-
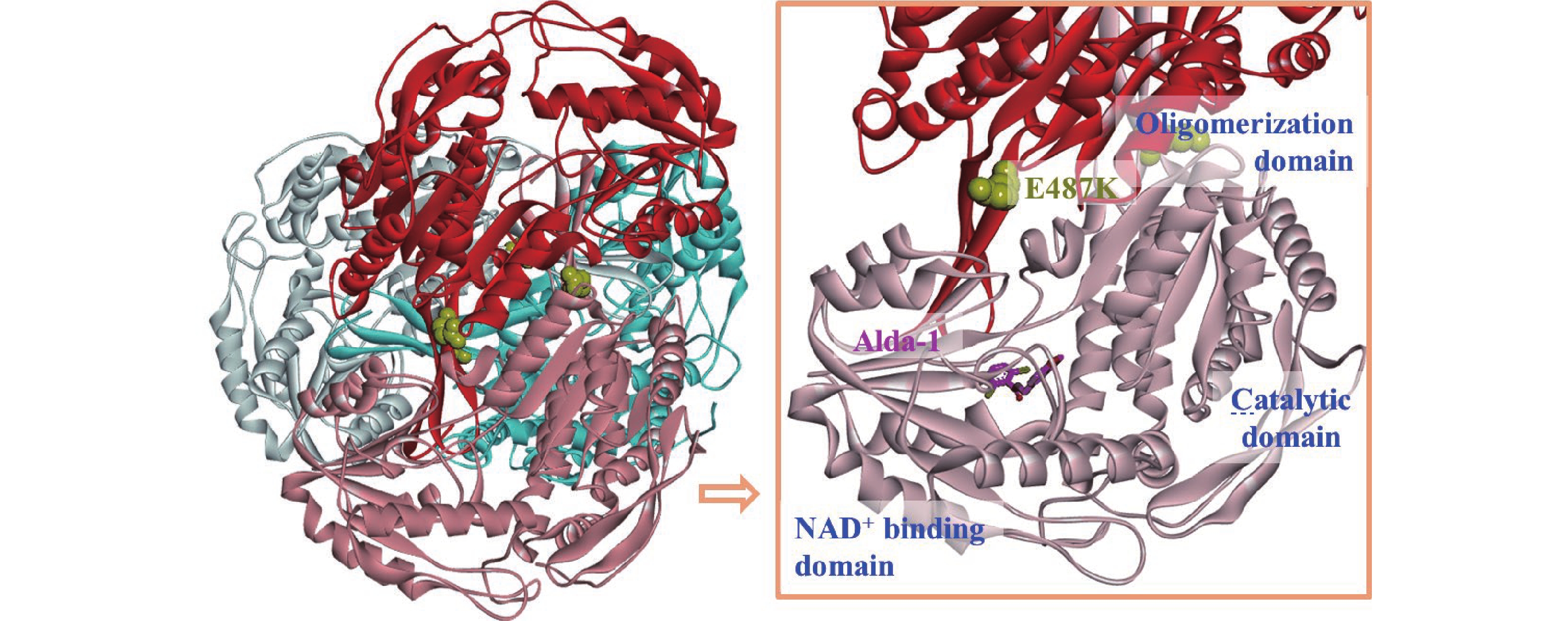
醛脱氢酶(ALDH)是I相反应中非常重要的氧化酶超家族之一,由一组NAD+依赖性酶组成,主要作用是不可逆的催化内源性和外源性醛,避免醛在人体内蓄积中毒[1]。在哺乳动物组织中均存在ALDH,其中肝脏表达水平最高,其次是肾脏、子宫和大脑,目前在人体内发现了19种不同的ALDH[2]。ALDH2是已知的19种ALDH中分布最广泛和表达最高的同工酶,是一种具有相同亚基的四聚体蛋白(图1),由位于12号染色体长臂(12q 24.2)上的ALDH2基因编码而成的517个氨基酸组成。其亚基均是由3个结构域组成:催化结构域、NAD+结合结构域和寡聚结构域[3](图1)。
ALDH2是对体内外活性醛代谢的最重要的一种酶,因其对酒精代谢解毒而闻名[4]。ALDH2代谢的活性醛主要有乙醛、丙烯醛、3,4-二羟基苯乙醛(DOPAL)、丙二醛(MDA)和4-羟基壬烯醛(4-HNE)等[5, 6]。其中很重要的是4-HNE,它是由人体脂质过氧化反应时活性氧(ROS)攻击双分子层细胞膜上的多不饱和脂肪酸而生成的有毒醛类物质,是目前研究最多的生物活性醛类物质之一[7, 8]。脂质过氧化以及它产生的4-HNE与许多疾病的发生有关,ALDH2能有效清除这些醛,因此是人体内重要的抗氧化应激损伤因子之一,越来越多被关注和研究。
ALDH2基因具有多态性,易发生基因突变,其点突变命名为ALDH2*2,是人类最常见的基因突变之一。全球估计有5.6亿人(约占世界人口的8%)都携带这种基因,尤其在东南亚人群中约有35%~45%为携带者。携带者分布中心位于我国南部地区,特别是我国长汀县约有65%的人口携带这种突变基因[9]。造成这种基因突变的原因是ALDH2基因在12外显子42421碱基上腺嘌呤取代鸟嘌呤,从而在转录翻译成ALDH2时其487位点的谷氨酸被赖氨酸(Glu 487 Lys)取代(图1),从而导致ALDH2结构不稳定以至活性降低。通常,ALDH2野生型和变异型等位基因有3种类型:野生型纯合子(ALDH2*1/*1)、杂合子(ALDH2*1/*2)和变异型纯合子(ALDH2*2/*2)。由于突变对编码ALDH2*1等位基因的野生型单体产生显性影响,所以凡是携带这种突变基因的人ALDH2活性均有所下降,ALDH2*1/*2相比于ALDH2*1/*1活性降低约50%,而ALDH2*2/*2活性几乎完全丧失[10]。
-
ALDH2是人体内重要的抗氧化应激损伤因子,突变基因携带者会增加与活性氧介导的氧化应激损伤相关疾病的风险。氧化应激损伤将导致脂质过氧化,这一过程会产生毒性醛,引发细胞稳态受损、酶失活、DNA损伤和细胞死亡,从而导致或加剧疾病的发生。研究不断发现与ALDH2密切相关的氧化应激损伤相关疾病,如心血管疾病、神经退行性疾病、肝脏疾病、癌症、糖尿病、范可尼贫血、骨质疏松症、疼痛等[3],下面将重点介绍最常见的几类疾病的相关研究。
-
心血管疾病是全球发病率以及死亡率最高的疾病,这其中主要包括心肌梗死、心脏肥厚和心力衰竭。研究发现,这些疾病的发生均与ROS诱导的应激损伤有关,ROS能使生物膜中的多不饱和脂肪酸过氧化,产生活性醛,从而影响人心肌的正常功能[11-13]。研究表明,心肌缺血再灌注损伤(IRI)与急性缺血性脑卒中(AIS)均与氧化应激产生过度4-HNE有关,而ALDH2是醛代谢解毒主要依赖,所以ALDH2介导的活性醛解毒是一种缺血再灌注损伤的内源性保护机制[14]。
近期研究发现,ALDH2还与心律失常相关。心房颤动(AF)是最常见的心律失常,其特征是过快的心房激活,不同步的心房收缩和不规则的心室率[15]。研究发现,ALDH2在 AF相关氧化应激反应中发挥心脏保护作用,同时ALDH2活性降低将导致AF的阈值水平低下,导致AF易感性增加。ROS诱导脂质过氧化产生活性醛,同时活性醛反过来会触发更高的ROS水平,ROS与醛类物质均可能导致心律失常。过量的ROS产生主要离子效应、肌细胞电偶联和异常分子机制的影响而与房颤相关,而活性醛会导致心肌细胞内ATP浓度严重下降,并引起与心律失常发展有关的电生理变化,也能显著抑制大鼠心室肌细胞内向整流钾电流(IK1)从而触发AF[16]。ALDH2与心律失常有着紧密联系,由于ALDH2对ROS与醛类物质均有抑制作用,所以激动ALDH2抗心律失常治疗途径可能有较好前景。
-
神经退行性疾病是由神经元或其髓鞘失去正常活性导致的,往往随年龄增长越来越严重,从而出现功能障碍,这其中最常见的是帕金森病(PD)和阿尔茨海默病(AD),它们的特征均是氧化应激诱导的脂质过氧化、线粒体功能障碍和醛产物的积累导致记忆丧失、认知能力下降和神经退行性变[3]。
PD是由黑质多巴胺能神经元缺失引起。大量研究表明,黑质多巴胺能神经元与强活性的DOPAL蓄积有关,动物实验证明DOPAL是一种神经毒素,注射DOPAL可诱发帕金森病[17, 18]。ALDH2是DOPAL代谢的关键酶,能将DOPAL转化为无毒的3,4-二羟基苯乙酸,所以ALDH2被认为对PD具有神经保护作用。
AD是另一种常见的以认知功能障碍为特征的神经退行性疾病。一些研究表明ALDH2与AD的发生有关,Ohsawa等[19]建立了携带ALDH2*2基因小鼠模型,发现由于ALDH2活性下降,小鼠的tau蛋白过度磷酸化而导致的4-HNE积累,使小鼠显示出与人类AD相似的年龄依赖性记忆障碍和神经病理。尸检报告发现,AD患者的颞皮层和壳核中的ALDH2活性明显高于健康对照组,这可能是因为AD患者大脑的ROS诱导氧化应激以至醛的增加,ALDH2活性的升高是为促进醛的代谢[20]。因此,较高的ALDH2活性被认为对AD的存活具有保护作用。
-
肝脏疾病,包括非酒精性脂肪性肝病(NAFLD)、肝炎、酒精性肝病(ALD)、药物性肝损伤(DILI)和原发性胆道胆管炎(PBC)等,每年全世界约有200万人死于该类疾病。有研究表明肝脏是ALDH2表达水平最高的器官,也是乙醇代谢的主要部位,而ALDH2是乙醇代谢的主要酶。此外,过量饮酒可增加细胞色素P450 2E1(CYP2E1)的表达和活性,CYP2E1的活化会促进ROS的形成进而导致乙醛的产生[21]。ALDH2活性对肝脏的影响是复杂的, ALD发生是长期大量饮酒导致的,这在很大程度上受到ALDH2变异的影响,ALDH2活性降低导致乙醇代谢过程中乙醛在肝脏蓄积,从而导致ALD。其他NAFLD、肝炎、DILI和PBC也均与ROS诱导的氧化应激有关,在这些肝脏疾病中ROS升高导致肝脏细胞脂质过氧化产生4-HNE。一组体内实验表明,敲除ALDH2基因将加重肝脏疾病,而相应提升ALDH2的表达水平可以延缓疾病进一步发展[22]。
-
研究表明,ALDH2与许多癌症发生有关,如肝癌、结直肠癌、胃癌、食管癌、肺癌、膀胱癌等。乙醛、4-HNE和MDA等有毒物质,在细胞中的积累会引起醛类诱导的DNA链间交联,从而进一步诱导这些癌症的发生和发展[23, 24]。对于庞大的ALDH2*2携带人群来说,ALDH2低活性更易引起毒性醛的蓄积,患上癌症风险更大。同时研究也证实了ALDH2在大多数肿瘤中相对于正常组织表达水平上存在缺陷,此外,ALDH2的缺失往往提示恶性表型和不良预后,有助于提高癌症患者的准确诊断和及时干预[25]。对于携带ALDH2*2癌症患者,可能不仅ALDH2活性低导致毒性醛蓄积诱导癌症的发展,还可能因患有癌症使得患者ALDH2表达降低,这种双重效应使得患者症状加重。鉴于此,对于与ALDH2相关的癌症,可将ALDH2作为新的靶点,激动ALDH2活性是一个可能的新治疗方法[26]。
-
近些年新型冠状病毒(SARS-CoV-2)引起的新冠肺炎持续大流行,在全球范围内造成严重的公共卫生威胁。现在对其治疗的方式大多还是以疫苗的预防作用为主,提前接种能够在很大程度上避免重症和死亡[27]。近几年的研究发现,ALDH2基因rs671多态性可能与免疫系统存在一些联系。随后在最新研究中发现ALDH2基因突变携带者在接种疫苗前后4个月,其体内SARS-CoV-2 刺突蛋白S1 IgG水平与rs671变异等位基因数量呈负相关。该研究结果首次表明了ALDH2的变异等位基因rs671与COVID-19 mRNA疫苗的免疫原性减弱有关[28]。因此,对突变基因携带者来说,提高ALDH2活性可能可以辅助疫苗作用,加强疫苗在病毒免疫应答流程快速产生抗体,这将对新冠后时代提供一个新的解决方式。
-
铁死亡是近年来发现的一种铁依赖的、非凋亡的新型细胞死亡模式,在其发生过程中通常伴有大量铁积累和脂质过氧化,其中最主要特征是累积大量ROS[29]。近期研究发现,在某些疾病发生时ALDH2与铁死亡之间存在着一些联系,在一定程度上,铁死亡的发生将使得细胞中ALDH2的表达减少,提高ALDH2活性能够减少细胞铁死亡。
急性肺损伤(ALI)是败血症的常见并发症,在2022年Cao等[30]采用盲肠结扎穿刺法(CLP)建立脓毒症所致小鼠肺损伤模型,经过CLP处理的小鼠肺组织形态遭到破坏,脂质过氧化损伤,铁含量增加,肺环加氧酶2(PTGS2)蛋白表达增加,同时谷胱甘肽过氧化酶4(GPX4)蛋白表达减少,还下调了ALDH2的表达。而在对照组,加入ALDH2激动剂处理后,发现ALDH2表达增加,肺损伤减轻,ROS水平降低,组织铁含量和PTGS2蛋白表达降低,GPX4蛋白表达升高,这表明了ALDH2的激活能抑制铁死亡。另一组加入铁死亡抑制剂铁抑素Fer-1处理组,ALDH2蛋白表达增加,铁死亡被抑制也促进ALDH2的表达。这些研究表明在急性肺损伤中ALDH2和铁死亡存在很大联系。
最近研究发现,在临床相关心脏骤停(CA)和心肺复苏(CPR)后的肺损伤存活猪模型中,均观察到肺铁死亡,其表现为铁过量和醛产物增加,抗氧化剂减少。然而,当使用ALDH2激动剂处理的CA/CPR组,肺铁含量和醛产物均降低了,同时抗氧化剂增加。ALDH2激动剂能够抑制铁死亡并有效缓解CA/CPR后的肺损伤,其可能是ALDH2激动剂治疗缓解CA/CPR后肺损伤的作用机制[31]。Yu等[32]发现CPR后肾和肠损伤猪模型中,肾脏和肠道细胞发生铁死亡,其肾脏和肠道中铁过量,MDA和4-HNE含量及酰基辅酶A合成酶长链家族成员4(ACSL4)表达显著增加,GPX4表达显著降低,然而用ALDH2激动剂处理的CPR组,上述变化显著逆转,铁死亡得到有效的抑制。由此可见,ALDH2激动剂处理可通过抑制细胞铁死亡来缓解CPR后肾脏和肠道损伤。
近期Zhu等[33]针对阿尔茨海默病APP/PS1小鼠模型,发现ALDH2能通过抑制ACSL4依赖的铁死亡从而缓解由AD引起的心血管功能障碍,结果还表明ALDH2可通过调节脂质过氧化和铁死亡在AD诱导的心脏异常中起重要保护作用。
综上所述,铁死亡与ALDH2之间的关系密切,但以上联系的具体机制仍有待进一步研究。目前铁死亡相关疾病的研究还有很多,涉及神经系统、心脏、肺部、肾脏、胰腺疾病等[34]。如果能够进一步明确ALDH2与铁死亡在生物作用机制上有直接关系,那提高ALDH2活性对铁死亡相关疾病的患者将是一种新的可能治疗手段。
-
ALDH2是人体氧化应激损伤防护的重要环节,提高ALDH2活性具有重要意义,对人数众多的突变基因携带者尤其重要。提高ALDH2活性有利于氧化应激损伤导致的毒性醛的代谢,具有降低心血管疾病、神经退行性疾病、癌症、肝脏疾病等的患病率的潜在作用,同时也可能改善这些疾病预后。最近研究又表明,提高ALDH2活性在某些疾病中能够缓解铁死亡。因此,ALDH2逐渐成为相关疾病治疗的潜在靶点。最近十多年,研究报道了多个类型的小分子激动剂,不过总体来说仍处于研究起步阶段。
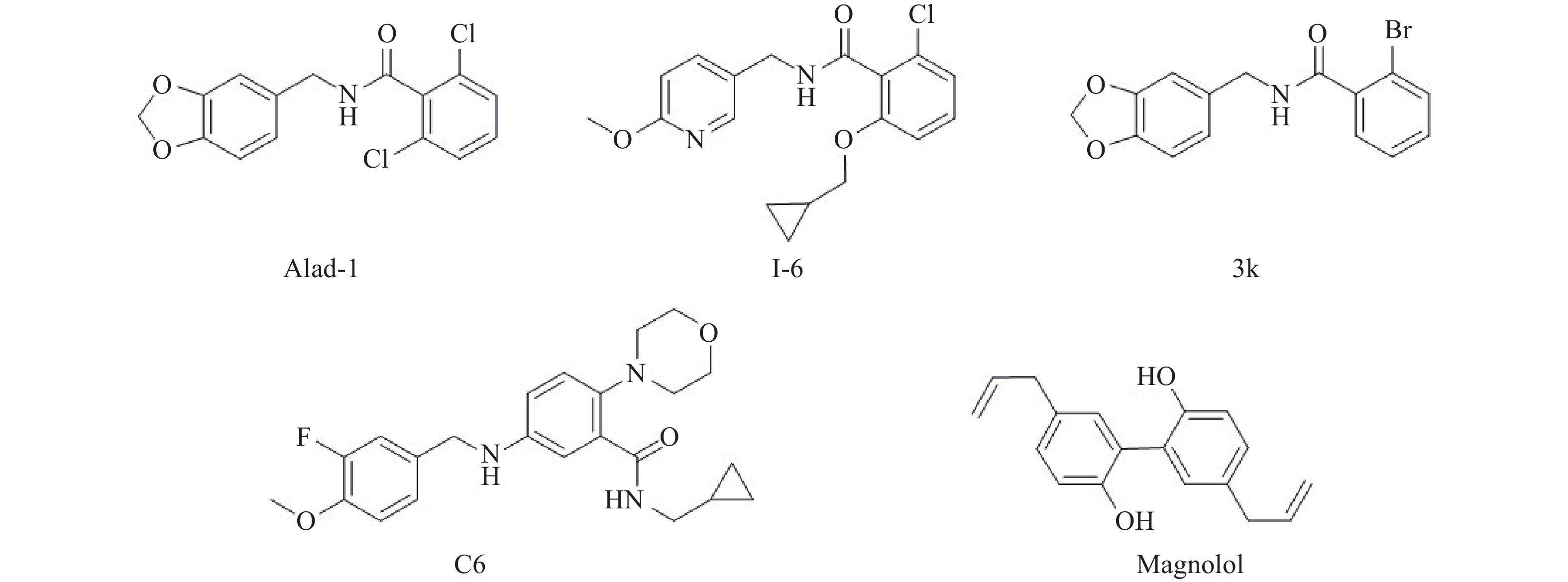
2008年Chen等[12]首次通过高通量筛选发现小分子Adla-1, 它能有效的对ALDH2野生型和ALDH2*2突变型产生激活作用。在体外实验中,Alda-1能将失活变体ALDH2*2 激动活性最高提高至10倍,同时也能最大程度的提高野生型ALDH2的活性至2倍。在体内实验中,能使缺血性脑损伤大鼠减少60%的梗死面积。研究证明,Alda-1与ALDH2催化区伸向蛋白表面的入口部位结合(图1),通过变构效应稳定ALDH2的结构从而发挥激动作用[35]。但是Alda-1的水溶性较差,且生物活性也有待提高(图2)。
针对Alda-1存在的问题,为了得到生物活性与水溶性更好的ALDH2激动剂,2018年本课题组Hu等[36]直接对Alda-1进行了结构修饰,以期提高它们的水溶性和生物活性。在合成所得到的三类新化合物中,化合物的水溶性均有所提高,其中两类化合物表现出较好的激动活性,其中化合物I-6活性最好。而后2020年Cheng等[37]鉴于Alda-1构效关系不完善以及活性不佳等问题,也对Alda-1进行了结构改造,设计并合成了系列Alda-1类似物,其中部分目标化合物的活性高于Alda-1,活性最好的化合物为3k。2021年,Lee等[38]研究发现了一种新的ALDH2激动剂AD-9308(结构未公开),它比Alda-1活性更好,且有较好水溶性和高选择性。研究发现AD-9308在治疗由4-HNE介导的糖尿病引起的心肌病时,能有效地激活ALDH2,从而降低糖尿病小鼠的血清4-HNE水平和心脏组织中的4-HNE蛋白加合物,同时改善心肌纤维化、炎症和细胞凋亡。这一研究结果表明了ALDH2激活对4-HNE介导疾病的治疗潜力,也表明了对新的ALDH2激动剂研发的重要性。
2021年,Chen等[39]发现厚朴中一种天然活性分子厚朴酚(Magnolol),能通过激动ALDH2活性从而抑制心脏成纤维细胞的增殖和胶原合成,进而抑制心肌纤维化,且有助于预防心血管疾病,包括心力衰竭。虽然其精确作用机制尚不清楚,不论作用位点是否与Alda-1一致,鉴于其源于天然产物,为ALDH2激动剂的发现提供了一个新方向。
2021年,本课题组通过计算机模拟筛选发现了一类全新骨架的ALDH2激动剂N-苄基苯胺类化合物C6[40],其在体外ALDH2活性实验中最大激动倍数为Alda-1的104%,在体内能使缺血性脑损伤大鼠模型减少约70%的梗死面积。该化合物的发现首先是通过两轮模拟筛选、基于药效团和结构平行筛选以及基于命中化合物的子结构搜索,发现N-苄基苯胺对ALDH2有激动活性,然后对其进行了结构优化,最终得到了具有良好体内外活性的化合物C6。
-
ALDH2不仅与氧化应激损伤相关疾病存在联系,也和铁死亡相关疾病存在间接联系,虽然有些机制尚不明确,但毫无疑问把ALDH2作为治愈或缓解相关疾病的靶点,是很有前景的。原因有两点,第一是在一些体内外实验中已经证明了激动ALDH2活性确实能够缓解相关疾病症状,而如果针对这些疾病以前的药物靶点存在耐药性或药效不好的问题,ALDH2不乏是一个全新的选择;第二是ALDH2基因突变的携带者广泛存在,特别是东亚人,对于这类人更易因为内源性醛的蓄积从而加重一些疾病的症状,激活ALDH2能够辅助相应疾病的治疗。目前,ALDH2小分子激动剂的研究还比较有限,报道的激动剂还存在结构类型少、生物活性不高和成药性有待提高等诸多问题。期望随着研究的深入,未来会有更多、更好的ALDH2小分子激动剂问世。
Progress on the relationship of aldehyde dehydrogenase 2 with human diseases and its small-molecule activators
-
摘要: 醛脱氢酶2(ALDH2)是人体内重要的抗氧化应激损伤因子之一,而较高比例的东亚人携带ALDH2失活突变基因。与ALDH2密切相关的疾病有很多,如心血管疾病、神经退行性疾病和肝脏疾病等。近期研究还发现ALDH2与铁死亡也有联系。正因如此,ALDH2逐渐成为上述相关疾病治疗的潜在靶点,研究者报道了其多个类型的小分子激动剂,展现出一定的应用前景。本文重点介绍ALDH2的结构、功能、与人类疾病的关系以及其激动剂的研究进展。Abstract: Aldehyde dehydrogenase 2 (ALDH2) is one of important factors against from the damage under oxidative stress in human body. A high proportion of East Asians carry ALDH2 inactive mutation gene. There are many diseases closely related to ALDH2, such as cardiovascular diseases, neurodegenerative diseases and liver diseases. Recent studies also have found that ALDH2 is associated with ferroptosis. Therefore, ALDH2 has becoming a potential target for the treatment of the above related diseases. Several types of small molecule activators with potential value of clinical application have been reported. The research progress on the structure and function of ALDH2 , the relationship with human diseases and its activators were summarized in this paper.
-
Key words:
- aldehyde dehydrogenase 2 /
- oxidative stress injury /
- mutant /
- ferroptosis /
- small-molecule activators
-
METRNL(Metrn-like)蛋白是近年来发现和证实的新的分泌蛋白[1-2],其与METRN构成了一个两蛋白的新蛋白家族。虽然最初的研究表明,该家族蛋白均可促进神经细胞轴突的生长[2-4],但两者表达差异很大,METRN在中枢神经系统中高特异性表达,而METRNL则在全身较为广泛地表达,提示其可能具有更广泛的生理功能[1-2, 5-6]。
最近的研究发现,METRNL对代谢具有重要的调节作用。其在脂肪组织中表达较高,特别是皮下脂肪,被认为是一种新的脂肪因子[1]。研究发现,该蛋白可以调节脂肪细胞的分化,脂肪细胞中METRNL过表达可提高全身胰岛素敏感性,减少脂肪炎症扩大脂肪细胞的体积等[7]。也有研究发现,METRNL可以在运动后由肌肉组织增加分泌,促进脂肪组织棕色化,从而提高代谢率,减轻体重和改善胰岛素敏感性[8]。这些研究提示,METRNL可能与提高胰岛素敏感性相关。
噻唑烷二酮类药物,如罗格列酮,可以通过激动PPARγ受体,提高胰岛素增敏性,被称为胰岛素增敏剂。但是这类药物与METRNL蛋白之间的关系,至今尚不清楚。我们前期的研究发现,白色脂肪组织中METRNL过表达可以提高PPARγ的表达,促进脂肪重构,降低白色脂肪炎症,但是激动PPARγ对METRNL表达的影响尚未有报道。
本研究拟通过高脂饮食(HFD)诱导的胰岛素抵抗小鼠模型,检测血液METRNL的浓度变化;通过给予胰岛素增敏剂罗格列酮治疗,构建胰岛素增敏动物模型,检测血液中METRNL的水平变化,从而明确激动PPARγ对血液METRNL水平影响,通过实时定量PCR检测不同组织中METRNL的表达,明确PPARγ通过何种组织调控METRNL的表达与血液浓度。
1. 材料和方法
1.1 动物处理
12周龄的雄性C57BL/6小鼠与小鼠饲料,均购自上海斯莱克实验动物有限公司。为检测胰岛素抵抗对METRNL表达的影响,分两组小鼠,每组8只,分别给予正常饮食(NCD)和HFD,均饲养4个月;为了检测胰岛素增敏对于METRNL表达的影响,分两组小鼠,每组8只,两组均先HFD饲养3个月,而后实验组的饲料中加入药物罗格列酮(胰岛素增敏组),剂量为10 mg/kg·d,治疗1个月,对照组继续HFD饲养1个月。
1.2 糖耐量实验
小鼠禁食18 h,腹腔注射30%葡萄糖溶液(2 g/kg),分别在0、30、60、90、120 min取尾静脉血,采用强生血糖仪(OneTouch Ultra)检测小鼠血糖水平。
1.3 酶联免疫吸附实验(ELISA)
戊巴比妥钠麻醉小鼠后(80 mg/kg),心脏取血收集血液,室温静置2 h,3000 r/min离心20 min,取上清液。采用小鼠METRNL ELISA试剂盒(购自美国R&D biosystem公司)检测血清中METRNL浓度。操作步骤参照试剂盒说明书。
1.4 荧光实时定量PCR实验
取小鼠附睾周围白色脂肪、肩胛骨间棕色脂肪、肝脏、腓肠肌、脑组织、肾脏、脾脏组织,采用TRIzol试剂(购自美国Invitrogen公司),按照说明书抽提组织总RNA,用RT-PCR逆转录试剂盒(购自中国TARARA公司)逆转录为cDNA。1 μg的cDNA用于检测METRNL的表达,GAPDH作为内参。采用2−ΔΔCt法与SYBR® Green PCR Master Mix(Applied Biosystems)试剂,反应条件为,95 ℃,5 min, 1个循环;95 ℃, 15 s,60 ℃,30 s,72 ℃,30 s,40个循环。相关引物序列见表1。
表 1 相关引物序列基因 上游序列(5'—3') 下游序列(5'—3') METRNL CTGGAGCAGGGAGGCTTATTT GGACAACAAAGTCACTGGTACA GAPDH GTATGACTCCACTCACGGCAAA GGTCTCGCTCCTGGAAGATG ERRα GCCG CGATGTCCTTTTGTG CTGTACTCGATGCTCCCTGC UCP-1 CACGGGGACCTACAATGCTT ACAGTAAATGGCAGGGGACG clec10a TGGTGTCTTGGTTTCCGTCC AGCTCCTAGCTCTCCTTGGC Mrc-1 CTCTGTTCAGCTATTGGACGC TGGCACTCCCAAACATAATTTGA Lipe GTTATGAGTGCGCTCCGAGA GAGCAAAGCTAGAGTCGGGG LPL GGTTGCGCGTAGAGAGGATG CTCACGCTCTGACATGCCTTC FABP4 AAGGTGAAGAGCATCATAACCCT TCACGCCTTTCATAACACATTCC CD36 ATGGGCTGTGATCGGAACTG TTTGCCACGTCATCTGGGTTT 1.5 统计学处理
所有数据均用(
$ \bar x \pm s $ )表示,用SPSS 10.0处理。两样本均数比较采用t检验。2. 结果
2.1 胰岛素增敏剂罗格列酮治疗改善了高脂饮食诱导的胰岛素抵抗
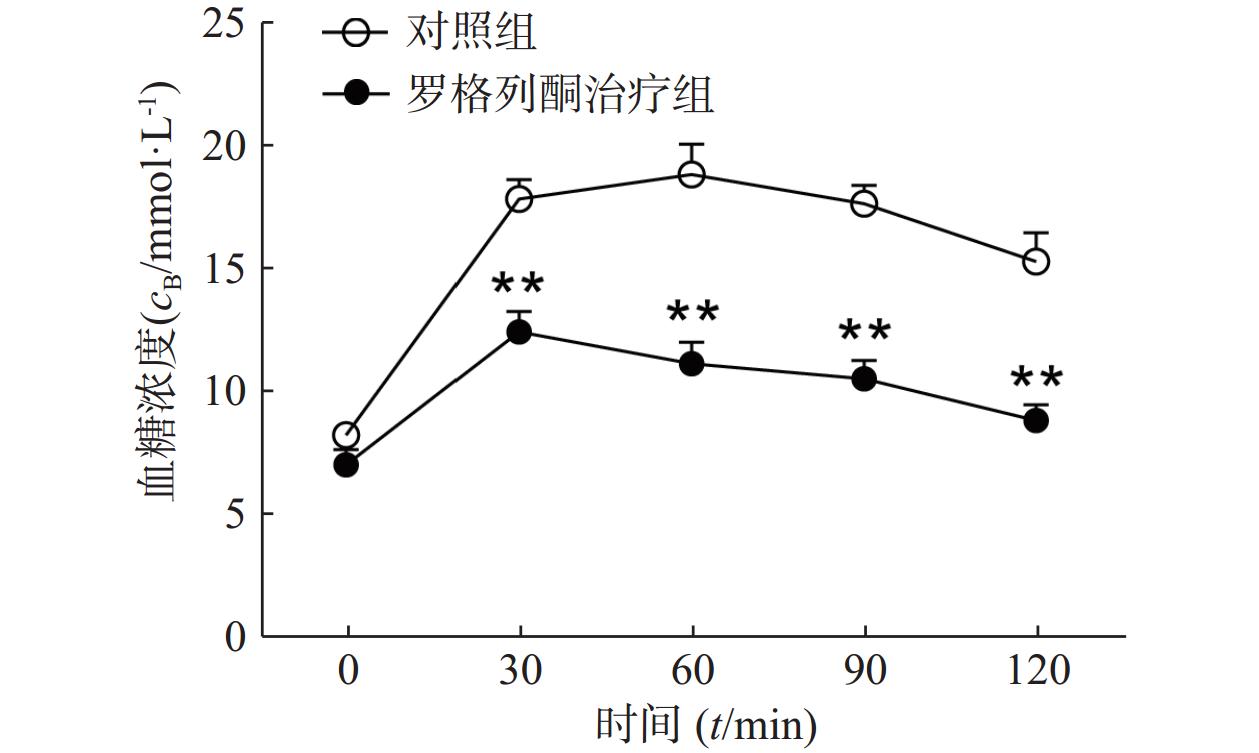
对HFD 3个月的小鼠采用罗格列酮治疗1个月后,葡萄糖耐量实验检测其糖耐量情况如图1所示,罗格列酮治疗组给予葡萄糖后30、60、90、120 min的血糖浓度均明显低于单纯HFD组(P<0.01),说明罗格列酮治疗明显改善了小鼠的糖耐量,提高了机体胰岛素敏感性。
2.2 胰岛素增敏剂罗格列酮治疗升高了胰岛素抵抗小鼠血液中METRNL的水平
取正常对照组、HFD组、HFD罗格列酮治疗组小鼠的血清,ELISA检测METRNL的水平,结果如图2所示,罗格列酮组血清中METRNL的浓度为(6 632±358) pg/ml,是单纯HFD组(4 271±310) pg/ml的1.6倍(P<0.05)。
2.3 胰岛素增敏剂罗格列酮治疗增加棕色脂肪组织和肾脏METRNL的表达
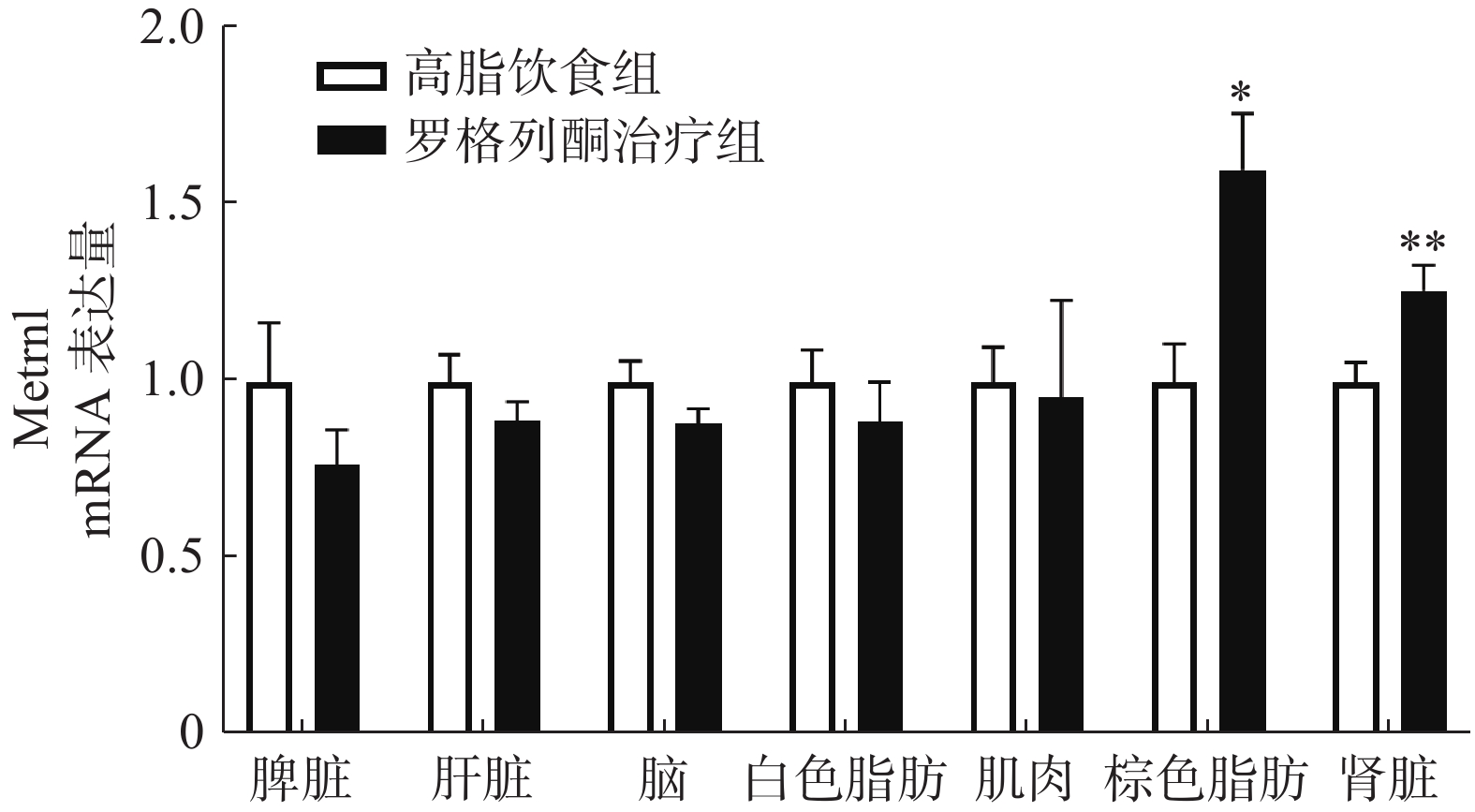
实时荧光定量PCR检测肌肉、肝脏、白色脂肪、棕色脂肪、脑、脾脏、肾脏等组织中METRNL的表达,结果如图3所示,与单纯HFD组相比,罗格列酮治疗组棕色脂肪组织METRNL表达升高1.6倍,肾脏组织METRNL表达升高1.3倍。
2.4 罗格列酮治疗促进了棕色脂肪代谢与棕色脂肪标记蛋白的表达
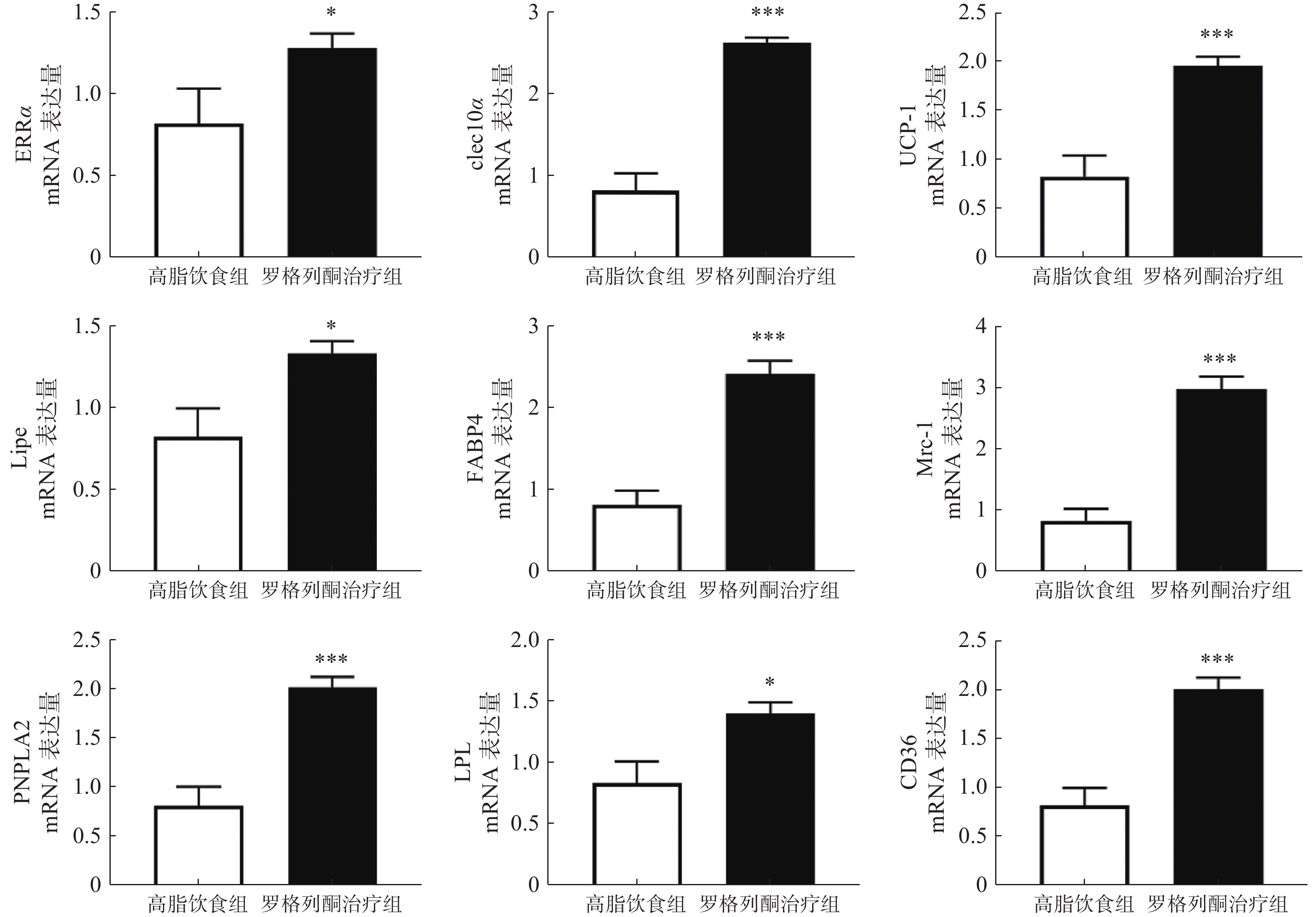
实时荧光定量PCR检测棕色脂肪组织代谢与棕色脂肪标记蛋白等mRNA表达情况,结果如图4所示,与单纯HFD组相比,罗格列酮治疗组ERRα、UCP-1、clec10a、Mrc-1、Lipe、LPL、FABP4、CD36、PNPLA2等因子表达显著升高。
3. 讨论
本研究通过HFD诱导胰岛素抵抗的肥胖小鼠,发现HFD可以导致METRNL血液水平升高。对肥胖小鼠不同组织METRNL表达的检测显示,脂肪组织METRNL表达显著升高。HFD诱导的胰岛素抵抗小鼠,给予胰岛素增敏剂罗格列酮治疗后,小鼠糖耐量改善,同时,血清METRNL的浓度也升高。这些结果说明,METRNL并非胰岛素敏感性的特异性指标,脂肪可能是使血液METRNL水平改变的主要组织之一。
Li等研究发现,肥胖小鼠的脂肪组织METRNL表达增加[7]。本研究也表明,METRNL的血液浓度在高脂诱导肥胖后升高。Löffler等研究发现,METRNL与脂肪细胞的肥大相关,而脂肪细胞肥大被认为与PPARγ活性的降低相关,是胰岛素抵抗的重要标志之一,进而认为METRNL是机体胰岛素抵抗的标志[9]。然而,在本研究中,胰岛素增敏剂罗格列酮治疗后小鼠胰岛素敏感性提高,同时METRNL表达也显著升高,可见METRNL血液浓度的升高并不能代表胰岛素抵抗的增加。
罗格列酮可以显著提高胰岛素的敏感性,故也称为胰岛素增敏剂。本研究表明,其可以显著提高METRNL的表达,而METRNL又具有促进白色脂肪棕色化和提高胰岛素敏感性的作用,所以METRNL可能参与介导了罗格列酮的胰岛素增敏作用。
我们前期的研究表明,增加白色脂肪表达可以提高血液中METRNL的水平。本研究发现,罗格列酮未促进高脂条件下白色脂肪METRNL的表达,在检测的7种组织中,罗格列酮显著提高了棕色脂肪和肾脏METRNL的表达,但是对脾脏、肝脏、肌肉、白色脂肪、脑组织METRNL的表达没有影响,说明罗格列酮可能主要通过棕色脂肪和肾脏提高血液METRNL浓度。此外,进一步实验发现,罗格列酮促进了棕色脂肪中代谢和棕色脂肪标记蛋白的表达,这与以往的研究结果一致[10],既往研究表明,METRNL可促进白色脂肪棕色化,提示罗格列酮促进棕色脂肪代谢的作用可能有METRNL蛋白参与。
本研究发现了胰岛素增敏剂罗格利酮治疗可能通过提高棕色脂肪和肾组织的METRNL表达来升高血清METRNL水平,提示METRNL可能参与了罗格列酮对糖尿病的治疗过程。
-
-
[1] LINDAHL R. Aldehyde dehydrogenases and their role in carcinogenesis[J]. Crit Rev Biochem Mol Biol, 1992, 27(4-5):283-335. doi: 10.3109/10409239209082565 [2] KOPPAKA V, THOMPSON D C, CHEN Y, et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application[J]. Pharmacol Rev, 2012, 64(3):520-539. doi: 10.1124/pr.111.005538 [3] CHEN C H, FERREIRA J C B, GROSS E R, et al. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities[J]. Physiol Rev, 2014, 94(1):1-34. doi: 10.1152/physrev.00017.2013 [4] YOSHIDA A, HSU L C, YASUNAMI M. Genetics of human alcohol-metabolizing enzymes[J]. Prog Nucleic Acid Res Mol Biol, 1991, 40:255-287. [5] BAGNARDI V, ROTA M, BOTTERI E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis[J]. Br J Cancer, 2015, 112(3):580-593. doi: 10.1038/bjc.2014.579 [6] CEDERBAUM A I. Alcohol metabolism[J]. Clin Liver Dis, 2012, 16(4):667-685. [7] VAISHNAV R A, SINGH I N, MILLER D M, et al. Lipid peroxidation-derived reactive aldehydes directly and differentially impair spinal cord and brain mitochondrial function[J]. J Neurotrauma, 2010, 27(7):1311-1320. doi: 10.1089/neu.2009.1172 [8] CARBONE D L, DOORN J A, KIEBLER Z, et al. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease[J]. J Pharmacol Exp Ther, 2005, 315(1):8-15. doi: 10.1124/jpet.105.088088 [9] LI H, BORINSKAYA S, YOSHIMURA K, et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant[J]. Ann Hum Genet, 2009, 73(3):335-345. doi: 10.1111/j.1469-1809.2009.00517.x [10] GROSS E R, ZAMBELLI V O, SMALL B A, et al. A personalized medicine approach for Asian Americans with the aldehyde dehydrogenase 2*2 variant[J]. Annu Rev Pharmacol Toxicol, 2015, 55:107-127. doi: 10.1146/annurev-pharmtox-010814-124915 [11] CHEN C H, SUN L H, MOCHLY-ROSEN D. Mitochondrial aldehyde dehydrogenase and cardiac diseases[J]. Cardiovasc Res, 2010, 88(1):51-57. doi: 10.1093/cvr/cvq192 [12] CHEN C H, BUDAS G R, CHURCHILL E N, et al. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart[J]. Science, 2008, 321(5895):1493-1495. doi: 10.1126/science.1158554 [13] RADOVANOVIC S, SAVIC-RADOJEVIC A, PLJESA-ERCEGOVAC M, et al. Markers of oxidative damage and antioxidant enzyme activities as predictors of morbidity and mortality in patients with chronic heart failure[J]. J Cardiac Fail, 2012, 18(6):493-501. doi: 10.1016/j.cardfail.2012.04.003 [14] FU S H, ZHANG H F, YANG Z B, et al. Alda-1 reduces cerebral ischemia/reperfusion injury in rat through clearance of reactive aldehydes[J]. Naunyn-Schmiedeberg’s Arch Pharmacol, 2014, 387(1):87-94. doi: 10.1007/s00210-013-0922-8 [15] STAERK L, SHERER J A, KO D, et al. Atrial fibrillation[J]. Circ Res, 2017, 120(9):1501-1517. doi: 10.1161/CIRCRESAHA.117.309732 [16] JIN J Y, CHEN J Y, WANG Y P. Aldehyde dehydrogenase 2 and arrhythmogenesis[J]. Heart Rhythm, 2022, 19(9):1541-1547. doi: 10.1016/j.hrthm.2022.05.008 [17] PANNETON W M, KUMAR V B, GAN Q, et al. The neuro-toxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis[J]. PLoS One, 2010, 5(12):e15251. doi: 10.1371/journal.pone.0015251 [18] WEY M C Y, FERNANDEZ E, MARTINEZ P A, et al. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson’s disease[J]. PLoS One, 2012, 7(2):e31522. doi: 10.1371/journal.pone.0031522 [19] OHSAWA I, NISHIMAKI K, MURAKAMI Y, et al. Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity[J]. J Neurosci, 2008, 28(24):6239-6249. doi: 10.1523/JNEUROSCI.4956-07.2008 [20] KIMURA M, YOKOYAMA A, HIGUCHI S. Aldehyde dehydrogenase-2 as a therapeutic target[J]. Expert Opin Ther Targets, 2019, 23(11):955-966. doi: 10.1080/14728222.2019.1690454 [21] HYUN J, HAN J, LEE C B, et al. Pathophysiological aspects of alcohol metabolism in the liver[J]. Int J Mol Sci, 2021, 22(11):5717. doi: 10.3390/ijms22115717 [22] YIN-CUI WU. The role of acetaldehyde dehydrogenase 2 in the pathogenesis of liver diseases[J]. Cell Signal, 2023, 102:110550. doi: 10.1016/j.cellsig.2022.110550 [23] CHANG J S, HSIAO J R, CHEN C H. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective[J]. J Biomed Sci, 2017, 24(1):19. doi: 10.1186/s12929-017-0327-y [24] HODSKINSON M R, BOLNER A, SATO K, et al. Alcohol-derived DNA crosslinks are repaired by two distinct mechanisms[J]. Nature, 2020, 579(7800):603-608. doi: 10.1038/s41586-020-2059-5 [25] MA B, LIU Z Q, XU H, et al. Molecular characterization and clinical relevance of ALDH2 in human cancers[J]. Front Med (Lausanne), 2022, 8:832605. [26] ZHANG H, FU L. The role of ALDH2 in tumorigenesis and tumor progression: targeting ALDH2 as a potential cancer treatment[J]. Acta Pharm Sin B, 2021, 11(6):1400-1411. doi: 10.1016/j.apsb.2021.02.008 [27] HADJ HASSINE I. Covid-19 vaccines and variants of concern: a review[J]. Rev Med Virol, 2022, 32(4):e2313. doi: 10.1002/rmv.2313 [28] MATSUMOTO A, HARA M, ASHENAGAR M S, et al. Variant allele of ALDH2, rs671, associates with attenuated post-vaccination response in anti-SARS-CoV-2 spike protein IgG: a prospective study in the Japanese general population[J]. Vaccines, 2022, 10(7):1035. doi: 10.3390/vaccines10071035 [29] XIE Y, HOU W, SONG X, et al. Ferroptosis: process and function[J]. Cell Death Differ, 2016, 23(3):369-379. doi: 10.1038/cdd.2015.158 [30] CAO Z Z, QIN H Q, HUANG Y H, et al. Crosstalk of pyroptosis, ferroptosis, and mitochondrial aldehyde dehydrogenase 2-related mechanisms in sepsis-induced lung injury in a mouse model[J]. Bioengineered, 2022, 13(3):4810-4820. doi: 10.1080/21655979.2022.2033381 [31] WU H B, XU S X, DIAO M Y, et al. Alda-1 treatment alleviates lung injury after cardiac arrest and resuscitation in swine[J]. Shock, 2022, 58(5):464-469. doi: 10.1097/SHK.0000000000002003 [32] YU Q, GAO J B, SHAO X B, et al. The effects of Alda-1 treatment on renal and intestinal injuries after cardiopulmonary resuscitation in pigs[J]. Front Med (Lausanne), 2022, 9:892472. [33] ZHU Z Y, LIU Y D, GONG Y, et al. Mitochondrial aldehyde dehydrogenase (ALDH2) rescues cardiac contractile dysfunction in an APP/PS1 murine model of Alzheimer’s disease via inhibition of ACSL4-dependent ferroptosis[J]. Acta Pharmacol Sin, 2022, 43(1):39-49. doi: 10.1038/s41401-021-00635-2 [34] LI J, CAO F, YIN H L, et al. Ferroptosis: past, present and future[J]. Cell Death Dis, 2020, 11(2):88. doi: 10.1038/s41419-020-2298-2 [35] PEREZ-MILLER S, YOUNUS H, VANAM R, et al. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant[J]. Nat Struct Mol Biol, 2010, 17(2):159-164. doi: 10.1038/nsmb.1737 [36] HU J, TIAN W, ZHOU R L, et al. Design, synthesis, and biological evaluation of new ALDH2 activators[J]. J Saudi Chem Soc, 2019, 23(3):255-262. doi: 10.1016/j.jscs.2018.07.001 [37] CHENG M C, LO W C, CHANG Y W, et al. Design, synthesis and the structure-activity relationship of agonists targeting on the ALDH2 catalytic tunnel[J]. Bioorg Chem, 2020, 104:104166. doi: 10.1016/j.bioorg.2020.104166 [38] LEE H L, HEE S W, HSUAN C F, et al. A novel ALDH2 acti-vator AD-9308 improves diastolic and systolic myocardial functions in streptozotocin-induced diabetic mice[J]. Antioxidants (Basel), 2021, 10(3):450. doi: 10.3390/antiox10030450 [39] CHEN L, Wu Y T, GU X Y, et al. Magnolol, a natural aldehyde dehydrogenase-2 agonist, inhibits the proliferation and collagen synthesis of cardiac fibroblasts[J]. Bioorg Med Chem Lett, 2021, 43:128045. doi: 10.1016/j.bmcl.2021.128045 [40] TIAN W, GUO J, ZHANG Q, et al. The discovery of novel small molecule allosteric activators of aldehyde dehydrogenase 2[J]. Eur J Med Chem, 2021, 212:113119. doi: 10.1016/j.ejmech.2020.113119 -





 下载:
下载:
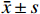

 下载:
下载: