C-8)、130.8 (C-9, C-13)、115.0 (C-10, C-12)、156.1 (C-11)、38.5 (C-14)、136.7 (C-15)、129.7 (C-16, C-20)、128.2 (C-17, C-19)、126.4 (C-18)。以上数据与文献[10]对比基本一致,故确定为cyclo-(L-Tyr-L-Phe)。
化合物5:白色固体,ESI-MS显示准分子离子峰m/z 277 [M+H]+。1H-NMR (600 MHz, DMSO)显示3个活泼氢质子信号δH 9.22 (1H, s, OH-4’),8.02 (2H, dd, J = 5.6, 2.5 Hz, NH-1, NH-4);4个芳香质子信号δH 6.90 (2H, d, J = 8.2 Hz, H-2’, H-6’),6.64 (2H, d, J = 8.2 Hz, H-3’, H-5’),提示分子中有1个对位二取代苯环;3个次甲基质子信号δH 4.06 (1H, q, J = 3.3 Hz, H-3),3.44 (1H, m, H-6),1.43 (1H, ov, H-8),2个亚甲基质子信号δH 1.43 (1H, m, H-7),1.23 (1H, m, H-7),2.69 (1H, q, J = 13.6, 4.8 Hz, H-11),3.01 (1H, q, J = 13.7, 3.7 Hz, H-11)以及2个末端甲基质子信号δH 0.63 (6H, ov, H3-9, H3-10)。13C-NMR (150 MHz, DMSO)结合DEPT谱显示其有15个碳信号,2个酮羰基碳δC 166.2,167.4,6个芳香碳,2个亚甲基碳δC 43.7,37.7,3个次甲基碳δC 55.7,52.3,21.4以及2个甲基碳δC 22.9,22.8。对其碳信号进行归属:δC 166.2 (C-2)、55.7 (C-3)、167.4 (C-5)、52.3 (C-6)、43.7 (C-7)、21.4 (C-8)、22.9 (C-9)、22.8 (C-10)、37.7 (C-11)、125.8 (C-1’)、131.2 (C-2’, C-6’)、114.8 (C-3’, C-5’)、156.4 (C-4’)。以上数据与文献[11]对比基本一致,故确定为cyclo-(L-Tyr-L-Leu)。
化合物6:白色固体,ESI-MS显示准分子离子峰m/z 259 [M+Na]+。1H-NMR (600 MHz, DMSO)显示有2个活泼氢质子信号δH 5.05 (1H, d, J = 4.5 Hz, OH-4)和4.39 (1H, q, J = 4.0 Hz, OH-13),3个甲基质子信号δH 0.88 (3H, d, J = 6.8 Hz, H3-12),0.98 (3H, s, H3-14)和δH 1.05 (3H, s, H3-15),5对亚甲基质子信号δH 2.14 (1H, m, H-3),1.23 (1H, m, H-3),1.74 (1H, m, H-9),1.60 (1H, m, H-9),1.44 (3H, m, H2-10, H-11),1.32 (1H, d, J = 10.4 Hz, H-11),3.95 (2H, m, H2-13),3个次甲基质子信号δH 1.68 (1H, m, H-2),4.57 (1H, m, H-4),1.77(1H, m, H-8)。13C-NMR (150 MHz, DMSO)结合DEPT谱共显示有15个碳信号,包括4个季碳δC 52.1,150.1,136.7,39.8;3个次甲基碳δC 35.2,69.8,46.4;5个亚甲基碳δC 42.4,23.8,28.7,36.4,57.1以及3个甲基碳δC 13.7,29.1,24.4。对其碳信号进行归属:δC 52.1 (C-1)、35.2 (C-2)、42.4 (C-3)、68.9 (C-4)、150.1 (C-5)、136.7 (C-6)、39.8 (C-7)、46.4 (C-8)、23.8 (C-9)、28.7 (C-10)、36.4 (C-11)、13.7 (C-12)、57.1 (C-13)、29.1 (C-14)、24.4 (C-15)。以上数据与文献[12]对比基本一致,故确定为albaflavenol B。
化合物7:白色结晶固体,ESI-MS显示准分子离子峰m/z 268 [M+H]+。1H-NMR (600 MHz, DMSO)可看出其有13个氢信号,包括5个活泼氢质子信号δH 3.56 (2H, m, NH2),5.49 (1H, s, OH-5’),5.36 (1H, t, J = 4.9 Hz, OH-2’)和5.23 (1H, s, OH-3’),4个连氧次甲基质子信号δH 4.56 (1H, s, H-2’),4.14 (1H, s, H-3’),3.96 (1H, m, H-4’)和5.90 (1H, d, J = 5.8 Hz, H-1’),1组亚甲基信号δH 3.66 (2H, m, H2-5’)以及2个低场区的烯氢质子信号δH 8.37 (1H, s, H-8)和8.21 (1H, s, H-2)。13C-NMR (150 MHz, DMSO)显示其共有10个碳信号,结合DEPT谱可推测有3个芳香季碳δC 149.9,119.8,154.3,2个连氮的芳香次甲基碳δC 151.7,138.6,4个次甲基碳δC 87.8,73.5,70.5,85.7,1个亚甲基碳δC 61.5。对其碳信号进行归属:δC 151.7 (C-2)、149.9 (C-4)、119.8 (C-5)、154.3 (C-6)、138.6 (C-8)、87.8 (C-1’)、73.5 (C-2’)、70.5 (C-3’)、85.7 (C-4’)、61.5 (C-5’)。以上数据与文献[13]对比基本吻合,故确定为β-adenosine。
化合物8:绿色无定型固体,ESI-MS显示准分子离子峰m/z 297 [M+H]+。1H-NMR (600 MHz, MeOD)显示有12个氢信号,包括1个活泼氢质子信号δH 8.37 (1H, s, H-11),1个甲氧基质子信号δH 3.79 (3H, s),1个甲基质子信号δH 1.25 (3H, d, J = 6.4 Hz, H3-13),3个低场区的芳香氢质子信号δH 8.31 (1H, d, J = 7.8 Hz, H-4),6.92 (1H, t, J = 8.0 Hz, H-5)和7.65 (1H, d, J = 8.0 Hz, H-6),以及2个次甲基质子信号δH 4.74 (1H, d, J = 3.2 Hz, H-8)和4.40 (1H, m, H-12),后与文献[14]对比发现其有4个活泼氢质子信号没有显示出来,而根据相关化学位移可确定其是同一个已知化合物。13C-NMR (150 MHz, MeOD)显示有13个碳信号,结合DEPT谱可推测有6个芳香碳δC 123.4,119.4,126.2,128.2,152.5,115.6,2个羰基碳δC 171.8,172.4,而δC 162.1为醛基碳,2个次甲基碳δC 59.4,68.4,1个甲基碳δC 20.5,以及1个甲氧基碳δC 52.9。对其碳信号进行归属:δC 115.6 (C-1)、152.5 (C-2)、128.2 (C-3)、126.2 (C-4)、119.4 (C-5)、123.4 (C-6)、171.8 (C-7)、59.4 (C-8)、172.4 (C-9)、52.9 (C-10)、162.1 (C-11)、68.4 (C-12)、20.5 (C-13)。以上数据与文献[14]对比基本吻合,故确定为N-formylantimyic acid methyl ester。
化合物9:白色粉末状固体,ESI-MS显示准分子离子峰m/z 499 [M+H]+。1H-NMR (600 MHz, DMSO)显示有19个氢信号:δH 8.20 (1H, s, H-11),6.82 (1H, s, H-10),6.32 (1H, dd, J = 10.5, 1.3 Hz, H-3),5.01 (1H, m, H-7),2.99 (1H, dd, J = 2.5, 15.8 Hz, H-8),2.80 (1H, dd, J = 10.3, 15.6 Hz, H-8),2.58 (1H, m, H-4),1.66 (1H, m, H-5),1.65 (3H, s, 2-Me),1.27 (1H, m, H-6),1.25 (1H, m, H-5),1.06 (3H, d, J = 6.5 Hz, 4-Me)和0.95 (3H, d, J = 5.9 Hz, 6-Me)。而13C-NMR (150 MHz, DMSO)结合DEPT谱显示只有14个碳信号:δC 166.0 (C-1),126.8 (C-2),147.4 (C-3),30.7 (C-4),37.6 (C-5),35.1 (C-6),74.5 (C-7),24.0 (C-8),149.4 (C-9),122.9 (C-10),151.3 (C-11),12.7 (2-Me),21.1 (4-Me)和16.2 (6-Me),说明这个化合物可能是一个具有对称结构的二聚体,通过与文献[15]中化合物conglobatin A对比后发现两者波谱数据完全吻合,故最终确定为conglobatin A。
3. 讨论
自上世纪发现青霉素以来,微生物中活性次生代谢产物一直是药物先导化合物的重要来源之一,据统计1940年—2019年间,科学家从微生物中开发出293种治疗不同疾病的临床药物[16]。但随着研究的深入,很多微生物及其次生代谢产物存在被重复开发和提取分离的问题,加之多重耐药性的产生,迫使人们需要开拓新的制造药物的微生物来源[5-6],而其中极地微生物资源是珍贵而特殊的。来自极地海洋等特殊生态环境的生物往往具有比陆地生物更为丰富的代谢途径和功能基因簇,增加了产生结构新颖且功能独特的次生代谢物的可能性。极地生物以微生物和一些能适应极端条件的海洋生物为主,然而与已报道的大量极地微生物相比,鲜有微生物活性天然产物相关研究报道,因此,极地微生物极具研究价值[17-18]。
笔者以一株采自北极海域海绵共附生放线菌Streptomyces sp. LHW11-07为研究对象,从其发酵浸膏中分离得到9个单体化合物1~9,包括环二肽化合物1~5,倍半萜化合物6,核苷类化合物7,以及两个其他结构类型化合物8和9,其中化合物1和2是首次分离于Streptomyces放线菌,而这些化合物的生物活性还有待进一步探究;本研究进一步丰富了该属放线菌的化学多样性,同时,为高值化开发利用极地微生物这一国家战略资源提供了物质基础和理论依据。
据文献报道,化合物1对所测试的病原性细菌和真菌均具有一定的对抗作用[19],化合物2测试了4种肿瘤细胞均无明显的细胞毒性[20],化合物3对海胆Strongylocentrotus intermedius胚胎具有细胞毒活性[9],化合物5具有抗炎活性并对H1N1和RSV病毒有一定的杀伤作用[21],化合物7作为一种内源性嘌呤核苷,具有降低血压、抑制血小板聚焦、舒张血管、减慢心律等生理活性[22],而化合物9可抑制癌细胞株的增殖,在体外对Trypanosoma brucei brucei GUTat 3.1表现出抗锥虫体活性等[23]。
-
表 1 测定8株白念珠菌对FLC的MIC值
菌株 MIC50(μg/ml) MIC80(μg/ml) SC5314 0.125 0.250 Y0109 0.250 0.500 9821 0.125 0.125 7879 0.125 0.125 7654 0.125 0.125 9296 0.250 0.250 9161 0.250 0.250 7781 0.125 0.125 -
[1] LOGAN C, MARTIN-LOECHES I, BICANIC T. Invasive candidiasis in critical care: challenges and future directions[J]. Intensive Care Med, 2020, 46(11):2001-2014. doi: 10.1007/s00134-020-06240-x [2] LASS-FLÖRL C, KANJ S S, GOVENDER N P, et al. Invasive candidiasis[J]. Nat Rev Dis Primers, 2024, 10(1):20. doi: 10.1038/s41572-024-00503-3 [3] ROSENBERG A, ENE I V, BIBI M, et al. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia[J]. Nat Commun, 2018, 9(1):2470. doi: 10.1038/s41467-018-04926-x [4] KE W X, XIE Y Y, CHEN Y Y, et al. Fungicide-tolerant persister formation during cryptococcal pulmonary infection[J]. Cell Host Microbe, 2024, 32(2): 276-289. e7. [5] DRUSEIKIS M, MOTTOLA A, BERMAN J. The metabolism of susceptibility: clearing the FoG between tolerance and resistance in Candida albicans[J]. Curr Clin Microbiol Rep, 2023, 10(2):36-46. doi: 10.1007/s40588-023-00189-3 [6] FENG Y R, LU H, WHITEWAY M, et al. Understanding fluconazole tolerance in Candida albicans: implications for effective treatment of candidiasis and combating invasive fungal infections[J]. J Glob Antimicrob Resist, 2023, 35:314-321. doi: 10.1016/j.jgar.2023.10.019 [7] RAUF A, ABU-IZNEID T, KHALIL A A, et al. Berberine as a potential anticancer agent: a comprehensive review[J]. Molecules, 2021, 26(23):7368. doi: 10.3390/molecules26237368 [8] 江涛, 曹煜, 赵秀华, 等. 22种中草药有效成分抗真菌研究及新剂型应用[J]. 中华皮肤科杂志, 1999, 32(5):316-318. doi: 10.3760/j.issn:0412-4030.1999.05.007 [9] LEE J S, JUNG W K, JEONG M H, et al. Sanguinarine induces apoptosis of HT-29 human colon cancer cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent pathway[J]. Int J Toxicol, 2012, 31(1):70-77. doi: 10.1177/1091581811423845 [10] AHSAN H, REAGAN-SHAW S, BREUR J, et al. Sanguinarine induces apoptosis of human pancreatic carcinoma AsPC-1 and BxPC-3 cells via modulations in Bcl-2 family proteins[J]. Cancer Lett, 2007, 249(2):198-208. doi: 10.1016/j.canlet.2006.08.018 [11] COWEN L E, SINGH S D, KÖHLER J R, et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease[J]. Proc Natl Acad Sci U S A, 2009, 106(8):2818-2823. doi: 10.1073/pnas.0813394106 [12] LEVIN-REISMAN I, RONIN I, GEFEN O, et al. Antibiotic tolerance facilitates the evolution of resistance[J]. Science, 2017, 355(6327):826-830. doi: 10.1126/science.aaj2191 [13] QUAN H, CAO Y Y, XU Z, et al. Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole[J]. Antimicrob Agents Chemother, 2006, 50(3):1096-1099. doi: 10.1128/AAC.50.3.1096-1099.2006 [14] LI D D, XU Y, ZHANG D Z, et al. Fluconazole assists berberine to kill fluconazole-resistant Candida albicans[J]. Antimicrob Agents Chemother, 2013, 57(12):6016-6027. doi: 10.1128/AAC.00499-13 [15] BERMAN J, KRYSAN D J. Drug resistance and tolerance in fungi[J]. Nat Rev Microbiol, 2020, 18(6):319-331. doi: 10.1038/s41579-019-0322-2 [16] IYER K R, ROBBINS N, COWEN L E. The role of Candida albicans stress response pathways in antifungal tolerance and resistance[J]. iScience, 2022, 25(3):103953. doi: 10.1016/j.isci.2022.103953 -





 下载:
下载:
 下载:
下载: