-
雷公藤为卫矛科雷公藤属植物雷公藤Tripterygium wilfordii Hook.f.的干燥根,是一种公认的同时具有较强药效和较强毒性的中药材,广泛用于治疗类风湿性关节炎、肾病综合征、系统性红斑狼疮等疾病[1]。但其毒性较大,不良反应高发且严重,常使患者不能耐受[2]。雷公藤的主要有效成分为二萜类、三萜类和生物碱类,研究表明各种成分均具有不同程度的抗炎、抗肿瘤和免疫抑制等活性[3-4],其中生物碱的毒性要小于二萜和三萜类成分,临床应用具有疗效好,不良反应较小的特点[5]。雷公藤次碱是雷公藤生物碱中含量较高的一种倍半萜类单体化合物。目前研究显示其具有良好的杀虫活性,其他药理活性和机理研究较少,特别是抗炎和免疫抑制活性方面。
本研究主要通过建立脂多糖(LPS)诱导的RAW264.7细胞炎症模型,探讨雷公藤次碱对LPS诱导的炎性因子NO,IL-1β,TNF-α和IL-6释放的影响,用免疫印迹法考察雷公藤次碱对TRAF6、IRAK、NF-κB、IκBα、JNK、ERK、p38等关键蛋白表达或磷酸化的影响,探讨其可能的抗炎作用机制,为促进雷公藤次碱的应用研究提供基础。
-
雷公藤次碱(纯度98%,上海纯优生物科技有限公司);DMEM细胞培养基、胎牛血清(Life Technologies GIBCO);Griess试剂、细胞计数盒-8 (CCK-8)(上海碧云天生物技术有限公司);脂多糖(LPS, Sigma-Aldrich);IL-1β、TNF-α和IL-6检测试剂盒(达科为生物技术有限公司);核质提取试剂盒(Dojindo Laboratories);TRAF6、IRAF、p-IRAF、p-p38、p38、p-JNK、JNK、p-ERK、ERK、p-IκBα、IκBα、NF-κB p65、β-actin、HRP山羊抗兔抗体(Cell Signaling Technology);RAW264.7细胞购自中国科学院上海细胞库。
-
RAW264.7细胞在含10%的新生小牛血清及100 U/ml青霉素、链霉素的DMEM高糖培养液中进行培养,培养条件为5%CO2、37 ℃,隔天换液,每日观察细胞的生长状况。实验时取对数生长期RAW264.7细胞,胰酶消化,加入新鲜培养基反复吹打成细胞混悬液,细胞增殖实验、细胞因子分泌含量测定调整细胞浓度为1×105 cells/ml,以每孔100 μl细胞悬液接种于96孔细胞培养板中,细胞蛋白表达测定调整细胞浓度到2.25×105 cells/ml,以每孔2 ml接种于6孔细胞培养板中,然后细胞置于37 ℃、5%CO2培养箱中培养过夜。在药物处理前2 h给予LPS溶液(使其终浓度为1 μg/ml)诱导RAW264.7细胞发生炎症反应建立模型。设空白组(不给予LPS和药物处理)、模型组(给予LPS处理)、药物组(给予LPS诱导后再给予相应浓度的药物进行处理),每组设6个复孔(n=6)。
-
设空白组和药物组,药物组分别给予终浓度为200、100、50和25 μmol/L的雷公藤次碱药液进行处理。细胞分别培养24和48 h,实验结束4 h前加入10 μl CCK-8试剂,结束后以酶标仪于450 nm处检测吸光度(A)。
-
设空白组、模型组和药物组,药物组细胞给予LPS诱导后分别给予终浓度为100、50、25 μmol/L的药液进行处理。细胞培养24 h后取出,吸取细胞上清液,Griess试剂法检测上清中NO光密度值,ELISA试剂盒检测上清中IL-1β、TNF-α和IL-6的光密度值。
-
设空白组、模型组和药物组,药物组细胞给予LPS诱导后分别给予终浓度为100、50、25 μmol/L的药液进行处理。细胞培养24 h后取出,用4 ℃预冷的PBS液洗涤各孔两次,然后,吸弃PBS液,每孔中加入200 μl 4 ℃预冷的RIPA提取细胞蛋白,在13000 r/min下离心20 min,弃去上清,得各样本。用Bradford法对蛋白定量;使用细胞核/质提取试剂盒制备用于检测p65/NF-κB的胞质和核提取物。每孔加入20 μg蛋白上样,在SDS-PAGE凝胶中电泳,蛋白转移到PVDF膜上,用BSA封闭2 h。分别用相应一抗4 ℃孵育过夜,然后用与辣根过氧化物酶偶联的二抗(1∶1000)孵育2 h。最后用 ECL超敏发光液显影,于凝胶成像仪中曝光,使用 LAS成像软件对条带进行定量分析
-
实验数据的统计分析用(
$ \bar x \pm s $ )表示,采用单因素方差分析法进行统计学处理。应用Alpha软件处理Western Blot实验所得目标带的光密度值,并以各自的β-actin蛋白条带灰度值与目标条带灰度值的比值作为蛋白相对表达水平值。 -
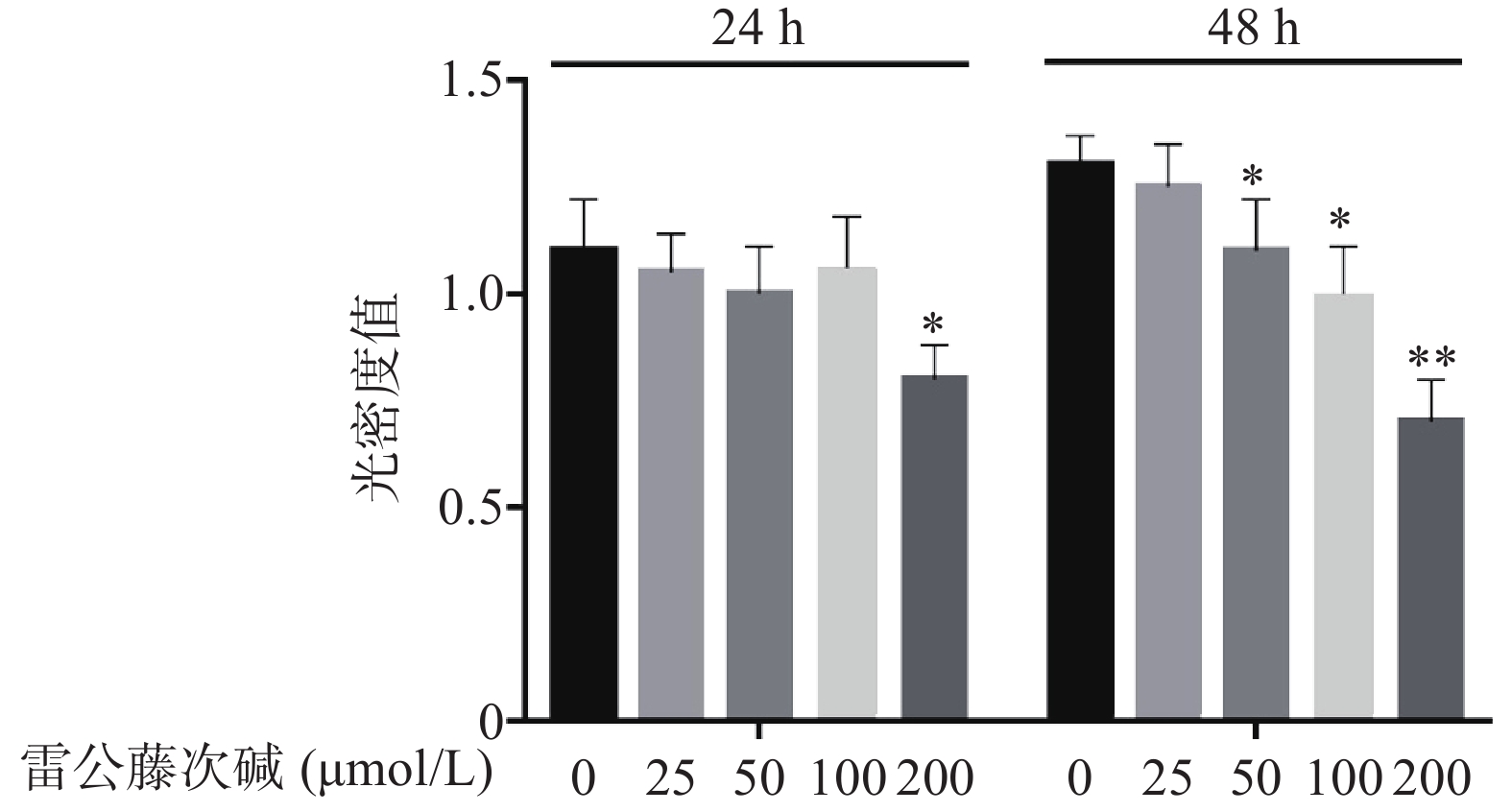
图1显示了不同浓度雷公藤次碱对RAW264.7细胞的细胞毒性作用。给予雷公藤次碱(25~200 μmol/L)孵育24 h后,200 μmol/L雷公藤次碱可显著抑制RAW264.7细胞活性;孵育48 h后,50、100和200 μmol/L雷公藤次碱均可显著抑制RAW264.7细胞的活性。
-
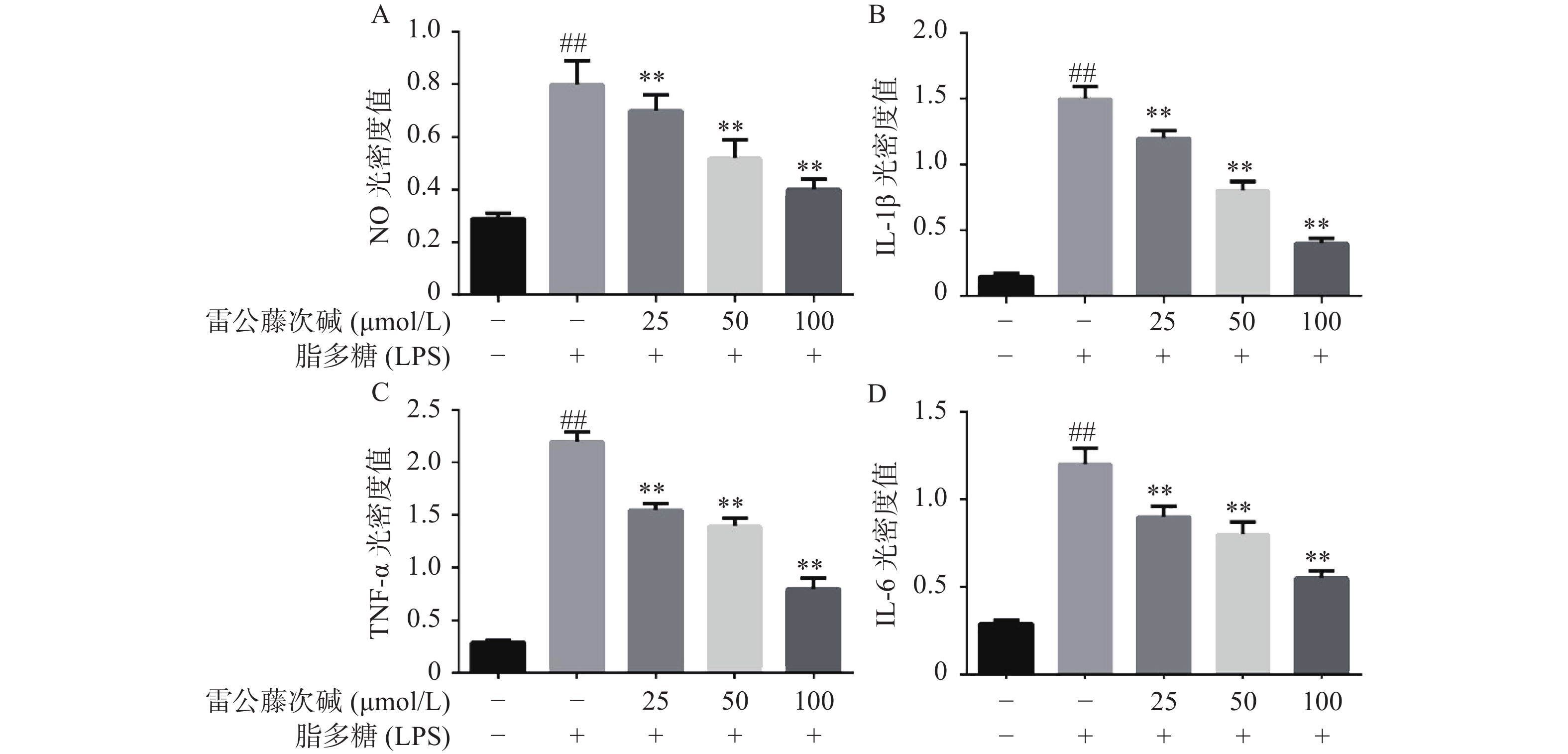
为了进一步研究雷公藤次碱体外抗炎活性,检测了雷公藤次碱对LPS刺激RAW264.7细胞分泌炎症因子的影响。结果显示(图2),给予LPS刺激后的RAW264.7细胞,NO、IL-1β、TNF-α和IL-6等促炎因子释放水平急剧增加(P<0.01),给予不同浓度雷公藤次碱(100、50和25 μmol/L)处理后,上述因子分泌水平呈剂量依赖性显著降低(P<0.01)。由此提示,雷公藤次碱的抗炎作用可能与抑制细胞释放NO、IL-1β、TNF-α和IL-6等促炎因子有关。
-
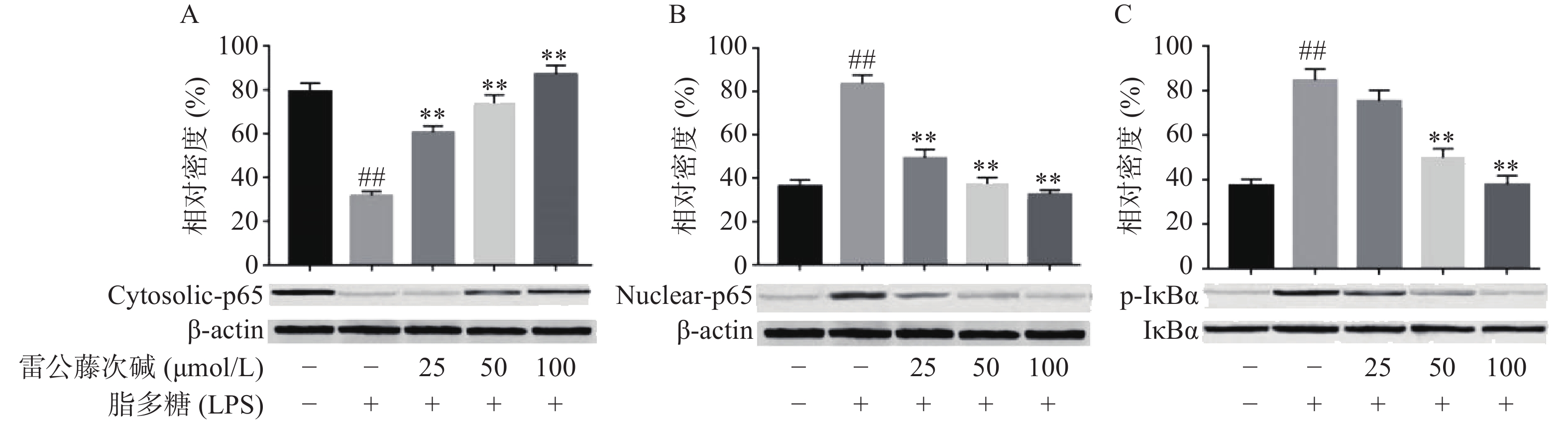
LPS是引发炎症的早期关键因素,可诱发多种细胞内信号事件,LPS-TLR4/MyD88信号通路可调节促炎基因表达,并控制炎症相关细胞因子的表达,其中IRAK及TRAF6是MyD88依赖性途径中的关键信号传导的分子。TLR4受体受LPS诱导后,可导致IRAK磷酸化从而脱离MyD88与TRAF6的结合体,使得游离的TRAF6活化后续信号转导途径[6]。为了评估雷公藤次碱对LPS激活TLR4/MyD88信号通路的影响,蛋白印迹法考察了雷公藤次碱对IRAK磷酸化和TRAF6表达的影响。结果显示(图3),与空白组相比,LPS刺激的模型组IRAK磷酸化和TRAF6表达明显增加(P<0.01);与模型组相比,雷公藤次碱各剂量组可显著抑制IRAK磷酸化和降低TRAF6表达(P<0.01)。
-
NF-κB是MyD88信号通路中重要的转录因子[7]。我们考察了雷公藤次碱对LPS诱导的RAW264.7细胞中NF-κB活化的潜在影响。如图4所示,RAW264.7细胞LPS刺激后,细胞核内NF-κB p65水平增高而细胞质内NF-κB p65水平降低,说明NF-κB p65活化后由细胞质进入到细胞核内,而给予雷公藤次碱处理后,细胞质内NF-κB p65水平增高而细胞核内NF-κB p65水平降低,说明雷公藤次碱以浓度依赖的方式显著地阻止了NF-κB p65从细胞质转运到细胞核。 NF-κB p65易位进入核内与TLR4途径中的IκBα磷酸化有关。因此,我们考察了雷公藤次碱对NF-κB抑制蛋白IκBα磷酸化的影响。结果表明,雷公藤次碱以浓度依赖的方式显著抑制LPS诱导的IκBα磷酸化和降解。
-
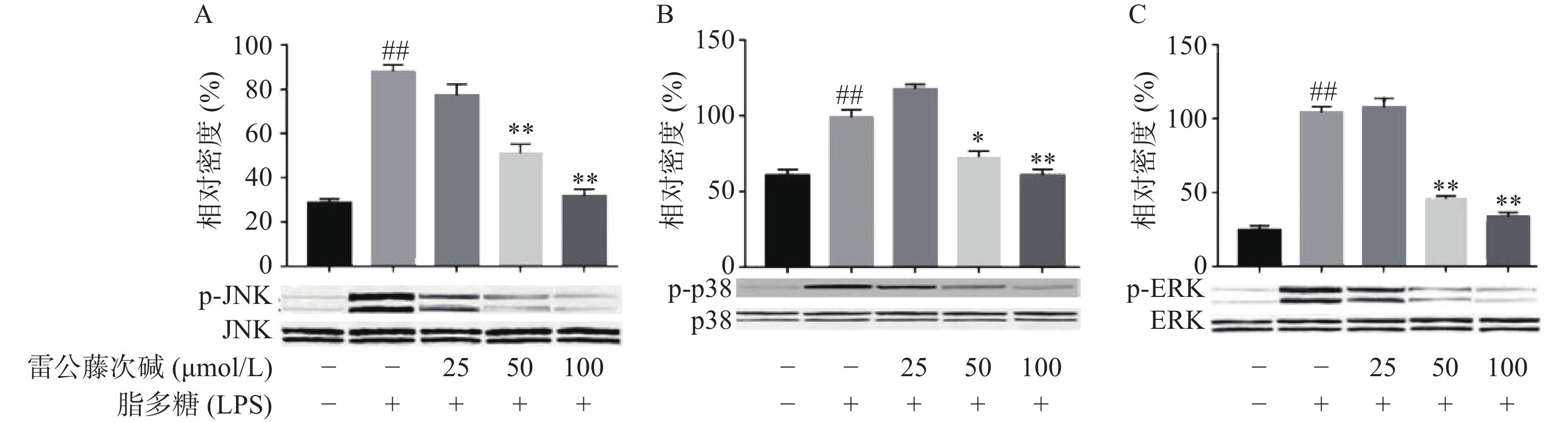
MAPKs是信号从细胞表面传导到细胞核内部的重要传递者。为了进一步研究雷公藤次碱是否调节MAPKs,考察了其对LPS诱导的RAW264.7细胞中ERK、p38和JNK等MAPKs磷酸化的影响。如图5所示,LPS暴露可显著促进RAW264.7细胞中ERK、JNK和p38的磷酸化。给予雷公藤次碱可以显著抑制LPS诱导的ERK、p38和JNK的磷酸化,但并未影响ERK、p38和JNK的表达。上述结果表明,MAPKs的磷酸化可能参与了雷公藤次碱对RAW264.7细胞中LPS刺激炎症的抑制作用。
-
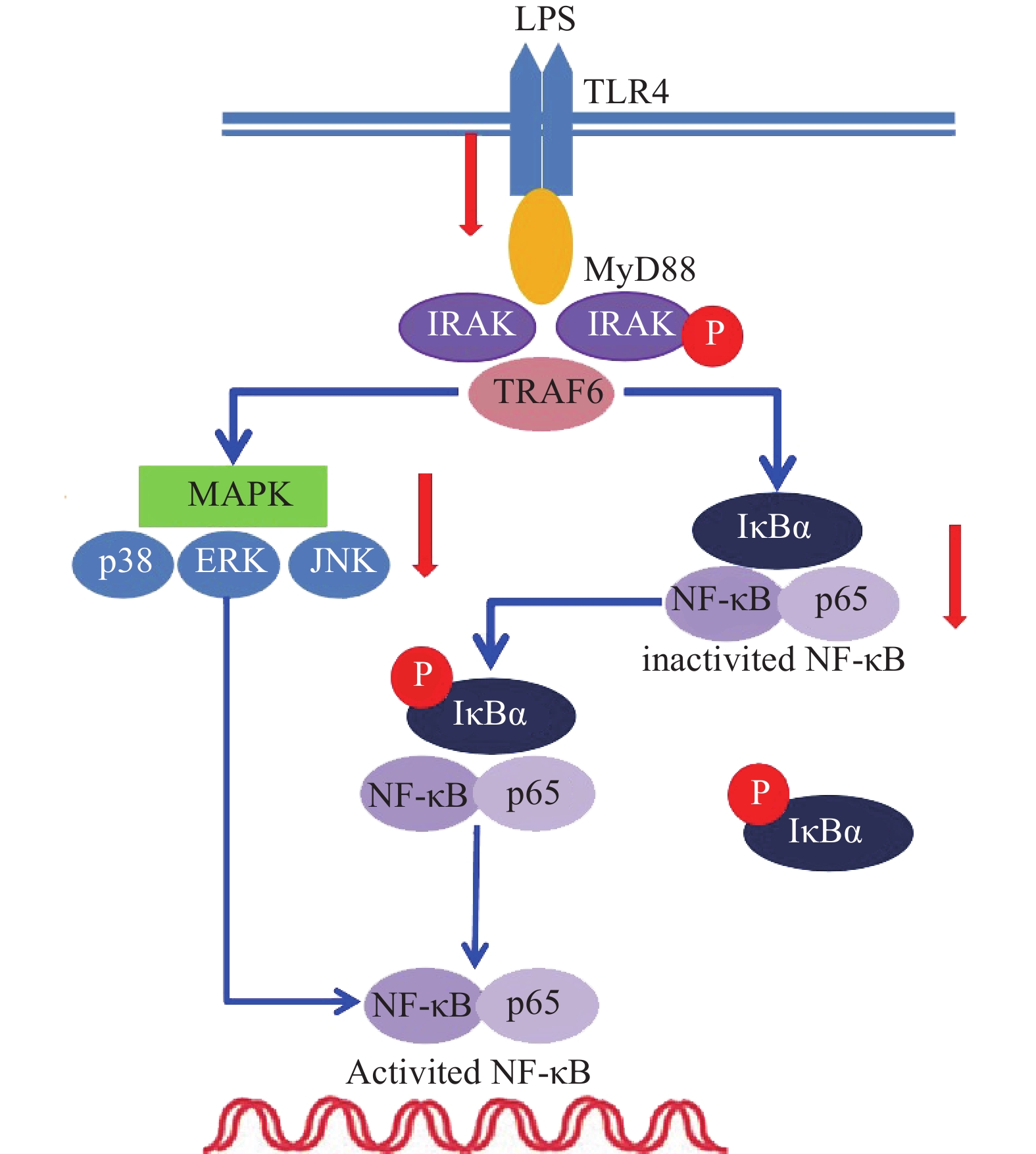
雷公藤次碱是雷公藤的代表性生物碱成分之一,但是自从被分离以来,其生物学活性除了杀虫外,其他方面了解甚少[8]。本研究显示,雷公藤次碱对LPS诱导的RAW264.7细胞炎症模型显示出显著的抗炎活性。雷公藤次碱可减少LPS刺激引起的NO、IL-1β、TNF-α及IL-6等促炎因子的产生,并抑制LPS诱导的TLR4/MyD88通路中信号传导的分子IRAK磷酸化及TRAF6表达,从而影响MAPKs和NF-κB的激活,如图6所示。
已有证据显示,LPS可引发多种细胞内信号事件,包括导致TLR4,NF-κB及p38,ERK和JNK等丝裂素活化蛋白激酶(MAPKs)途径激活[9-10]。研究发现,LPS诱导后RAW264.7细胞Toll样受体4(TLR4)的表达明显增高,说明LPS是或可引导TLR4的配体与TLR4结合,二者结合后可启动细胞内一系列信号级联反应,最终诱导目的基因的表达。TLRs亚型中除TLR3外,均需要髓样分化因子88(MyD88)来激活下游释放信号分子的通路,这种途径成为MyD88依赖性途径。静息状态下,MyD88与IRAK及TRAF6形成细胞信号转导复合物,受到诱导后导致IRAK磷酸化,IRAK磷酸化后TRAF6从信号转导复合物中解离出来,TRAF6的活化可引起两条不同的信号转导途径,一条为MAPK家族(包括p38、JNK),另一条为活化MP3K(mitogen activated protein kinase kinase kinase)家族成员NIK(NF-κB inducing kinase),后者的磷酸化激活IκB激酶,导致IκB的磷酸化而使NF-κB/IκB复合物解离,NF-κB由此活化转位进入细胞核[11]。在本研究中,数据显示雷公藤次碱可以抑制IRAK磷酸化和TRAF6的表达。
NF-κB是一种多效性的转录因子,参与调控炎症反应、免疫、肿瘤等相关的细胞因子、趋化因子、黏附分子、炎症介质等的转录过程。IκB是其抑制蛋白,正常情况下二者结合而使NF-κB处于失活状态,IκBα是IκB的亚分子,受到LPS刺激,IκBα磷酸化增加,从而导致游离NF-κB p65释放并从细胞质转移至细胞核,与DNA上的相应位点结合,导致特定靶基因(如TNF-α、IL-1β和IL-6)的转录表达[12-13]。在本研究中,数据显示雷公藤次碱可抑制IκBα磷酸化,并以浓度依赖的方式阻止NF-κB p65由细胞质进入细胞核,从而阻止炎症相关因子的转录表达。
此外,NF-κB的易位也受ERK、p38和JNK等丝裂素活化蛋白激酶途径调节,该途径可控制炎症反应过程中细胞因子的合成和释放。丝裂素活化蛋白激酶激活后,细胞质或细胞核中的转录因子又被激活,从而触发引起生物学反应的靶基因的表达,包括促炎性介质的表达。本研究显示,LPS诱导的RAW264.7细胞中,雷公藤次碱可显著抑制ERK、JNK和p38的磷酸化。
Wilforine inhibits LPS-induced inflammatory response in RAW264.7 cells by regulating the TLR4/MyD88/TRAF6 signaling pathway
-
摘要:
目的 探讨雷公藤次碱抗炎活性及其作用机制。 方法 用细胞计数盒-8 (CCK-8)法考察雷公藤次碱对小鼠单核巨噬细胞白血病细胞RAW 264.7 增殖活性的影响,用酶联免疫吸附法(ELISA)检测雷公藤次碱对脂多糖(LPS)诱导的RAW264.7细胞分泌细胞因子一氧化氮(NO)、白细胞介素-1β(IL-1β)、 肿瘤坏死因子α(TNF-α)和白细胞介素6(IL-6)的影响,用免疫印迹法考察雷公藤次碱对白介素受体相关激酶(IRAK)、肿瘤坏死因子受体相关蛋白6(TRAF6)、核因子κB抑制因子α(IκBα)、核因子κB(NF-κB p65)、分裂原激活的蛋白激酶p38(p38)、c-Jun氨基末端激酶(JNK)、细胞外调节蛋白激酶(ERK)在LPS刺激的RAW264.7细胞中的表达及其磷酸化的影响。 结果 雷公藤次碱在25、50、100 μmol/L浓度下对RAW264.7细胞无显著毒性,并可显著抑制细胞因子NO、IL-1β、TNF-α和IL-6含量。免疫印迹法检测结果显示雷公藤次碱可显著抑制IRAK及TRAF6的表达;显著抑制ERK、P38和JNK的磷酸化;抑制IκBα的降解,降低NF-κB p65的核转运水平。 结论 雷公藤次碱具有体外抗炎活性,其作用机制可能介导TLR4/MyD88/TRAF6信号通路。 Abstract:Objective To investigate the anti-inflammatory effect and mechanism of wilforine. Methods Anti-inflammatory activity of wilforine was investigated in LPS-induced RAW264.7 cells. The cytokines production of RAW264.7 cells was analyzed by ELISA assay and the cell viability was assessed by CCK-8 method. The expression of TRAF6, the phosphorylation of IRAK, p38, ERK and JNK, the degradation of inhibitory κBα (IκBα) and the nuclear translocation of NF-κB p65 were further investigated by western blot. Results Triptolide had no significant toxicity to RAW264.7 cells at concentrations of 25, 50 and 100 μmol/L. and could significantly inhibit the contents of cytokines NO, IL-1β, TNF-α and IL-6. Wilforine significantly decreased the expression of TRAF6 and phosphorylation of IRAK, and inhibited the phosphorylation of ERK, p38, and JNK and degradation of IκBα, and reduced the level of nuclear translocation of NF-κB p65. Conclusion The anti-inflammatory activity of wilforine of LPS-induced RAW264.7 cells is probably via TLR4/MyD88/TRAF6 signaling pathway. -
Key words:
- Tripterygium wilfordii /
- wilforine /
- anti-inflammation /
- TLR4 /
- MyD88 /
- NF-κB
-
水合氯醛,又称水合三氯乙醛,具有镇静、催眠、抗惊厥等作用,临床上以溶液口服或灌肠方式给药。但水合氯醛稳定性较差,临床使用制成溶液制剂有效期很短,其主要的杂质有三氯甲烷、甲酸、氯乙酸等,其中三氯甲烷是原料生产与储存过程中比较重要的降解杂质[1]。国内较多数的文献对水合氯醛及其制剂的稳定性研究采用滴定法或紫外法[2-3],此方法准确度差,专属性不足,不能准确评估水合氯醛及其制剂的质量状况。目前,《中国药典》以及国外各国药典收载的水合氯醛及其制剂的质量标准中有关的检查方法专属性均较低[4-7]。
各国药典中鉴别、酸度、炽灼残渣与溶液澄清度是水合氯醛药物的基本理化分析项,醇合三氯乙醛则为控制本品合成过程中产生的一种杂质。水合氯醛在生产、运输过程中可能产生的降解杂质有甲酸、三氯甲烷、氯乙酸、二氯乙酸、三氯乙酸等,现有的法定标准对此部分降解产物未进行控制,有关物质项存在缺失。本文旨在对主要降解产物进行定量检测,如甲酸、三氯甲烷、氯乙酸、二氯乙酸、三氯乙酸进行检测方法开发,其中,水合氯醛通过碳碳键断裂降解成等比例甲酸、三氯甲烷,检测甲酸或检测三氯甲烷均可。
三氯甲烷主要采用气相、气质色谱法[8-10]进行检查,氯乙酸等卤代羧酸可采用液相色谱法和离子色谱法[11-13]分析,水合氯醛专属性较强的检测方法为气相色谱法[10-14]。因卤代羧酸具有沸点高、强极性特点,不易气化,无法直接进样进行气相色谱分析,需进行衍生化处理,故本文将卤代羧酸检测方法与三氯甲烷检测方法分别开发建立。
1. 实验部分
1.1 仪器与试剂
Thermo trace 1300 GC(赛默飞气相色谱仪);电子天平 ME-204E(梅特勒科技);三氯甲烷 (北京通广试剂公司);氯乙酸、二氯乙酸、三氯乙酸(标准品,CATO);正庚烷(fisher);甲醇、硫酸、硫酸钠;水合氯醛(青岛宇龙海藻药业,批号20190403)。
1.2 色谱条件
色谱柱温:40 ℃保持3 min;以5 ℃/min的速度升至80 ℃,以20 ℃/min升至160 ℃,再以30 ℃/min升至250 ℃;进样口温度:150 ℃;检测器:ECD,温度280 ℃;色谱柱:DB-5.625 30 m×0.25 mm,0.25 μm;进样体积1 μl。
1.3 供试品与对照品溶液配制
分别确定水合氯醛检测三氯甲烷和卤代羧酸的样品处理方法,详见表1。
表 1 三氯甲烷与卤代羧酸检测样品前处理方法溶液名称 前处理1 前处理2 供试品溶液(测定卤代羧酸) / 称取水合氯醛约10 mg,置50 ml具塞试管中,精密加入甲醇2 ml,20%的硫酸乙醇溶液2 ml(V/V),无水硫酸钠0.1 g,密塞,漩涡使溶解,置50 ℃水浴加热40 min,取出后迅速冲凉,精密加入4 ml正庚烷,漩涡混匀,加入
20 ml水,振摇,静置20 min,分层,取上清液即得。卤代羧酸对照品溶液 / 分别称取氯乙酸、二氯乙酸、三氯乙酸适量,分别用甲醇配制成浓度为100 μg/ml的储备液,取1 ml置10 ml量瓶中,用甲醇定容至刻度即得;取上述溶液各1 ml,置50 ml具塞试管中,精密加入1 ml甲醇,20%的硫酸乙醇溶液2 ml(V/V),无水硫酸钠0.1 g,密塞,漩涡使溶解,置50 ℃水浴加热
40 min,取出后迅速冲凉,精密加入4 ml正庚烷,漩涡混匀,加入20 ml水,振摇,静置20 min,分层,取上清液即得各对照品溶液。供试品溶液(测定三氯甲烷) 称取水合氯醛约10 mg,置10 ml具塞试管中,加甲醇溶液完全,定容后过滤取续滤液即得。 称取水合氯醛约100 mg,置10 ml具塞试管中,加入饱和氯化钠溶液
0.35 ml,氯化钠0.5 g,正己烷5 ml,密塞,振摇提取,静置20 min,取正己烷层,取续滤液即得。三氯甲烷对照品溶液 称取三氯甲烷适量,甲醇溶解至
60 μg/ml即得。称取三氯甲烷适量,用正己烷配置成浓度为20 μg/ml的储备液;取1 ml置10 ml量瓶中,用正己烷定容至刻度即得。 2. 结果与讨论
2.1 卤代羧酸检测分析方法验证
2.1.1 系统适用性试验
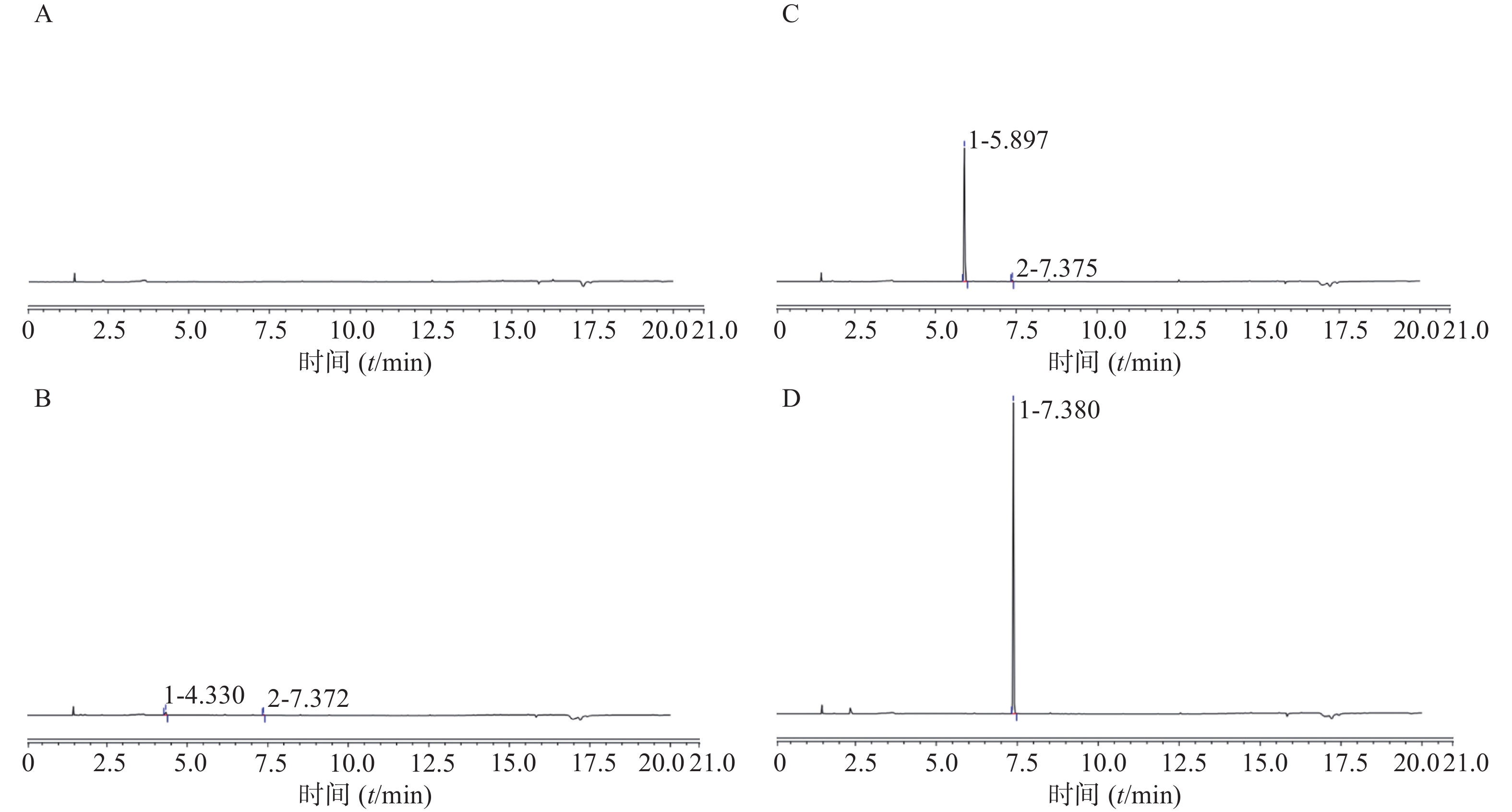
按照图1方法,配制用于氯乙酸、二氯乙酸、三氯乙酸检测的对照品溶液,并按照“1.2色谱条件”进样检测,结果如下。
对照品溶液浓度分别为氯乙酸 9.95 μg/ml,二氯乙酸 13.49 μg/ml,三氯乙酸9.45 μg/ml,连续进样5针,氯乙酸、二氯乙酸、三氯乙酸的保留时间分别为4.33、5.89和7.38 min,各峰面积RSD分别为0.75%、1.00%、1.00%,满足系统适用性要求。衍生化转化目标物更容易被气化,氯乙酸、二氯乙酸、三氯乙酸经衍生化后分别以氯乙酸乙酯、二氯乙酸乙酯、三氯乙酸乙酯形式被检测。
2.1.2 线性关系考察
分别取氯乙酸、二氯乙酸、三氯乙酸对照品适量,加甲醇溶解稀释制成浓度为10 μg/ml的储备液,分别取储备液适量置具塞试管中,按照表1衍生化处理后,取正庚烷层,即得各自的工作曲线样品溶液,按照“1.2色谱条件”进样检测,以峰面积为纵坐标,相应卤代羧酸浓度为横坐标,进行线性回归。结果见表2,并按照信噪比大于3和大于10分别规定检测限和定量限。
表 2 氯乙酸、二氯乙酸、三氯乙酸线性试验结果样品名称 线性方程 回归系数 r 线性范围(μg/ml) 检测限(μg/ml) 定量限(μg/ml) 氯乙酸 Y=0.0042X+0.0002 0.9997 0.25~5 0.025 0.05 二氯乙酸 Y=0.1106X+0.005 0.9998 0.125~2.5 0.05 0.125 三氯乙酸 Y=0.32X+0.0164 0.9998 0.0625~2.5 0.01 0.0625 将上述信噪比满足定量限的各对照品溶液连续进样5针,氯乙酸、二氯乙酸、三氯乙酸峰面积RSD为别为5.27%、1.94%、1.15%,显示各目标物的定量限浓度下检测精密度满足要求(RSD<15%)。
2.1.3 重复性试验
按表1前处理方法进行配制供试品溶液和对照品溶液,平行取6份供试品溶液,连续采集,记录并计算各供试品溶液中氯乙酸、二氯乙酸、三氯乙酸的含量,氯乙酸均未检出,二氯乙酸含量RSD为4.43%,三氯乙酸含量结果RSD为4.56%,按照本方法进行卤代羧酸含量检测,含量结果RSD均小于6%。 按照《中国药典》2020年版“通则9101分析方法验证指导原则”要求[15],本供试品中卤代羧酸的含量(10 ppm)重复性要求RSD小于6%。本方法重复性满足要求。
2.1.4 准确度试验
采用加样回收率进行方法准确度考察,精密称取10 mg水合氯醛原料药10份,分别置10个50 ml锥形瓶中,编号1~3加入混合对照品溶液1.2 ml,编号4~6加入混合对照品溶液1 ml,编号7~9加入混合对照品溶液0.8 ml,上述混合对照品溶液中,氯乙酸浓度为 9.95 μg/ml,二氯乙酸 浓度为13.49 μg/ml,三氯乙酸浓度为9.45 μg/ml,第10份不加混合对照品溶液,同步取样混合对照品1 ml置50 ml锥形瓶中,得到11份样品,按照表2衍生化处理,取正庚烷层进样检测,结果如表3。
表 3 氯乙酸、二氯乙酸、三氯乙酸准确度试验结果编号 原有量(m/mg) 加入量(m/μg) 测得量(m/μg) 回收率(%) 氯乙酸 二氯乙酸 三氯乙酸 氯乙酸 二氯乙酸 三氯乙酸 氯乙酸 二氯乙酸 三氯乙酸 1 10.23 3.00 3.00 3.00 3.14 2.88 3.11 104.5 95.45 95.82 2 10.17 3.00 3.00 3.00 2.72 2.85 2.99 90.64 94.35 91.75 3 9.97 3.00 3.00 3.00 2.61 2.70 3.35 86.99 89.44 103.7 4 10.00 2.50 2.50 2.50 2.46 2.38 2.51 98.25 94.44 91.13 5 9.95 2.50 2.50 2.50 2.13 2.44 2.50 85.09 96.70 90.44 6 10.08 2.50 2.50 2.50 2.52 2.35 2.48 100.9 93.22 89.86 7 10.11 2.00 2.00 2.00 1.71 1.89 2.03 85.53 93.40 89.49 8 9.88 2.00 2.00 2.00 1.82 1.86 2.05 91.01 91.88 90.83 9 10.01 2.00 2.00 2.00 1.78 1.88 2.09 88.82 92.87 92.85 按照本方法进行卤代羧酸含量检测准确度试验,回收率结果符合相关要求。 按照《中国药典》2020年版“通则9101分析方法验证指导原则”要求,本供试品中卤代羧酸的含量(10 ppm)回收率限度要求为80%~115%。本方法准确度满足要求。
2.1.5 稳定性试验
取“2.1.1”项下的各对照品溶液,分别于24 h内不同时间进样检测,结果显示氯乙酸、二氯乙酸、三氯乙酸峰面积RSD分别为4.0%、2.1%、1.6%,表明本方法样品溶液24 h内稳定性良好。
2.2 三氯甲烷检测方法学验证
2.2.1 专属性考察
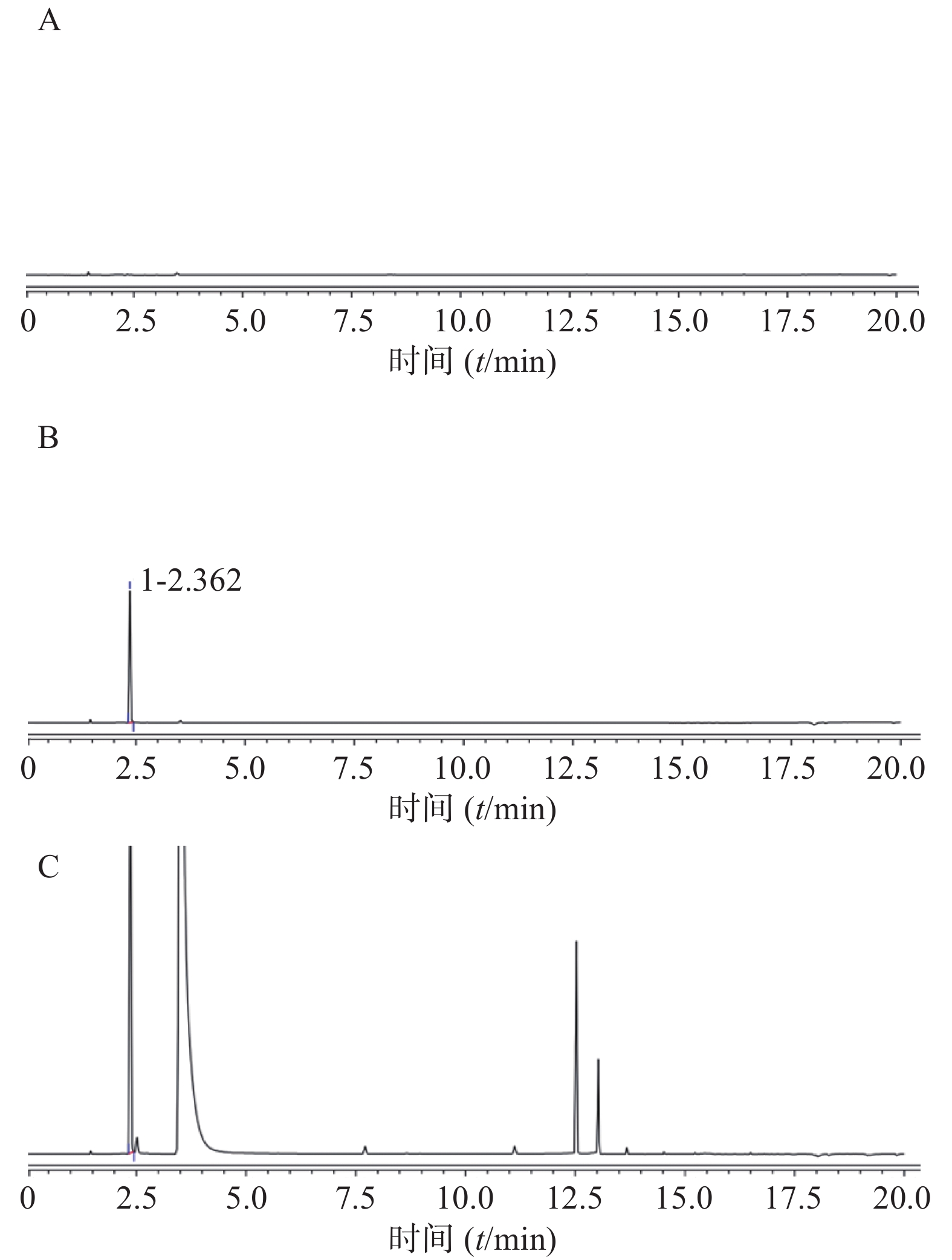
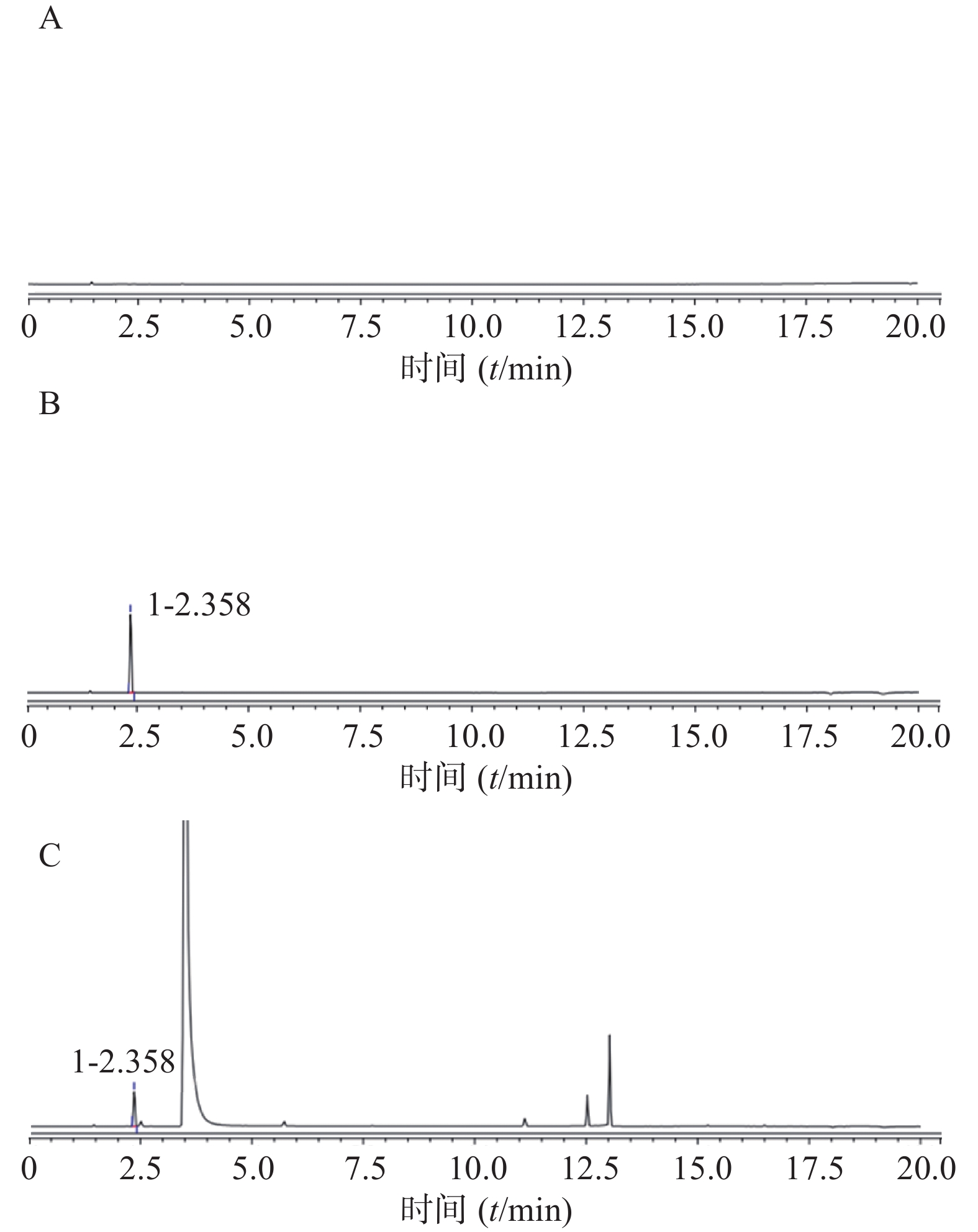
分别按照表1中两种前处理方式配制对照品溶液、供试品溶液。前处理1测定色谱图见图2,前处理2测定色谱图见图3。在上述色谱条件下,两种前处理使用的正己烷和甲醇均不干扰三氯甲烷的测定,三氯甲烷保留时间约为2.5 min。
2.2.2 重复性与系统适用性试验
分别按照前处理1和前处理2方法制备6份供试品;另取三氯甲烷对照品适量,分别用甲醇溶解稀释至60 μg/ml;用正己烷溶解稀释至2 μg/ml。将以上供试品与对照品,按照“1.2 色谱条件”进样检测,结果见表4。
表 4 三氯甲烷检测方法重复性试验样品名称 前处理方法1 前处理方法2 峰面积 含量(%) 峰面积 含量(%) 对照品溶液 1.5129 / 0.2863 / 供试品1 0.7824 3.19 0.0608 0.00212 供试品2 1.4486 5.91 0.0616 0.00215 供试品3 1.6091 6.56 0.0596 0.00208 供试品4 0.6147 2.51 0.0582 0.00203 供试品5 0.8345 3.40 0.0653 0.00228 供试品6 1.0321 4.21 0.0679 0.00237 RSD(%) 37.46 5.89 因水合氯醛对热不稳定,熔点低57 ℃,在高温条件下碳碳键断裂产生三氯甲烷和甲酸,直接进样或顶空进样均可导致水合氯醛降解而使检测结果偏高,不能反映其实际三氯甲烷含量。而且依据ICH Q3C(R6)残留溶剂指导原则,三氯甲烷属于药品生产中应限制使用的溶剂(二类溶剂),其限度要求是60 ppm(即残留上限0.006%),结合本品原料药检测结果与重复性结果,前处理方法2检测结果准确真实。
连续进样5针正己烷配制的三氯甲烷对照品溶液,峰面积RSD为2.45%,表明本法的系统适用性试验结果良好。
2.2.3 线性与范围
精密称取三氯甲烷对照品,用正己烷溶解稀释至20 μg/ml,分别取其中0.2、0.4、0.8、1.0、1.5 ml置10 ml量瓶,用正己烷稀释至刻度即得工作曲线样品,按照“1.2 色谱条件”进样,以峰面积为纵坐标,相应浓度为横坐标进行线性回归,得线性方程Y=0.1094X+0.0222, r =0.9998,表明三氯甲烷在浓度0.2408~3.011 μg/ml范围内线性良好。将三氯甲烷对照品溶液逐级稀释,得0.02、0.001 μg/ml进样分析,信噪比分别为14和4,分别为定量限和检测限。
2.2.4 三氯甲烷检测准确度试验
取水合氯醛原料药,按表2中前处理方法2配制,平行9份,编号1~3加入0.6 ml对照品溶液,编号4~6加入对照品储备液0.5 ml,编号7~9加入对照品储备液0.6 ml,分别置10 ml离心管中,加入饱和氯化钠0.5 ml,氯化钠0.5 g,4.4 ml正己烷,振摇溶解,静置30 min,分别取正己烷层进样检测,结果见表5。
表 5 三氯甲烷检测准确度试验样品编号 加入量(m/μg) 检测值(m/μg) 本底值(m/μg) 回收率(%) 供试品1 1.2042 2.30 1.19 91.53 供试品2 1.2042 2.18 1.19 81.70 供试品3 1.2042 2.39 1.19 99.71 供试品4 10.79 11.77 1.81 92.32 供试品5 10.79 11.60 1.81 90.71 供试品6 10.79 11.79 1.81 92.46 供试品7 12.948 13.25 1.81 88.29 供试品8 12.948 12.81 1.81 84.94 供试品9 12.948 14.04 1.81 94.41 按照本方法进行三氯甲烷含量检测的准确度试验,回收率符合《中国药典》相关要求。
2.2.5 三氯甲烷检测溶液稳定性
取三氯甲烷对照品、水合氯醛原料药,按表2中前处理方法2进行配制对照品溶液、供试品溶液,按“1.2色谱条件”进样检测,考察24 h内不同时间样品的稳定性,结果显示供试品与对照品稳定性均小于5.0%,稳定性良好。
2.3 水合氯醛稳定性试验
水合氯醛具有较强的挥发性与引湿性,在高温条件下挥发与降解性能均增强,结合《中国药典》2020年版药品稳定性试验技术要求,对本品进行影响因素试验与加速试验考察。将水合氯醛置样品瓶(封口)中,分别置温度25 ℃/湿度75%,温度40 ℃/湿度75%,温度60 ℃/湿度60%以及温度25 ℃/湿度60%、光照强度4500 lx条件下考察,并于不同时间点检测杂质含量变化,结果见表6。
表 6 水合氯醛稳定性试验结果放置条件 时间(t/d) 检测结果(%) 三氯乙酸 二氯乙酸 三氯甲烷 氯乙酸 温度25 ℃/湿度75% 10 0.001 0.003 0.004 未检出 30 0.001 0.003 0.004 未检出 温度40 ℃/湿度75% 10 0.001 0.003 0.152 未检出 30 0.001 0.003 0.323 未检出 温度60 ℃/湿度60% 10 0.001 0.004 0.546 未检出 30 0.001 0.004 0.871 未检出 温度25 ℃/湿度60%、
光照强度4500 lx10 0.001 0.003 0.045 未检出 30 0.001 0.003 0.151 未检出 以上数据显示,水合氯醛原料受温度和光照影响较大,主要的降解产物为碳碳键断裂的水解产物三氯甲烷,卤代羧酸未有明显的改变。三氯甲烷是主要的降解产物,其中三氯甲烷为二类毒性溶剂,但目前水合氯醛的国内外法定标准中均无三氯甲烷的含量检测和限度要求,且水合氯醛本身具有较大的毒性,则其降解产物的限度仍需结合长期稳定性结果和临床使用的安全性、有效性进行综合评估。另本次试验采用称量瓶封口存放,密封性不佳,会加速水合氯醛的挥发水解,本品应室温避光密封保存;在制剂研发和存储运输过程中,应严格控制温度与光照影响。
3. 结论
本文建立了气相色谱法测定水合氯醛原料药中杂质三氯甲烷、氯乙酸、二氯乙酸、三氯乙酸。利用检测目标物的理化性质,采用不同前处理方法进行三氯甲烷、卤代羧酸的检测,色谱峰形良好,方法简单、准确度高、重复性良好,可避免水合氯醛检测过程中的自身降解,准确高效的检测药物中杂质含量,满足目前市售原料及其制剂的检测需求。
本法中,三氯甲烷检测限浓度为供试品浓度的万分之0.005(0.5 ppm);氯乙酸检测限浓度为供试品浓度的万分之0.1(10 ppm);二氯乙酸检测限浓度为供试品浓度的万分之0.2(20 ppm);三氯乙酸检测限浓度为供试品浓度的万分之0.04(4 ppm)。本品多次检测以及稳定性试验中均未出现氯乙酸,说明本品中确无高于检出限的残留,也不易降解产生。
通过水合氯醛的稳定性实验发现,本品在温度、光照条件下均会发生降解,三氯甲烷为主要的降解产物,故进行本品相关制剂的研发、检测、生产和运输过程中需要严格控制生产工艺与储存条件,保证产品的质量稳定,保障用药安全性。
-
-
[1] 白雪, 付瑞嘉, 乐世俊, 等. 雷公藤治疗类风湿性关节炎研究进展[J]. 中草药, 2020, 51(1):265-275. doi: 10.7501/j.issn.0253-2670.2020.01.034 [2] 姚骥如, 孙莹, 罗顺葵, 等. 雷公藤多苷的临床应用进展[J]. 中国新药与临床杂志, 2010, 29(3):179-182. [3] 胡攀勇, 李振麟, 濮社班, 等. 雷公藤研究进展[J]. 中国野生植物资源, 2013, 32(2):1-3,7. doi: 10.3969/j.issn.1006-9690.2013.02.001 [4] ZHANG Y Q, XU W, LI H, et al. Therapeutic effects of total alkaloids of Tripterygium wilfordii Hook f. on collagen-induced arthritis in rats[J]. J Ethnopharmacol,2013,145(3):699-705. doi: 10.1016/j.jep.2012.11.018 [5] 刘莉, 闫君, 舒积成, 等. 雷公藤生物碱类成分及其药理活性研究进展[J]. 天然产物研究与开发, 2019, 31(12):2170-2181. doi: 10.16333/j.1001-6880.2019.12.022 [6] LI T, LI F, LIU X Y, et al. Synergistic anti-inflammatory effects of quercetin and catechin via inhibiting activation of TLR4-MyD88-mediated NF-κB and MAPK signaling pathways[J]. Phytother Res,2019,33(3):756-767. doi: 10.1002/ptr.6268 [7] 鲍璐璐, 崔立红. TLR4/MyD88/NF-κB信号通路的研究进展[J]. 胃肠病学和肝病学杂志, 2019, 28(5):568-572. doi: 10.3969/j.issn.1006-5709.2019.05.019 [8] 师宝君, 姬志勤, 张继文, 等. 昆明山海棠的杀虫活性及有效成分[J]. 昆虫学报, 2007, 50(8):795-800. doi: 10.3321/j.issn:0454-6296.2007.08.007 [9] LAI J L, LIU Y H, LIU C, et al. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways[J]. Inflammation,2017,40(1):1-12. doi: 10.1007/s10753-016-0447-7 [10] LIU F, SUN G Q, GAO H Y, et al. Angelicin regulates LPS-induced inflammation via inhibiting MAPK/NF-κB pathways[J]. J Surg Res,2013,185(1):300-309. doi: 10.1016/j.jss.2013.05.083 [11] 单佳铃, 程虹毓, 文乐, 等. TLR/MyD 88/NF-κB信号通路参与不同疾病作用机制研究进展[J]. 中国药理学通报, 2019, 35(4):451-455. doi: 10.3969/j.issn.1001-1978.2019.04.002 [12] 崔嵩, 刘学锋, 吴斌. NF-кB在肿瘤中的研究进展[J]. 现代肿瘤医学, 2009, 17(1): 134-137. 范慧婕, 谭章斌, 赵晓山, 等. 四逆散通过MAPKs/NF-κB途径保护脂多糖致Raw264.7的细胞炎症[J]. 暨南大学学报(自然科学与医学版), 2019, 40(1): 10-18. [13] KAMINSKA B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy: from molecular mechanisms to therapeutic benefits[J]. Biochim Biophys Acta,2005,1754(1-2):253-262. doi: 10.1016/j.bbapap.2005.08.017 -





 下载:
下载:
 下载:
下载: