-
表面等离子共振(SPR)[1, 2]是指当偏振光以特定角度照射金属或其他导电材料时,在介质(通常是玻璃和液体)与金属之间的界面发生全反射,然后产生消逝波,同时入射光激发金属中的自由电子进行纵向振动,形成等离子波,消逝波与金属膜表面的等离子波发生共振的现象。SPR传感器是一种基于SPR现象发展而来的分析技术,SPR传感器表面结合的生物分子质量的变化使共振波长或共振角度发生偏移,从而能够对生物标记物进行定量监测[3]。SPR传感器可通过传感器芯片实时、原位和动态测量各种生物分子,如多肽、蛋白质、寡核苷酸、寡聚糖,以及病毒、细菌、细胞、小分子化合物之间的相互作用,无须进行标记,也无须纯化各种生物组分,具有灵敏度高和耗样量低等突出优点。
识别元件也被称为目标受体,是生物传感器的重要组成部分。在SPR传感器中,识别元件被固定在传感器芯片上,参与特异性识别和捕获目标分析物,与传感器的选择性能密切相关,是实现生物传感器特异性检测的关键[4]。目前识别元件主要包括抗体、核酸适配体(Apt)、分子印迹聚合物(MIP)、蛋白质和金属纳米粒子等,分别能够通过对不同的生物样品的特异性结合实现检测。本文简要介绍了SPR传感器中不同识别元件的原理、检测范围、优势以及局限性,重点分析近些年国内外将不同识别元件的SPR传感器应用于医药领域研究的研究成果,如表1所示。
表 1 基于不同识别元件的SPR传感器在医药领域中的应用
识别元件 分析物 检测限 KD值 线性范围 参考文献 抗体 杆状病毒抑制因子5 6.25 pg/ml 12.1 nmol/L — [5] NS1蛋白 0.8 nmol/L — — [6] 马血红素肌红蛋白 — (270 ± 14)nmol/L — [7] 脱辅基肌红蛋白 — (350 ± 13.5)nmol/L — [7] 白介素-8 — 82.2 nmol/L — [9] 吡噻菌胺 10 ng/ml 0.82 nmol/L — [10] 诺氟沙星 0.02 ng/ml — — [11] 三唑磷 0.096 ng/ml — 0.98~8.29 ng/ml [12] 适配体 β淀粉样蛋白 0.2 pmol/L — — [14] 糖化血红蛋白 2.55 nmol/L — — [15] 溶菌酶 2.4 nmol/L — — [16] 肌钙蛋白T 3.13 nmol/L — — [17] 新霉素B 5 nmol/L 6.5 μmol/L 10 nmol/L~100 µmol/L [19,20] 伊马替尼 — 0.7~10 µmol/L [21] 军团菌 104.3 CFU/ml — — [22] 副溶血性弧菌 2.4 nmol/L — [23] MIP 多巴胺 — — 0.01~0.50 ppb [31] 肌红蛋白 4.72 ng/ml — — [27,28] 牛血清蛋白 8.5 nmol/L — — [29] CA-125 0.01 U/ml — 0.1 ~500 U/ml [30] 蛋白质 PD-1蛋白 10 nmol/L — — [33] 刺突蛋白 — 0.37 nmol/L — [35] 金属纳米粒子 Cu2+ 0.24 μg/L — 0.5 ~35 μg/L [37] Hg2+ 8 nmol/L — 20~100 nmol/L [38] Hg2+ 9.98 nmol/L — — [39] Cu2+ 200 nmol/L — 10~250 μmol/L [40] 注:“—”表示在原文中未说明。 -
由于抗体对蛋白质、病毒、细菌、细胞以及小分子等具有特异性结合,利用抗体作为识别元件检测生物样品目前在SPR传感器中应用最广泛、最普遍[41]。随着抗体研发的发展,抗体作为识别元件也更广泛地应用到各种生物样品的检测中,并且其检测限不断降低,检测范围不断增大。
-
Jena等[5]基于无标记SPR免疫传感器利用杆状病毒抑制因子5抗体检测狗血清中杆状病毒抑制因子5的含量,方法如图1所示,KD为12.1 nmol/L,检测限低至6.25 pg/ml,具有高灵敏度和特异性。患乳腺肿瘤犬的血清杆状病毒抑制因子5的平均含量为(110.02±9.77)pg/ml,健康狗血清中检测到的平均含量为(30.28±2.99)pg/ml,这表明SPR传感器在犬科乳腺肿瘤的诊断中具有重要的潜在应用价值。
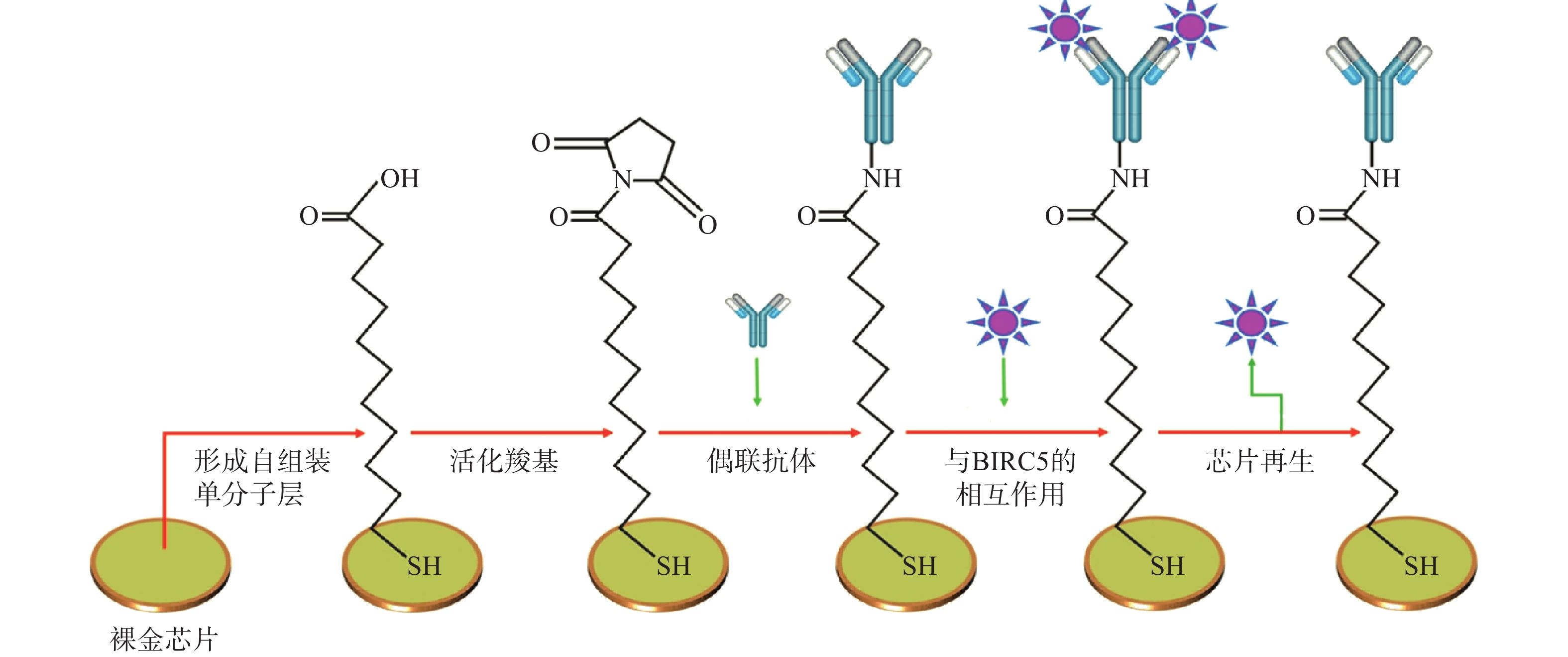
图 1 裸金 SPR 传感器表面的制作和利用SPR传感器检测杆状病毒抑制因子5[6]
Widoretno等[6]为了检测登革热感染,在研究中利用SPR传感器检测非结构蛋白(NS1)抗体和NS1蛋白的相互作用,与目前可用的经典检测方法——酶联免疫吸附测定和逆转录聚合酶链反应进行了比较,在对从印度尼西亚登革热患者收集的26个样本的检测中,SPR能够以更低的浓度和更短的时间正确识别所有16名阳性感染者。酶联免疫吸附测定法对NS1的检测限为8 nmol/L,而SPR传感器检测限低至0.8nmol/L。
Mihoc等[7]将蛋白水解表位提取质谱法和SPR生物传感器结合,利用肌红蛋白的单克隆抗体检测出马血红素肌红蛋白的KD为(270±14)nmol/L,脱辅基肌红蛋白的KD为(350±13.5)nmol/L,并用蛋白水解法通过质谱确定了单克隆抗体和多克隆抗体的抗原表位。Lupu等[8, 9]同样以该法,利用白介素-8的单克隆抗体对白介素进行检测,通过蛋白水解法确定了单克隆抗体的抗原表位。
-
Ceballos-Alcantarilla等[10]为了检测吡噻菌胺,开发了其单克隆抗体作为识别元件,优化并验证了基于单克隆抗体的SPR直接免疫测定法,用于快速分析葡萄、葡萄汁和葡萄酒样品中的吡噻菌胺。通过SPR检测的样品结果与液相二级质谱的结果进行比较,发现了极好的相关性(r=0.995),并且在葡萄、葡萄汁和葡萄酒样品中的检测限低至 10 ng/ml。
Acaroz等[11]采用单克隆抗体作为识别元件,利用SPR检测氟喹诺酮类药物,在优化条件下,该测定表现出非常高的灵敏度,例如,诺氟沙星的检测限可低至0.02 ng/ml。为了证明该方法的适用性,对人工污染的牛奶、肉类、鱼类和虾样品进行了检测,平均回收率分别为88.9%、76.2%、74.4%和77.9%。这些数据表明,该测定方法具有广泛的适用性。
可见,抗体作为一种最常见的特异性很强的识别元件,广泛应用于各种生物分子的检测,包括蛋白质、小分子等,并且具有较高的灵敏度和特异性,能够识别极少量的目标分子,然而抗体也存在制备过程繁琐、易产生交叉反应和易失活等一些缺点。基于抗体作为识别元件的SPR传感器能够进行灵敏精确的生物检测,尤其在感染性疾病的早期诊断和监测中显示出高效性和准确性。
-
核酸适配体[13]是基于指数富集的配体系统进化(SELEX)技术从随机寡核苷酸文库中经过8 ~ 20轮不断筛选获得的对靶物质具有很高特异性和亲和力的寡核苷酸序列。这一小段经体外筛选得到的寡核苷酸序列能与相应的配体进行高亲和力和强特异性的结合,它的出现为生物医学界提供了一种新的高效快速识别的研究平台,并在许多方面展示了良好的应用前景。基于核酸适配体的SPR生物传感器因其简单、可行、可替代抗体和检测成本低等特点而受到广泛关注。
-
Zheng等[14]开发了一种基于双适配体的SPR传感器,用于定量检测β淀粉样蛋白40(Aβ40)聚集体。这种基于双适配体的SPR传感器避免了空间位阻和表位的限制,Aβ40低聚物的检测限低至0.2 pmol/L,并且其操作简单、识别元件的成本较低。该SPR传感器可成功应用于检测人工脑脊液和未稀释的真实脑脊液中的Aβ40聚集体。Sun等[15]利用适配体具有选择性高、制备简单、保存方便等优点,采用适配体SPR传感器进行糖化血红蛋白(HbA1c)检测,明显提高了HbA1c测量的准确性和灵敏度。
Mihai等[16]使用基于适配体的SPR传感器对葡萄酒中的过敏原蛋白溶菌酶进行无标记检测,并优化了适配体用量、电泳缓冲液的组成、适配体与蛋白质的孵育时间、流速和样品预处理方式。结果表明,基于适配体的SPR传感器检测葡萄酒中的溶菌酶时具有高准确度和灵敏度,检测限低至2.4 nmol/L,表现出良好稳定性、选择性。并分别在不同化合物(槲皮素、没食子酸、儿茶素、表儿茶素、咖啡酸)存在下检测溶菌酶,其响应值与相同浓度的标准溶液响应值相比明显降低,证明了溶菌酶与槲皮素等酚类化合物具有相互作用。
Torrini等[17]利用肌钙蛋白T的适配体作为识别元件构建了SPR传感器,通过传感器对肌钙蛋白T进行检测,使用三明治法的适配体SPR传感器表现出更好的选择性,展现了适配体代替抗体作为识别元件的可行性。
-
Tenaglia等[18]构建了基于核酸适配体的SPR传感器,直接检测人血清中的妥布霉素,对血药浓度进行监测。结果表明,使用针对妥布霉素的适配体,可以定量检测接受妥布霉素治疗并进行治疗药物监测的患者获得的临床样本中抗生素的浓度,并且基于适配体的直接检测与临床使用的质谱法具有良好的相关性(r=0.99)。
Luan等[19]利用以核酸适配体为识别元件的SPR传感器筛选和检测氨基糖苷类抗生素,通过监测镀金膜上激光的角度变化,获得实时结合曲线。待测物质中抗生素与其适配体结合的解离常数和化学计量值均可使用SPR传感器的响应进行测量。检测了牛奶中新霉素B,线性范围为10 nmol/L~100 µmol/L,检测限低至5 nmol/L。
Tartaggia等[21]为了在临床实践中实施抗癌药物伊马替尼的治疗药物监测,依靠适配体作为识别元件,利用SPR传感器进行检测,检测方法具有出色的选择性,并且不受人血浆样品成分的干扰。SPR检测的结果与高效液相色谱-质谱联用技术的结果相比具有良好的相关性(r=0.9999)。其根据监管指南收集了可靠的验证数据,并将该方法与标准分析技术进行了比较,从而为接受伊马替尼治疗的患者的治疗药物监测开发了一种可行的适配体传感器。
-
Saad等[22]介绍了一种新的针对军团菌的检测策略——基于表面等离子共振成像(SPRi)的滴定测定法,该测定法使用嗜肺军团菌适配体,能有效避免传统SPR用于全细胞细菌检测存在的不足。与传统检测方法通过细菌培养(7~15 d)和逆转录聚合酶链反应(60~90 min)相比,该检测方法仅需30 min即可给出结果。没有标记或信号放大策略的情况下可以实现104.3 CFU/ml检测极限,并且信号放大策略的开发将进一步提高SPRi的滴定测定的灵敏度和准确性,以实现在环境监测或即时诊断等多个领域监测细菌。
Ahn等[23]将副溶血性弧菌的核酸适配体用于基于SPR的生物传感器,开发了快速、简单、特异的检测方法来检测副溶血性弧菌。由于副溶血性弧菌的某些细胞外成分形成了二级和三级结构,基于适配体的SPR生物传感器平台可以清楚地识别副溶血性弧菌,KD为2.4 nmol/L。基于核酸适配体的SPR传感器为诊断弧菌感染提供了一个快捷的检测平台,具有巨大的病原体检测潜力。
核酸适配体具有一些天然优势,例如:易于体外大量合成、重复性好、稳定性高且易于贮存;同时,适配体靶标广泛,包括农药、组织、细胞、病毒、蛋白质、毒素、维生素等;适配体易于被标记荧光且活性不受影响,因此易于结合应用其他检测技术。基于核酸适配体为识别元件的SPR传感器,在环境检测中,能够实时和高效的检测污染物、毒素等;在临床诊断中,能够高灵敏度和特异性的检测病原体、癌症标志物或者其他生物分子,并且其操作简便易行、检测成本较低。因此,核酸适配体有望成为优良的识别元件,也有望在SPR领域作为抗体的替代品。
-
分子印迹技术[24, 25]是在特定的环境(溶剂体系)中,以模板分子(待识别的目标分子)与功能单体发生相互作用形成主客体复合物,在交联剂的作用下功能单体交联聚合并围绕模板分子形成三维网络空间结构,随后模板分子被洗脱,结合位点暴露,三维空间形成印迹孔穴,即MIP。MIP在结合力、官能团、空间形状和大小等方面与目标分子高度互补,对模板分子具有“记忆效应”,可以从复杂的混合物中结合目标分子。因此,MIP具备了生物抗体的两个最重要特性——识别和结合特定目标分子的能力。
-
Torrini等[26]以去甲肾上腺素为单体,开发并优化了一种高选择性和高灵敏度的MIP,通过SPR生物传感器利用竞争法检测促性腺激素释放素(图2),实现了实时、无标签的SPR生物检测。通过测试一系列在分子量和主序列方面从高到低相似度的肽验证其选择性,并且在选择性、可重复使用性和成本方面与高效液相和商业酶联免疫吸附测定试剂盒进行了直接比较,结果表明,SPR传感器成本更低,且无需高度培训人员。与抗体相比较,MIP可适应更复杂的环境,显示了以MIP为识别元件的SPR传感器的整体优良特性。
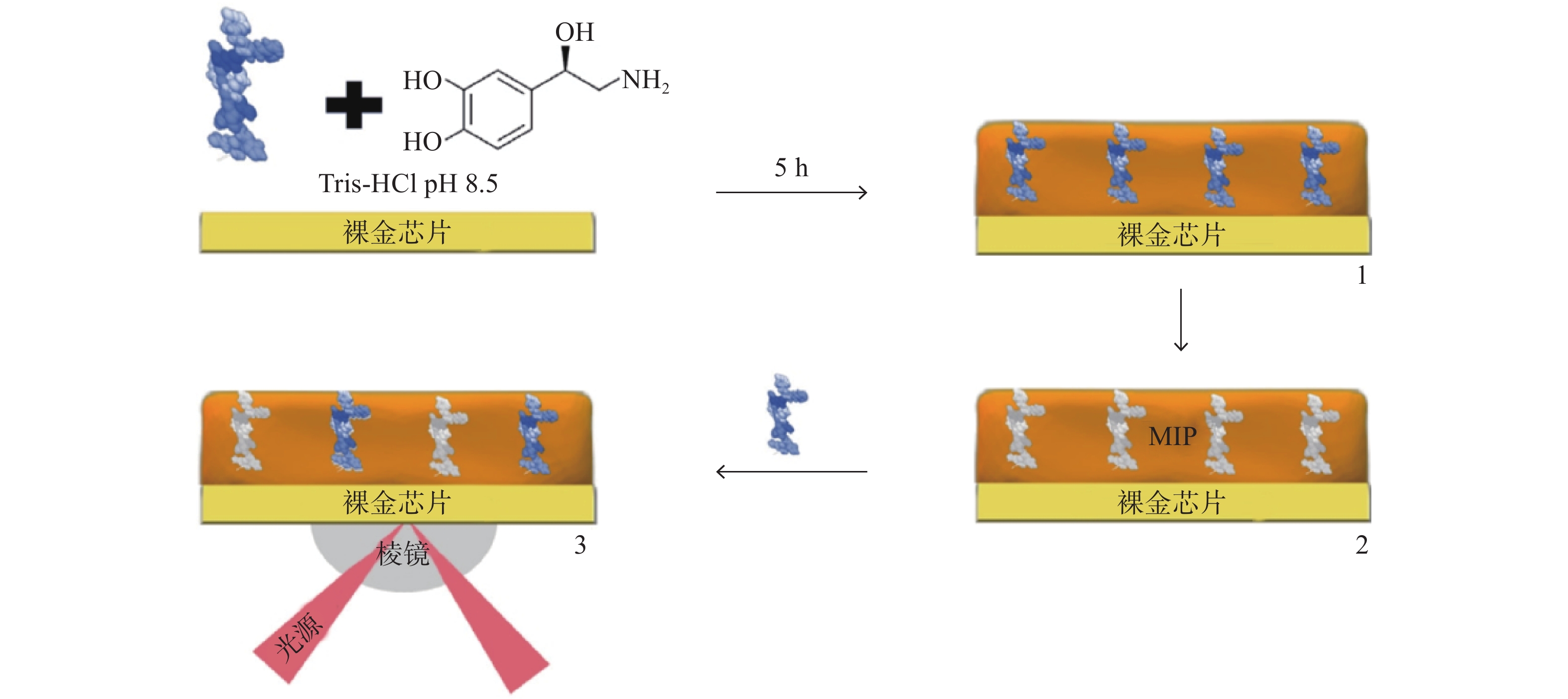
图 2 利用SPR金芯片表面压印后检测促性腺激素释放素[27]
Osman[27, 28]利用分子印迹技术制备了SPR传感器,用于检测人血清中的肌红蛋白。通过在SPR传感器的金表面涂上印有肌红蛋白的聚纳米粒子MIP,制备了基于SPR的MIP传感器,并用不同浓度的肌红蛋白溶液评估了MIP/SPR纳米传感器检测肌红蛋白的能力,结果表明,检测限和定量限分别为4.72 ng/ml和15.74 ng/ml,具有很高的灵敏度。为了确证SPR传感器的选择性,对肌红蛋白、溶菌酶、细胞色素C和牛血清白蛋白的进行了竞争性检测,结果显示,SPR传感器对肌红蛋白有很高的选择性。
Arcadio等[29]利用一种基于聚甲基丙烯酸甲酯覆盖的金纳米膜,与用于检测牛血清蛋白的MIP受体相结合,利用SPR传感器对牛血清蛋白进行检测,检测限低至8.5 nmol/L,并有进一步提高灵敏度的潜力。开发的SPR芯片可连接简易的光源和光谱仪,为SPR设备的小型化和便携化提供了可能。
Rebelo[30]利用糖类抗原125(CA-125)制备MIP,以此为识别元件通过SPR传感器检测CA-125。结果表明,该SPR传感器具备良好的选择性和较高的灵敏度,检测限低至0.01 U/ml,线性浓度范围为0.1~500 U/ml。并且可被成功地应用于人工血清样品的CA-125分析,回收率为91%~105%,平均相对误差为5.8%。可见,SPR传感器具有灵敏度高、无需标签、实时检测以及样品消耗低的突出优势。
-
Deniz等[31]制备了多巴胺压印的聚纳米颗粒MIP,并以未用多巴胺分子压印的聚纳米颗粒MIP作为对照,利用SPR传感器对水溶液和生物样品中的多巴胺进行了选择性和敏感性检测,在0.01~0.5 ppb浓度范围内获得了良好的线性关系,相关系数r分别为0.990 8(0.01~0.075 ppb)和0.990 9(0.075~0.5 ppb),用0.5 ppb多巴胺溶液评估该SPR传感器的可重复性,测定的相对标准偏差小于1.7%。基于SPR的多巴胺检测技术,有效避免了传统技术样品制备繁琐、成本高、设备复杂等使用限制,并且不需要耦合过程,传感器制备简单,可被成功应用于检测人工血浆和生理血清样品中的多巴胺。
-
Cennamo等[32]针对检测SARS-CoV-2病毒的需要,设计了一种特定的MIP受体,并与SPR相结合,用于SARS-CoV-2刺突蛋白的S1亚单位的识别。结果表明,该光学-化学传感器的灵敏度高于逆转录聚合酶链反应,并且检测时间短(10 min),其结果初步证明,MIP/SPR是一种能快速进行SARS-CoV-2病毒检测的方法,可在大量样本上进一步验证。
MIP近些年来在合成上已得到很大的创新和进步,与天然生物分子相比,不但具有同等的识别特异性,还具备了更高的稳定性,并且成本低、制备简单,在取代天然生物分子作为生物传感器识别元件上具有潜在的优势。基于MIP的SPR传感器因其高度选择性和灵敏度,已被广泛应用于包括环境污染物、药物分子等在内的多种小分子检测领域,可以在早期疾病诊断或生物研究中特异性识别生物标志物。
-
蛋白质作为识别元件可用于检测蛋白质-蛋白质相互作用、蛋白质-小分子的特异性结合,其优点是种类丰富、易于表达、特异性好,并且蛋白质作为药物靶点的主要组成,使基于蛋白质作为识别元件的SPR传感器在筛选药物中可以直接针对关键的生物分子,提高药物疗效和选择性,缺点在于蛋白质的纯化成本较高且费时、稳定性欠佳。
Yang等[33]为了无标记检测多价蛋白质结合及其动力学过程,利用pNIPAm-co-AAc纳米凝胶与目标分子形成多价蛋白质复合物,将其作为SPR传感器识别元件,可以无标记定量测量多价蛋白结合与单价或非特异性蛋白质-蛋白质相互作用,且不会产生增强响应(图3)。该方法用于检测程序性死亡受体(PD-1)抗体与两种PD-1蛋白多价蛋白结合时,检测限可低至10 nmol/L。该方法能够从复杂的介质中分析双特异性或多价靶分子,而无需或最大限度地减少预处理程序,对多价蛋白结合及其动力学的简便而准确的检测开辟了一条新的途径。
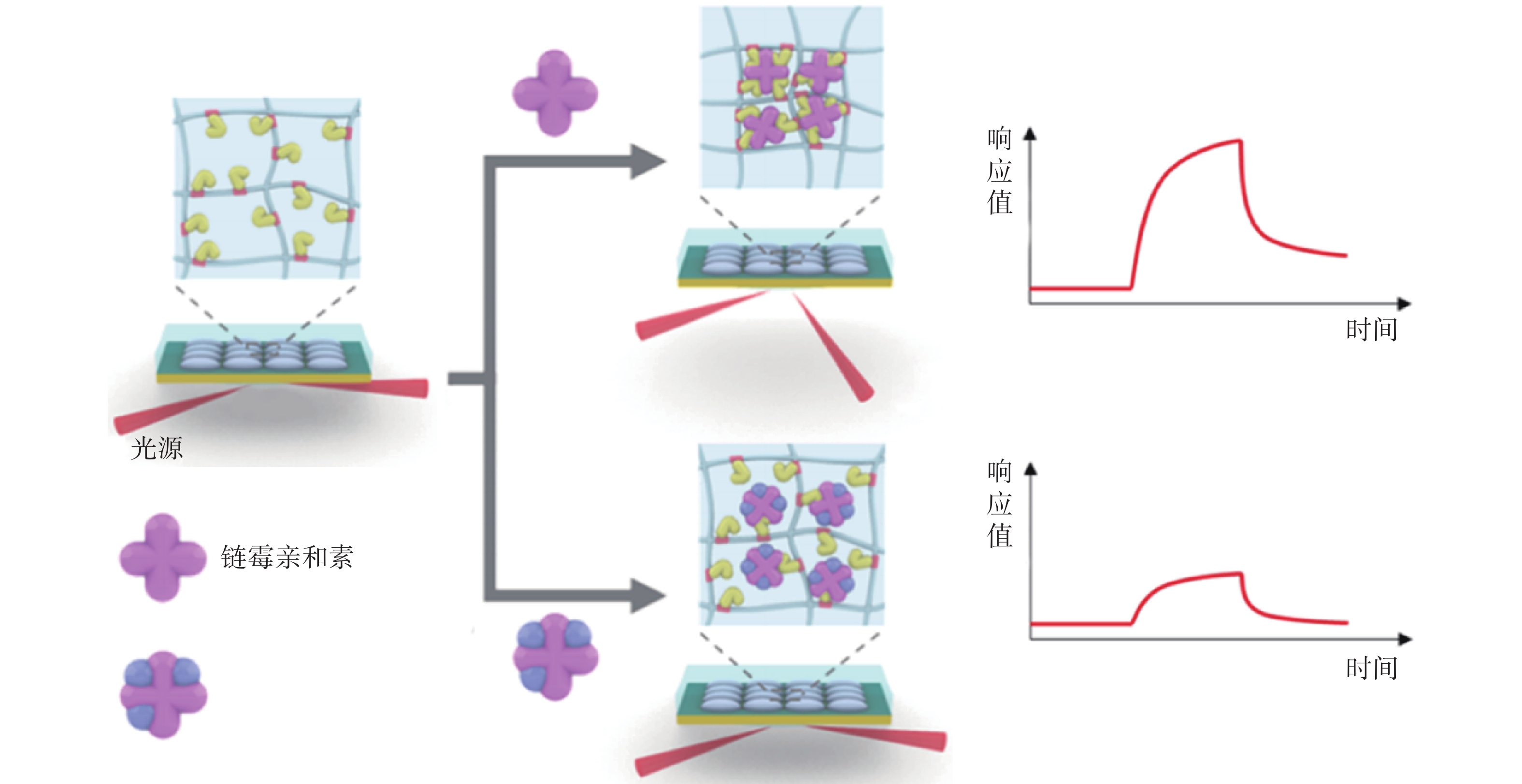
图 3 使用纳米凝胶与目标分子形成的多价蛋白质复合物对单价/多价分析物进行 SPR 分析[34]
Vachali[34]使用标准胺偶联方法将叶黄素的类固醇生成急性调节域蛋白3固定在传感器表面,利用SPR传感器来研究类胡萝卜素及其结合蛋白之间的相互作用,该方法简单灵活,有助于快速和可重复地获取结合数据。由于SPR是一种非破坏性方法,有助于最大限度地减少样品使用量。此外,SPR也已经开始用于与类胡萝卜素结合的其他大分子的研究,以表征蛋白的功能。
Zhu等[35]先用血管紧张素酶2(ACE2)作为识别元件,利用SPR传感器验证了ACE2与刺突蛋白结合结构域的相互作用,测得KD为0.37 nmol/L。然后分别将ACE2和严重急性呼吸系统综合症冠状病毒2刺突蛋白作为识别元件,通过将含不同浓度(0~125 μmol/L)化合物的20 nmol/L刺突蛋白溶液连续流过ACE2表面,观察信号响应,筛选出阻断作用最强的化合物02B05(去甲泽拉木醛),测定了02B05-ACE2和02B05-刺突蛋白的结合亲和力分别为1.736 μmol/L和1.039 μmol/L,竞争实验结果表明,筛选出的化合物02B05可有效阻断刺突蛋白与ACE2蛋白的结合。
蛋白质作为在SPR传感器中常用的识别元件,在研究蛋白质-蛋白质、蛋白质-小分子相互作用,靶向药物筛选上具有较大潜力,并且灵敏度高、特异性强,但同抗体一样,仍然存在稳定性欠佳、易失活、保存要求高等特性。利用蛋白质作为识别元件的SPR传感器能够在药物开发过程中帮助筛选和评估候选药物分子与目标蛋白质之间的结合情况。此外,在生物医学研究领域,在探索蛋白质间相互作用、研究蛋白质与其他分子的结合机制等方面的应用,有助于揭示生物分子网络和信号传导路径的复杂性。
-
由于金属纳米粒子的局域共振与传感薄膜的SPR之间存在共振耦合,因此金属纳米粒子可增强SPR传感器的信号[36],在研究中可作为SPR传感器的放大标签。基于金属纳米粒子的SPR传感器可以大大提高其光谱吸收强度,在检测领域具有广阔的应用前景。
Peng等[37]开发了一种基于银纳米粒子的SPR传感器,用于自动比色检测水样中的痕量Cu2+。该方法对Cu2+的测定具有高灵敏度和良好的选择性。Cu2+浓度为10 μg/L的样品的相对标准偏差(RSD,n=7)为1.21%,25 μg/L的样品的RSD为1.03%。该方法具有简单、快速、低成本和灵敏的特点,已成功应用于水质研究和食品安全,并被证明对现场监测和污染调查具有巨大的潜在价值。
Janani等[38]报道了一种利用银纳米颗粒作为探针的SPR技术对Hg2+进行高灵敏度和特异性检测。在396 nm处的吸光度与20~100 nmol/L Hg2+存在着极好的线性关系(r=0.996),具有较高的灵敏度,并且在其他金属离子的检测中表现出了良好特异性,检测水平不受pH、温度和含盐量的影响,在检测和定量测定各种水样中的Hg2+离子方面表现出高效性。
金属纳米粒子,特别是金纳米粒子和银纳米粒子,因其独特的光学性质而在增强SPR传感器信号方面扮演了重要角色。这些纳米粒子通过局部表面等离子体共振效应能够显著增强传感器的信号,为检测信号的放大提供了一种独特的方法。尤其在重金属离子检测领域,基于金属纳米粒子的SPR传感器显示出了极高的灵敏度和优良的选择性,这使得它们成为检测痕量污染物的强大工具。然而,尽管金属纳米粒子在增强SPR传感器性能方面具有显著的优势,它们的应用仍然面临一些挑战,其制备和功能化过程需要精确控制,以确保它们具有适当的尺寸、形状和表面化学性质,而这一过程往往复杂且成本较高,限制了基于金属纳米粒子的SPR传感器在更广泛应用中的推广。
-
抗体是最广泛应用的识别元件,是基于抗原-抗体特异性识别的原理进行检测,但其在检测目标、成本和稳定性方面存在一定限制;核酸适配体合成简便、稳定性高且易于标记,但其更容易受到环境(如:离子强度、盐浓度和pH值)的影响,限制了其在测试中的广泛应用;MIP具有耐热、耐化学试剂和机械性能优良优势,且成本较低,但其制备过程复杂、耗时较长以及模板分子渗漏等问题仍需解决;蛋白质作为识别元件具有靶向筛选药物的潜力,但其存在稳定性差、成本高等局限性;金属纳米粒子作为信号放大标签,在重金属离子检测方面具有独特优势,但应用范围受限、制备要求高(表2)。
表 2 不同识别元件的优缺点及应用范围
识别元件 优点 缺点 应用范围 抗体 高特异性和灵敏度,能够识别极小量的目标分子,适用范围广泛 制备过程繁琐,成本较高,易产生交叉反应,稳定性有限 广泛应用于各种生物分子的检测以及感染性疾病的早期诊断 适配体 制备简单、成本低,稳定性高 结合亲和力可能低于抗体;可能受环境因素的影响 检测小分子、蛋白质、细菌等 MIP 耐热、耐化学试剂、机械性能优良,成本较低,可重复使用 识别特异性和亲和力可能不如生物识别元件;制备过程复杂、耗时,可能存在模板分子渗漏问题 检测小分子、某些蛋白质 蛋白质 高特异性和灵敏度,可通过基因工程方法定制 稳定性差,成本高,制备和纯化过程复杂 分析蛋白-蛋白相互作用,靶向药物筛选 金属纳米粒子 可以显著增强SPR传感器的信号,尤其适用于放大检测信号 应用范围受限,制备要求高 主要用于重金属离子的检测 本文综述了基于不同识别元件的SPR传感器在临床医药领域中的研究进展,识别元件是SPR传感器的重要组成部分,SPR传感器的发展依赖于识别元件的发展。各种识别元件都在SPR传感器中展现了不同的优势和潜力,相信随着对识别元件的深入研究,SPR传感器不仅可以继续发挥其无需标记、实时快速的优势,而且将在准确度、灵敏度上进一步提高,在医药研究中不断拓展更加广泛的应用领域。
Research and Application Progress on Recognition Components for Surface Plasmon Resonance Sensors in the Pharmaceutical Field
-
摘要: 表面等离子共振(SPR)传感器是一种基于光学的检测技术,基于SPR的生物传感器可实时动态检测生物样品,具有无需标记和高灵敏度等卓越特点,是表征分子间相互作用的重要工具。识别元件是SPR传感器的关键组成部分,能够特异性识别和捕获目标分析物,与传感器的选择性能有密切关系。综述采用抗体、适配体、分子印迹聚合物、蛋白质和金属纳米粒子作为识别元件的SPR传感器在医药研究中的进展。Abstract: Surface plasmonresonance (SPR) sensoris anoptical detection technique enables real-time and dynamic monitoring of biological samples. SPR-based biosensors have remarkable characteristics such as label-free detection and high sensitivity, making them important tools for studying molecular interactions. The recognition element, which plays a critical role in SPR sensors, allows for specific identification and capture of target analytes, closely influencing the selectivity performance of the sensor. The progress on SPR sensors in pharmaceutical research were reviewed, which focused on the application of recognition elements such as antibodies, aptamers, molecularly imprinted polymers, and metal nanoparticles.
-
Key words:
- surface plasmonresonance /
- recognition element /
- antibodies /
- aptamers /
- molecularly imprinted polymer
-
高海拔地区最大的环境特征是大气压降低,从而使机体从空气中摄取的氧气含量减少。大脑是最耗氧的器官之一,对缺氧异常敏感,这种生理反应使机体处于缺氧环境中可能导致严重的脑损伤,如学习和记忆障碍[1-3]。近年来,治疗或改善低压低氧引起的中枢神经系统(CNS)损伤在高原医学领域引起了越来越多的关注[4-5]。有研究表明,急性暴露在缺氧环境中会加速活性氧(ROS)的积累,并在海马和皮层区域引发氧化应激,而氧化应激是高原学习记忆障碍的主要诱因[6-8],随着进入高海拔地区学习、工作和旅游的人群日益增加,寻找能够改善高原缺氧记忆损伤的药物成为高原缺氧损伤防治研究的重点。
利舒康胶囊为国药准字(Z20025932)药品,其主要成分为红景天、手掌参、甘青青兰、烈香杜鹃四味藏药辅以黄柏、甘草,经提取加工而制成,具有抗缺氧、抗疲劳、增强体力等作用,主要用于防治各种急、慢性高原病。临床实践表明,该药还可用于慢性缺血缺氧症侯群、脑梗死伴眩晕症、血管性痴呆、非痴呆性血管性认知功能障碍等缺血缺氧性相关疾病的治疗,其作用机制可能与抑制脂质过氧化,清除自由基减轻氧化应激有关[9]。本研究利用大型低压氧舱模拟高原海拔7500 m缺氧72 h,从行为学、组织形态学和Keap1/Nrf2/HO-1信号通路等方面,探讨利舒康胶囊对高原学习记忆损伤的干预作用,以期进一步阐明其可能的作用机制,为扩大利舒康胶囊的药理作用和临床适用范围提供理论依据。
1. 材料与方法
1.1 实验动物
本研究符合《实验动物福利伦理指南》的规定,并取得中国人民解放军联勤保障部队第九四〇医院伦理委员会的认可(审批编号:2021KYLL205)。实验动物选取体重质量为18~22 g的SPF级Balb/C雄性小鼠60只,购于兰州大学动物科,饲养于中国人民解放军联勤保障部队第九四〇医院动物实验科[合格证号:SCXK(京)2016-0006]。
1.2 主要材料和仪器
Anti-Keap1抗体、Anti-Nrf2抗体、Anti-HO-1抗体、Anti-Caspase-3抗体、Anti-Bcl-2抗体、Anti-Bax抗体(abcam公司);β-Actin单克隆抗体(中杉金桥公司);RM-200八臂迷宫分析测试系统(成都泰盟科技有限公司);DYC-1703070大型低压氧舱(贵州风雷航空军械有限公司);Tissuelyser-24多样品组织研磨机(上海净信实业发展有限公司);免疫印迹分析仪( BIO-RAD公司)。
1.3 方法
1.3.1 八臂迷宫实验检测高原缺氧小鼠的学习记忆能力
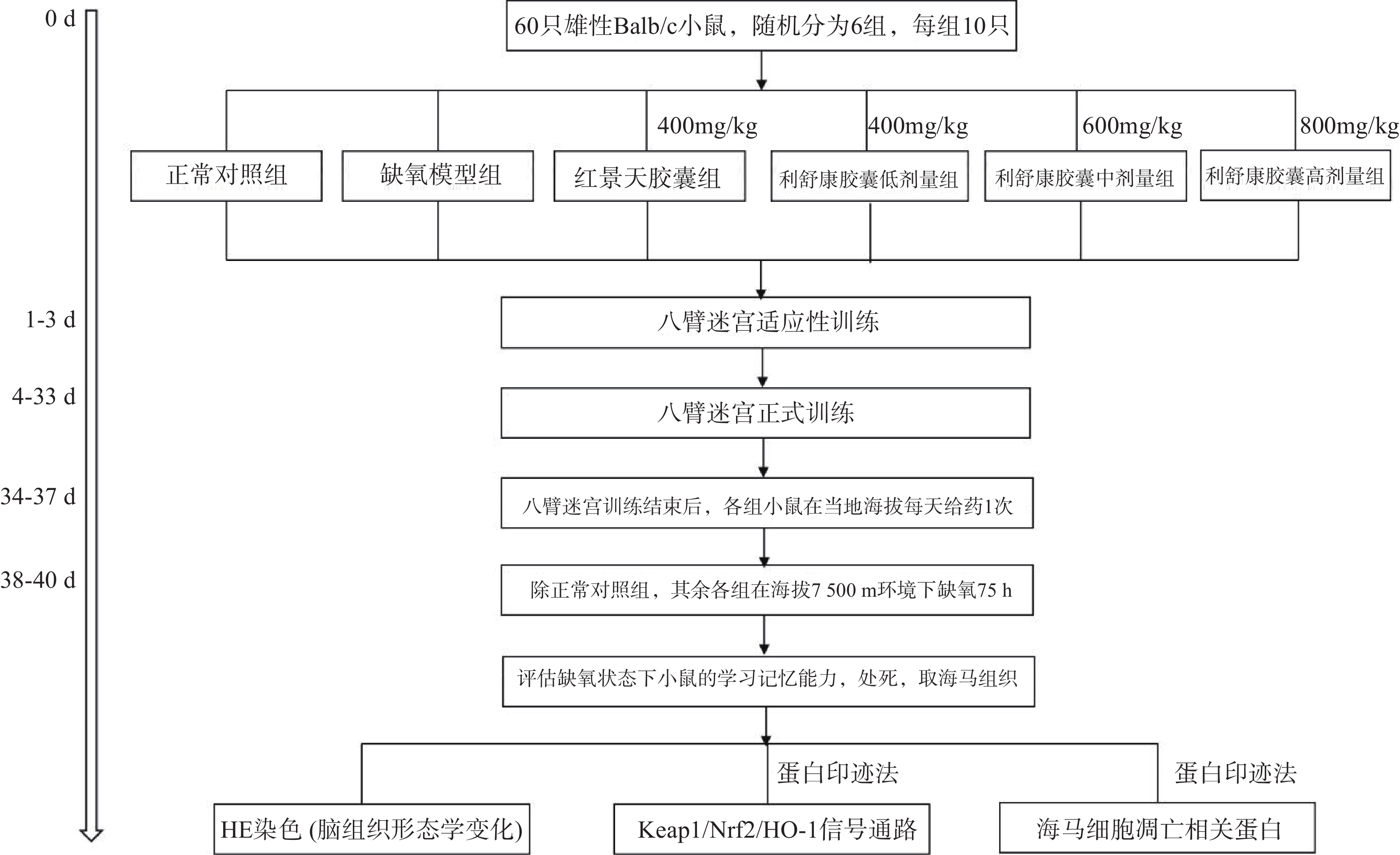
海马体对空间工作记忆和参考记忆都是至关重要的,Xu等[10]研究发现八臂迷宫以食物作为诱饵,并在食物臂上贴上信号图,使实验动物可以在参考记忆任务中优先进入下一个选择的手臂的轨迹,在工作记忆任务中优先进入先前访问过的手臂的轨迹。Li和Du等[11-12]研究表明,八臂迷宫模型能很好地反映高原缺氧环境下小鼠的空间工作记忆情况和参考记忆情况。本实验将60只Balb/C雄性小鼠(体重18~22 g)进行适应性训练3 d(将小鼠置于迷宫中,八个臂均放入饵料自由进食5 min),适应性训练结束后,称重,将小鼠随机分为正常对照组、缺氧模型组、红景天胶囊组[400 mg/kg],利舒康胶囊低剂量组[400 mg/kg]、中剂量组[600 mg/kg]、高剂量组[800 mg/kg],每组10只,在之后的饲养中给予小鼠少许食物,第4 d开始正式实验,训练时,在1、3、4、7(食臂末端有信号图)食臂中放入饵料,将一只小鼠置于迷宫中央用塑料罩罩住,30 s后打开使其自由进食,训练总时间为5 min,5 min内吃完则系统自动停止,5 min内仍未吃完则实验终止。测试指标为:①工作记忆错误(WME),即在同一个训练中小鼠再次进入已经放置饵料的臂;②参考记忆错误(RME),即小鼠进入没有放置饵料的臂;③小鼠探究总时间(TT),即小鼠吃完所有饵料所花的时间;④错误总次数(TE)。小鼠训练成功的标准为:WME=0;RME≤1。每次训练结束后清理小鼠排泄物,立即用75%酒精擦拭八臂迷宫内部祛除气味干扰。连续训练30 d。
1.3.2 低压低氧实验
在小鼠训练30 d后,第31 d开始对所有实验小鼠进行灌胃给药,每天一次。正常对照组和缺氧模型组给予等体积的生理盐水,连续给药7 d(舱外给药4 d+舱内给药3 d),期间所有小鼠正常在当地海拔进行八臂迷宫训练。在灌胃给药第4 d后停止八臂迷宫训练,除正常对照组外,将其余各组小鼠放入大型低压低氧动物实验舱内,借助实验舱可以模拟不同海拔高度。小鼠以极快的速度(10 m/s)从当地海拔上升到海拔7500 m高度。之后每天上午实验员进入实验舱内(舱内海拔由7500 m降至3500 m,下降速度为20 m/s),在模拟海拔3 500 m处对小鼠进行灌胃给药,连续3 d。每天给药完毕后,将海拔上升至预定海拔7500 m处,小鼠在舱内自由进食和摄水,正常对照组小鼠同时饲养于动物实验科(当地海拔1500 m)。缺氧结束后将舱内海拔降至3500 m,实验人员进入舱内,检测高原损伤后小鼠的学习记忆能力,评价利舒康胶囊对高原学习记忆损伤的干预作用。实验流程如图1。实验结束后麻醉小鼠,取出海马组织,按照测定要求对其进行处理。
1.3.3 HE染色观察高原缺氧小鼠脑组织形态学变化
各组取2只小鼠,取脑组织,经生理盐水漂洗后放入10%甲醛溶液中浸泡,1周后,将样品送至联勤保障部队第940医院病理科,进行石蜡包埋,组织切片,染色以及光镜观察。
1.3.4 Western blot法检测高原缺氧小鼠海马中Nrf2途径蛋白含量及凋亡相关蛋白含量
将实验小鼠大脑取出,剖出海马体后,用BCA法测定其蛋白浓度,之后加入缓冲液,100 ℃水浴5 min,流水冷却后重复水浴5 min使其充分变性。以β-action为内参,配制10% SDS聚丙烯酰氨凝胶进行电泳、转膜和抗体孵育。次日使用ECL液进行显影,Image J图像分析软件对条带进行分析。其中一抗稀释比例为:β-action(1: 2000)、Keap1(1∶1000)、Nrf2(1∶2 000)、HO-1(1∶2 000)、Caspase-3(1∶2 000)、Bax(1∶1000)、BCL-2(1∶1000)。
1.4 统计学处理
采用SPSS 13.0统计软件分析结果,符合正态分布的实验数据以
$ \bar x \pm s $ 表示。多组间比较采用单因素方差分析,P<0.05表示差异有统计学意义。2. 结果
2.1 利舒康胶囊对高原缺氧小鼠学习记忆的干预作用
与正常对照组相比,缺氧模型组小鼠WME、RWE、TE、TT均显著升高(P<0.01);与缺氧模型组相比,利舒康胶囊三个剂量组小鼠四项指标均有不同程度降低,其中利舒康胶囊高剂量组800 mg/(kg·d)具有显著性差异(P<0.05或P<0.01),表明高原低氧缺氧能导致小鼠空间记忆障碍,而利舒康胶囊高剂量组能够增强高原缺氧小鼠的短期记忆和长期记忆能力,改善大鼠认知,有望对高原缺氧导致的学习记忆损伤患者提供预防和治疗。结果见表1和图2。
表 1 利舒康胶囊对高原缺氧小鼠空间记忆的影响($ \bar x \pm s $ ,n=10)组别 剂量(mg/kg) WME(次) RME(次) TT(秒) TE(次) 正常对照组 − 0.43±0.35 0.50±0.41 63.47±9.64 1.50±0.17 缺氧模型组 − 3.00±1.58## 2.70±1.52## 119.99±43.00## 3.10±0.48## 红景天胶囊组 400 0.57±0.35** 0.79±0.49* 81.78±28.20 1.83±0.29#** 利舒康胶囊低剂量组 400 2.43±0.89#▲ 1.36±0.56 99.45±25.27## 3.06±0.58##▲▲ 利舒康胶囊中剂量组 600 2.14±1.14#▲ 1.43±1.10 98.75±30.51 3.04±0.52##▲▲ 利舒康胶囊高剂量组 800 1.07±0.61* 0.71±0.49** 69.38±31.69* 1.73±0.37** # P<0.05, ## P<0.01, 与正常对照组比较;* P<0.05, ** P<0.01,与缺氧组比较 ;▲P<0.05, ▲▲P<0.01,与红景天胶囊组比较 2.2 利舒康胶囊对缺氧小鼠脑组织形态学的影响
海马体在学习和记忆中发挥关键作用,尤其容易受到缺氧损伤。在本研究中,小鼠脑组织病理学切片结果显示(见图3),正常对照组小鼠海马神经元密集,细胞核大而圆,神经元呈层状有序分布,各层细胞形态饱满而数量多,细胞排列整齐且致密;缺氧模型组小鼠海马神经元细胞分布受到破坏,细胞形态不规则,部分细胞缺失或有空泡(黑色箭头),出现明显核固缩(蓝色箭头),细胞排列紊乱,CA1区有较多不规则形状的深染细胞(绿色箭头),表明脑组织出现神经元死亡;经过红景天胶囊和利舒康胶囊药物干预后,缺氧模型组脑组织损伤有所改善;神经元细胞排列整齐,核固缩现象减轻(蓝色箭头),深染细胞数量减少(绿色箭头),少有细胞缺失或空泡(黑色箭头),细胞形态较好,排列整齐,且利舒康胶囊高剂量组脑组织形态趋于正常。
2.3 利舒康胶囊对高原缺氧小鼠海马组织中Keap1/Nrf2/HO-1信号通路蛋白水平的影响
与正常对照组比较,缺氧模型组小鼠海马组织中Keap1蛋白含量显著升高(P<0.01),Nrf2、HO-1蛋白含量显著降低(P<0.01);与缺氧模型组相比,利舒康胶囊组Keap1蛋白含量降低(P<0.01),Nrf2、HO-1蛋白含量显著升高(P<0.01),其中以利舒康胶囊高剂量组蛋白水平变化最为显著,且效果优于阳性药红景天胶囊组。结果见图4和表2。
表 2 各组小鼠脑组织蛋白表达比较($ \bar x \pm s $ ,n=3)分组 剂量
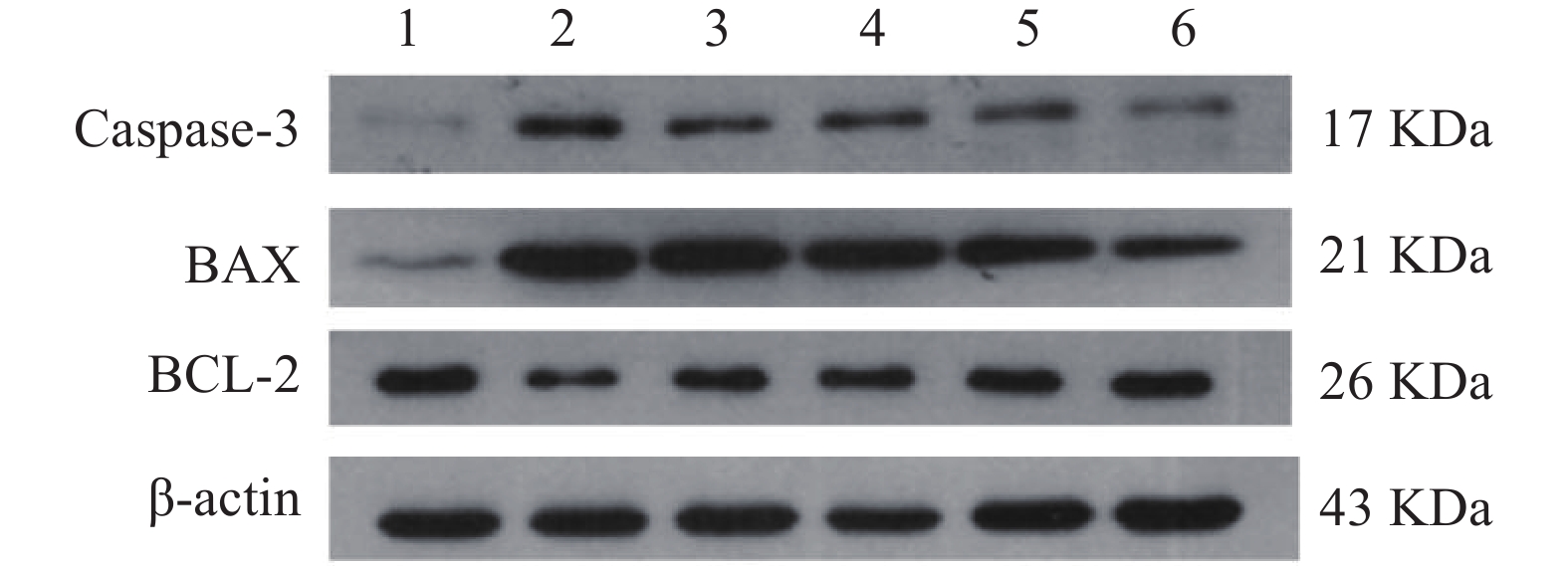
(mg/kg)Keap1 Nrf2 HO-1 Caspase-3 Bax BCL-2 正常对照组 − 0.36±0.08 0.76±0.08 1.03±0.14 0.03±0.01 0.09±0.02 0.79±0.09 缺氧模型组 − 0.91±0.15## 0.45±0.03## 0.31±0.05## 0.95±0.12## 0.99±0.08## 0.31±0.02## 红景天胶囊组 400 0.44±0.03** 0.92±0.15** 0.86±0.03 0.75±0.16## 0.73±0.11##** 0.65±0.02#** 利舒康胶囊低剂量组 400 0.42±0.03** 0.77±0.09** 0.67±0.02## 0.86±0.17## 0.81±0.05##** 0.62±0.02##** 利舒康胶囊中剂量组 600 0.41±0.04** 0.83±0.09** 0.98±0.13 0.64±0.06## 0.79±0.06##** 0.73±0.09** 利舒康胶囊高剂量组 800 0.33±0.02**▲▲ 1.21±0.11##**▲▲ 1.23±0.08**▲▲ 0.46±0.01##**▲▲ 0.52±0.05##**▲ 0.76±0.11** # P<0.05, ## P<0.01, 与正常对照组比较;* P<0.05, ** P<0.01,与缺氧组比较;▲P<0.05, ▲▲P<0.01,与红景天胶囊组比较 2.4 利舒康胶囊对缺氧小鼠海马细胞凋亡相关蛋白Caspase-3、Bax和Bcl-2的影响
与正常对照组相比,缺氧模型组小鼠海马组织中Caspase-3和Bax的表达显著升高(P<0.01),Bcl-2蛋白表达显著降低(P<0.01);与缺氧模型组相比,利舒康胶囊组Caspase-3和Bax蛋白表达显著降低(P<0.01),BCL-2蛋白表达显著升高(P<0.01),其中利舒康胶囊高剂量组蛋白水平变化尤为显著,且效果优于阳性药红景天胶囊组。结果见图5和表2。
3. 讨论
缺氧会引起机体组织细胞形态结构、功能和代谢的异常变化[13]。大脑对缺氧刺激异常敏感,短期暴露于高海拔地区的急性低压低氧会导致认知能力下降,尤其是空间学习、短期记忆和工作记忆能力下降。高原脑缺氧可引起中枢神经系统氧化性损伤,大量证据表明,氧化应激和细胞凋亡是神经元变性和死亡的主要诱因,进而造成机体认知功能损伤,且这种损伤为不可逆缺陷[14]。在本研究中,采用八臂迷宫实验方法检测高原缺氧环境下小鼠的空间记忆能力。通过利舒康胶囊高剂量组干预治疗后,WME、RME、TE和TT与缺氧模型组相比显著减少(P<0.05或P<0.01),表明利舒康胶囊能明显提高高原缺氧环境下小鼠的空间记忆能力。海马体是大脑中对缺氧最敏感的区域之一,也是形成空间记忆的关键区域[15]。HE染色结果显示,与正常对照组相比,缺氧模型组小鼠海马神经元细胞形态不规则,部分细胞缺失或有空泡;经利舒康胶囊干预后,细胞形态较好,排列整齐,少有细胞缺失或空泡。提示进行利舒康胶囊药物干预可以修复高原低压低氧造成的海马组织神经元损伤,防止了小鼠因暴露在高海拔环境中而导致的空间记忆障碍。
Keap1/Nrf2/HO-1信号通路是机体内保持细胞稳态的防御系统,在预防由氧化应激引起的细胞损伤中起重要作用,Nrf2是一种具有细胞保护和抗氧化活性的诱导蛋白,可被视为氧化应激反应的主调控因子[16-18],HO-1可以催化血红素的分解,从而抑制中性粒细胞、淋巴细胞和巨噬细胞介导的炎性反应。在正常条件下,Keap1形成E3泛素连接酶的一部分,紧密调控Nrf2的活性,这时的Nrf2活性处在相对较低水平。在氧化应激条件下,Nrf2通过与细胞质中含有半胱氨酸残基的阻遏蛋白Keap-1分离而被激活,进而激活其相关的抗氧化酶HO-1表达,达到抵抗氧化应激的作用[19]。本文研究结果显示,与正常对照组相比,缺氧模型组海马细胞中Keap1蛋白表达升高(P<0.01)、Nrf2和HO-1蛋白表达下降(P<0.01),表明机体在缺氧环境中,ROS积累导致氧化还原状态失衡。经利舒康胶囊干预后,与缺氧模型组相比,Keap1蛋白表达下降,且具有显著性差异(P<0.01),Nrf2和HO-1蛋白表达均显著升高(P<0.01),且利舒康胶囊高剂量组治疗效果最好,本课题组前期对利舒康胶囊对高原脑损伤防治的研究结果显示[20],机体暴露在高原环境下,活性氧(ROS)大量累积导致氧化性损伤,利舒康胶囊能降低脑组织内MDA含量,提高SOD的活力,具有良好的抗氧化应激的作用。因此我们推测,利舒康胶囊可以通过调控Keap1/Nrf2/HO-1信号通路,发挥抗氧化作用。
氧化应激和炎症在脑损伤中起关键作用,导致细胞凋亡。细胞凋亡是器官生长、发育和维持正常体内平衡的重要过程,也是脑缺氧后神经元死亡的主要形式[21]。海马锥体神经元的凋亡可导致学习和记忆障碍[22]。Caspase-3作为凋亡激活剂是重要的凋亡调节因子,是缺氧诱导的脑细胞凋亡的重要标志,抗凋亡基因Bcl-2和促凋亡基因Bax可能参与病理性凋亡和坏死细胞死亡途径[23-25]。在本研究中,与正常对照组相比,缺氧模型组Caspase-3蛋白含量和Bax/Bcl-2比值表达显著增加(P<0.01),说明缺氧可引起脑组织细胞凋亡。而利舒康胶囊可通过降低Caspase-3蛋白表达和Bax/Bcl-2比值来逆转这些变化,抑制了氧化应激引起的脑细胞凋亡和炎症引发的脑损伤,且利舒康胶囊高剂量组优于阳性药红景天胶囊组。
综上所述,利舒康胶囊可以改善高原缺氧小鼠脑损伤,提高小鼠在高原缺氧环境下的抗氧化能力,其机制可能是通过调控Keap1/Nrf2/HO-1信号通路发挥抗氧化应激作用,减轻缺氧引起的ROS积累;同时通过抗氧化和抗凋亡机制在低压低氧暴露期间为海马神经元提供神经保护作用,从而改善缺氧诱导的小鼠空间记忆损伤。
利益冲突
所有作者均声明不存在利益冲突。
-
图 1 裸金 SPR 传感器表面的制作和利用SPR传感器检测杆状病毒抑制因子5[6]
图 2 利用SPR金芯片表面压印后检测促性腺激素释放素[27]
图 3 使用纳米凝胶与目标分子形成的多价蛋白质复合物对单价/多价分析物进行 SPR 分析[34]
表 1 基于不同识别元件的SPR传感器在医药领域中的应用
识别元件 分析物 检测限 KD值 线性范围 参考文献 抗体 杆状病毒抑制因子5 6.25 pg/ml 12.1 nmol/L — [5] NS1蛋白 0.8 nmol/L — — [6] 马血红素肌红蛋白 — (270 ± 14)nmol/L — [7] 脱辅基肌红蛋白 — (350 ± 13.5)nmol/L — [7] 白介素-8 — 82.2 nmol/L — [9] 吡噻菌胺 10 ng/ml 0.82 nmol/L — [10] 诺氟沙星 0.02 ng/ml — — [11] 三唑磷 0.096 ng/ml — 0.98~8.29 ng/ml [12] 适配体 β淀粉样蛋白 0.2 pmol/L — — [14] 糖化血红蛋白 2.55 nmol/L — — [15] 溶菌酶 2.4 nmol/L — — [16] 肌钙蛋白T 3.13 nmol/L — — [17] 新霉素B 5 nmol/L 6.5 μmol/L 10 nmol/L~100 µmol/L [19,20] 伊马替尼 — 0.7~10 µmol/L [21] 军团菌 104.3 CFU/ml — — [22] 副溶血性弧菌 2.4 nmol/L — [23] MIP 多巴胺 — — 0.01~0.50 ppb [31] 肌红蛋白 4.72 ng/ml — — [27,28] 牛血清蛋白 8.5 nmol/L — — [29] CA-125 0.01 U/ml — 0.1 ~500 U/ml [30] 蛋白质 PD-1蛋白 10 nmol/L — — [33] 刺突蛋白 — 0.37 nmol/L — [35] 金属纳米粒子 Cu2+ 0.24 μg/L — 0.5 ~35 μg/L [37] Hg2+ 8 nmol/L — 20~100 nmol/L [38] Hg2+ 9.98 nmol/L — — [39] Cu2+ 200 nmol/L — 10~250 μmol/L [40] 注:“—”表示在原文中未说明。 表 2 不同识别元件的优缺点及应用范围
识别元件 优点 缺点 应用范围 抗体 高特异性和灵敏度,能够识别极小量的目标分子,适用范围广泛 制备过程繁琐,成本较高,易产生交叉反应,稳定性有限 广泛应用于各种生物分子的检测以及感染性疾病的早期诊断 适配体 制备简单、成本低,稳定性高 结合亲和力可能低于抗体;可能受环境因素的影响 检测小分子、蛋白质、细菌等 MIP 耐热、耐化学试剂、机械性能优良,成本较低,可重复使用 识别特异性和亲和力可能不如生物识别元件;制备过程复杂、耗时,可能存在模板分子渗漏问题 检测小分子、某些蛋白质 蛋白质 高特异性和灵敏度,可通过基因工程方法定制 稳定性差,成本高,制备和纯化过程复杂 分析蛋白-蛋白相互作用,靶向药物筛选 金属纳米粒子 可以显著增强SPR传感器的信号,尤其适用于放大检测信号 应用范围受限,制备要求高 主要用于重金属离子的检测 -
[1] WEIX X, YINM, ZHANGL, et al. Surface Plasmon Resonance(SPR)biosensor for detection of mycotoxins: a review[J]. JImmunolMeth, 2022, 510:113349. [2] MULYANTIB, NUGROHOHS, WULANDARIC, et al. SPR-based sensor for the early detection or monitoring of kidney problems[J]. IntJBiomater, 2022, 2022:9135172. [3] SWAMIS, KAYENATF, WAJIDS. SPR biosensing: cancer diagnosis and biomarkers quantification[J]. MicrochemJ, 2024, 197:109792. doi: 10.1016/j.microc.2023.109792 [4] 张泽, 张颖聪, 于洪伟, 等. 生物传感器识别元件的种类及其在临床检验中的研究进展[J]. 临床检验杂志, 2020, 38(10):767-771. [5] JENA S C, SHRIVASTAVA S, SAXENA S, et al. Surface plasmon resonance immunosensor for label-free detection of BIRC5 biomarker in spontaneously occurring canine mammary tumours[J]. Sci Rep, 2019, 9(1):13485. doi: 10.1038/s41598-019-49998-x [6] Widoretno, SJAHRURACHMAN A, DEWI B E, et al. Surface plasmon resonance analysis for detecting non-structural protein 1 of dengue virus in Indonesia[J]. Saudi J Biol Sci, 2020, 27(8):1931-1937. doi: 10.1016/j.sjbs.2020.06.018 [7] MIHOC D, LUPU L M, WIEGAND P, et al. Antibody epitope and affinity determination of the myocardial infarction marker myoglobin by SPR-biosensor mass spectrometry[J]. J Am Soc Mass Spectrom, 2021, 32(1):106-113. doi: 10.1021/jasms.0c00234 [8] WIEGAND P, LUPU L, HÜTTMANN N, et al. Epitope identification and affinity determination of an inhibiting human antibody to interleukin IL8(CXCL8)by SPR- biosensor-mass spectrometry combination[J]. J Am Soc Mass Spectrom, 2020, 31(1):109-116. doi: 10.1021/jasms.9b00050 [9] LUPU L M, WIEGAND P, HOLDSCHICK D, et al. Identification and affinity determination of protein-antibody and protein-aptamer epitopes by biosensor-mass spectrometry combination[J]. Int J Mol Sci, 2021, 22(23):12832. doi: 10.3390/ijms222312832 [10] CEBALLOS-ALCANTARILLAE, ABAD-FUENTESA, AGULLÓC, et al. Immunochemical method for penthiopyrad detection through thermodynamic and kinetic characterization of monoclonal antibodies[J]. Talanta, 2021, 226:122123. doi: 10.1016/j.talanta.2021.122123 [11] ACAROZU, DIETRICHR, KNAUERM, et al. Development of a generic enzyme-immunoassay for the detection of fluoro (quinolone)-residues in foodstuffs based on a highly sensitive monoclonal antibody[J]. Food AnalMeth, 2020, 13(3):780-792. [12] GUOY R, LIUR, LIUY, et al. A non-competitive surface plasmon resonance immunosensor for rapid detection of triazophos residue in environmental and agricultural samples[J]. Sci Total Environ, 2018, 613-614:783-791. doi: 10.1016/j.scitotenv.2017.09.157 [13] HERMANN T, PATEL D J. Adaptive recognition by nucleic acid aptamers[J]. Science, 2000, 287(5454):820-825. doi: 10.1126/science.287.5454.820 [14] ZHENG Y, GENG X H, YANG X H, et al. Exploring interactions of aptamers with Aβ40amyloid aggregates and its application: detection of amyloid aggregates[J]. Anal Chem, 2020, 92(3):2853-2858. doi: 10.1021/acs.analchem.9b05493 [15] SUN D P, WU Y, CHANG S J, et al. Investigation of the recognition interaction between glycated hemoglobin and its aptamer by using surface plasmon resonance[J]. Talanta, 2021, 222:121466. doi: 10.1016/j.talanta.2020.121466 [16] MIHAII, VEZEANUA, POLONSCHIIC, et al. Label-free detection of lysozyme in wines using an aptamer based biosensor and SPR detection[J]. SensActuat B Chem, 2015, 206:198-204. [17] TORRINI F, PALLADINO P, BRITTOLI A, et al. Characterization of troponin T binding aptamers for an innovative enzyme-linked oligonucleotide assay(ELONA)[J]. Anal Bioanal Chem, 2019, 411(29):7709-7716. doi: 10.1007/s00216-019-02014-7 [18] TENAGLIA E, FERRETTI A, DECOSTERD L A, et al. Comparison against current standards of a DNA aptamer for the label-free quantification of tobramycin in human sera employed for therapeutic drug monitoring[J]. J Pharm Biomed Anal, 2018, 159:341-347. doi: 10.1016/j.jpba.2018.06.061 [19] LUAN Y X, WANG N, LI C, et al. Advances in the application of aptamer biosensors to the detection of aminoglycoside antibiotics[J]. Antibiotics, 2020, 9(11):787. doi: 10.3390/antibiotics9110787 [20] DE-LOS-SANTOS-ALVAREZ N, LOBO-CASTAÑÓN M J, MIRANDA-ORDIERES A J, et al. SPR sensing of small molecules with modified RNA aptamers: detection of neomycin B[J]. Biosens Bioelectron, 2009, 24(8):2547-2553. doi: 10.1016/j.bios.2009.01.011 [21] TARTAGGIA S, MENEGHELLO A, BELLOTTO O, et al. An SPR investigation into the therapeutic drug monitoring of the anticancer drug imatinib with selective aptamers operating in human plasma[J]. Analyst, 2021, 146(5):1714-1724. doi: 10.1039/D0AN01860K [22] SAADM, CASTIELLO F R, FAUCHERS P, et al. Introducing an SPRi-based titration assay using aptamers for the detection of Legionella pneumophila[J]. SensActuat B Chem, 2022, 351:130933. [23] AHN J Y, LEE K A, LEE M J, et al. Surface plasmon resonance aptamer biosensor for discriminating pathogenic bacteria Vibrio parahaemolyticus[J]. J Nanosci Nanotechnol, 2018, 18(3):1599-1605. doi: 10.1166/jnn.2018.14212 [24] CHEN L X, XU S F, LI J H. Recent advances in molecular imprinting technology: current status, challenges and highlighted applications[J]. Chem Soc Rev, 2011, 40(5):2922-2942. doi: 10.1039/c0cs00084a [25] 成琛, 史楠, 姜霄震. 分子印迹光学生物传感器的研究进展[J]. 高校化学工程学报, 2020, 34(3):572-581. [26] TORRINI F, PALLADINO P, BALDONESCHI V, et al. Sensitive ‘two-steps’ competitive assay for gonadotropin-releasing hormone detection via SPR biosensing and polynorepinephrine-based molecularly imprinted polymer[J]. Anal Chim Acta, 2021, 1161:338481. doi: 10.1016/j.aca.2021.338481 [27] OSMAN B, UZUN L, BEŞIRLI N, et al. Microcontact imprinted surface plasmon resonance sensor for myoglobin detection[J]. Mater Sci Eng C Mater Biol Appl, 2013, 33(7):3609-3614. doi: 10.1016/j.msec.2013.04.041 [28] ATAYNO, OSMANB, AKGOLS, et al. Preparation of molecularly imprinted SPR nanosensor for myoglobin detection[J]. CurrApplPolymSci, 2018, 2(2):102-111. [29] ARCADIOF, ZENIL, PERRIC, et al. Bovine serum albumin protein detection by a removable SPR chip combined with a specific MIP receptor[J]. Chemosensors, 2021, 9(8):218. doi: 10.3390/chemosensors9080218 [30] REBELOT S C R, COSTA R, BRANDÃOA T S C, et al. Molecularly imprinted polymer SPE sensor for analysis of CA-125 on serum[J]. Anal Chim Acta, 2019, 1082:126-135. doi: 10.1016/j.aca.2019.07.050 [31] TÜRKMEND, BAKHSHPOURM, GÖKTÜRKI, et al. Selective dopamine detection by SPR sensor signal amplification using gold nanoparticles[J]. New JChem, 2021, 45(39):18296-18306. [32] CENNAMO N, D’AGOSTINO G, PERRI C, et al. Proof of concept for a quick and highly sensitive on-site detection of SARS-CoV-2 by plasmonic optical fibers and molecularly imprinted polymers[J]. Sensors, 2021, 21(5):1681. doi: 10.3390/s21051681 [33] YANG H M, TEOH J Y, YIM G H, et al. Label-free analysis of multivalent protein binding using bioresponsive nanogels and surface plasmon resonance(SPR)[J]. ACS Appl Mater Interfaces, 2020, 12(5):5413-5419. doi: 10.1021/acsami.9b17328 [34] VACHALI P P, LI B X, BARTSCHI A, et al. Surface plasmon resonance(SPR)-based biosensor technology for the quantitative characterization of protein–carotenoid interactions[J]. Arch Biochem Biophys, 2015, 572:66-72. doi: 10.1016/j.abb.2014.12.005 [35] ZHU Z L, QIU X D, WU S, et al. Blocking effect of demethylzeylasteral on the interaction between human ACE2 protein and SARS-CoV-2 RBD protein discovered using SPR technology[J]. Molecules, 2020, 26(1):57. doi: 10.3390/molecules26010057 [36] ZENGS W, YUX, LAWW C, et al. Size dependence of Au NP-enhanced surface plasmon resonance based on differential phase measurement[J]. SensActuat B Chem, 2013, 176:1128-1133. [37] PENG J J, LIU G K, YUAN D X, et al. A flow-batch manipulated Ag NPs based SPR sensor for colorimetric detection of copper ions (Cu 2+) in water samples[J]. Talanta, 2017, 167:310-316. doi: 10.1016/j.talanta.2017.02.015 [38] JANANI B, SYEDA, THOMASA M, et al. Enhanced SPR signals based on methylenediphosphonic acid functionalized Ag NPs for the detection of Hg (II) in the presence of an antioxidant glutathione[J]. JMolLiq, 2020, 311:113281. [39] YUAN H Z, SUN G Y, PENG W, et al. Thymine-functionalized gold nanoparticles (Au NPs) for a highly sensitive fiber-optic surface plasmon resonance mercury ion nanosensor[J]. Nanomaterials, 2021, 11(2):397. doi: 10.3390/nano11020397 [40] DEYMEHKARE, ALITAHERM, KARAMIC, et al. Synthesis of SPR Nanosensor using Gold Nanoparticles and its Application to Copper(II) Determination[J]. Silicon, 2018, 10(4):1329-1336. doi: 10.1007/s12633-017-9608-z [41] MAURIZ E, GARCÍA-FERNÁNDEZ M C, LECHUGA L M. Towards the design of universal immunosurfaces for SPR-based assays: a review[J]. Trac Trends Anal Chem, 2016, 79:191-198. doi: 10.1016/j.trac.2016.02.006 -





 下载:
下载:
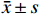






 下载:
下载:


