-
脓毒症作为机体对感染反应失调的器官功能障碍综合征,常累及肾脏,是危重患者发生急性肾损伤最常见的原因之一。脓毒症引起的急性肾损伤不仅使住院死亡率增加6~8倍,还与远期慢性肾脏病的发展和远期死亡风险增加有关[1-3]。通常认为脓毒症相关急性肾损伤的发病过程与过度炎症反应、血流动力学障碍、凝血功能障碍、小管上皮细胞损害等有关[1],然而,脓毒症相关急性肾损伤的机制尚未完全阐明。近年来,代谢组学作为反应疾病内环境与内源性代谢物之间关系的一种方法,被广泛应用于各种疾病,因此,利用代谢组学探究脓毒症相关急性肾损伤中内源性代谢物的变化有助于进一步理解其发病机制。黄连作为常见中药,具有抗炎、抗氧化等功能,被广泛用于各种疾病[4]。既往研究发现黄连可以通过减轻炎症改善脓毒症诱导的急性肝损伤[5],而黄连是否对脓毒症相关急性肾损伤存在保护作用还有待进一步研究。本研究旨在研究黄连对脓毒症相关肾损伤的影响,并利用代谢组学探讨其潜在机制。
-
C57BL/6小鼠(上海斯莱克实验动物有限责任公司);黄连生药获于安徽亳州;生化指标试剂盒(南京建成生物工程研究所有限公司);Agilent 7890A /5975C气相色谱-质谱联用仪(美国);Agilent J&W Scientific HP-5ms(30 m × 0.25 mm,0.25 μm)毛细管色谱柱。
-
黄连生药由海军军医大学药学院孙连娜教授鉴定;根据《中国药典》(2015年版)准备黄连水提取物。
-
采用盲肠结扎穿孔术(CLP)建立脓毒症相关急性肾损伤模型。将18只小鼠随机分为3组,每组6只。具体分组如下,假手术组(Sham组):仅对小鼠切开缝合,并给予等体积生理盐水灌胃;模型组(CLP组):小鼠行CLP,并给予等体积生理盐水灌胃;给药组(RCE组):小鼠行CLP,并给予黄连提取物100 mg/kg灌胃。
-
干预完成24 h后,对所有小鼠行内眦静脉取血,4 ℃冰箱静置40 min后,以3000 r/min离心5 min,取血清置于−80 ℃冰箱保存,以行生化指标检测。取血后处死小鼠,行心脏灌流,切取肾脏置于−80 ℃冰箱中保存,以行代谢组学分析。
-
利用试剂盒检测血清肌酐(Scr)、尿素氮(BUN)水平。
-
取出−80 ℃冰箱中储存的肾组织,取皮髓交界部分,称取50 mg加入内标甲醇1 ml,再加入碾磨珠,4 ℃,70 Hz碾磨120 s,再置入4 ℃离心机中,以12 000 r/min离心10 min,取上清液200 μl置离心管中,温和氮气吹干。加入15 mg/ml的甲氧胺吡啶溶液50 μl,涡旋30 s,置于70 ℃的烘箱中反应60 min,再加入N-甲基-N-三甲基硅基三氟乙酰胺(含1%的三甲基氯硅烷)50 μl,涡旋1 min,室温反应30 min,再加入100 μl正庚烷,涡旋30 s,4 000 r/min离心5 min,取上清液100 μl上样。余上清液每个取10 μl混合成质量控制样本(QC),涡旋后取100 μl上样。
采用气相色谱-质谱(GC-MS)进行代谢组学分析。仪器参数设定为:进样口温度280 ℃,EI离子源温度230 ℃,四极杆温度150 ℃,高纯氦气(纯度<99.999%)作为载气,不分流进样,进样量1.0 μl。升温程序为:初始温度80 ℃,维持2 min,10 ℃/min的速度升至320 ℃,并维持6 min。采用全扫描模式进行质谱检测,质谱检测范围为50~550 m/z。采用随机顺序进行连续样本分析,避免因仪器信号波动造成的影响。
-
在R软件平台下采用自写的程序代码进行数据预处理,包括基线过滤、峰识别和积分,然后在TagFinder软件下进行保留时间校正、峰对齐和质谱碎片归属等分析,最后在Excel软件中进行后期编辑,包括来自于柱流失和样本制备造成的杂质峰剔除和定量离子选择等,将最终结果组织为二维数据矩阵,包括变量(保留时间及质荷比)、观察量(样本)和积分面积。本项目共得到1 234个物质(每组样本至少存在80%以上的物质)。将中心化和归一化后的数据导入SIMCA-P V11.0进行主成分分析(PCA)和偏最小二乘法-判别式分析(PLS-DA)进行排列测试以评估模型的质量,生成变量重要性投影(VIP),表示对每种代谢物离子的群间区分的贡献。选择VIP 值>1.0的代谢物用于进一步分析。
所有数据以(
$\bar x$ +s)形式表示,使用one-way ANOVA 和 post hoc Tukey’s 检验,通过 SPSS 17.0 软件计算P值,以P<0.05为差异有统计学意义。 -
假手术组、模型组及给药组Scr水平分别为(9.83±1.95)、(50.83±13.53)、(29.67±4.96)μmol/L;BUN水平分别为(8.08±0.84)、(27.67±5.22)、(16.33±2.69)mmol/L。模型组较假手术组Scr水平、BUN水平均上调;而给药组较模型组均下调,差异均有统计学意义(P<0.05,表1),表明黄连提取物在脓毒症相关急性肾损伤中可改善肾功能。
表 1 黄连提取物对小鼠外周血生化指标的影响
组别 Scr (μmol/L) BUN (mmol/L) 假手术组 9.83±1.95 8.08±0.84 模型组 50.83±13.53* 27.67±5.22* 给药组 29.67±4.96# 16.33±2.69# *P<0.05,与假手术组比较;#P<0.05,与给药组比较。 -
按照上述色谱-质谱条件,分别对各组样品进样分析,得到典型的总离子流图(图1)。
-
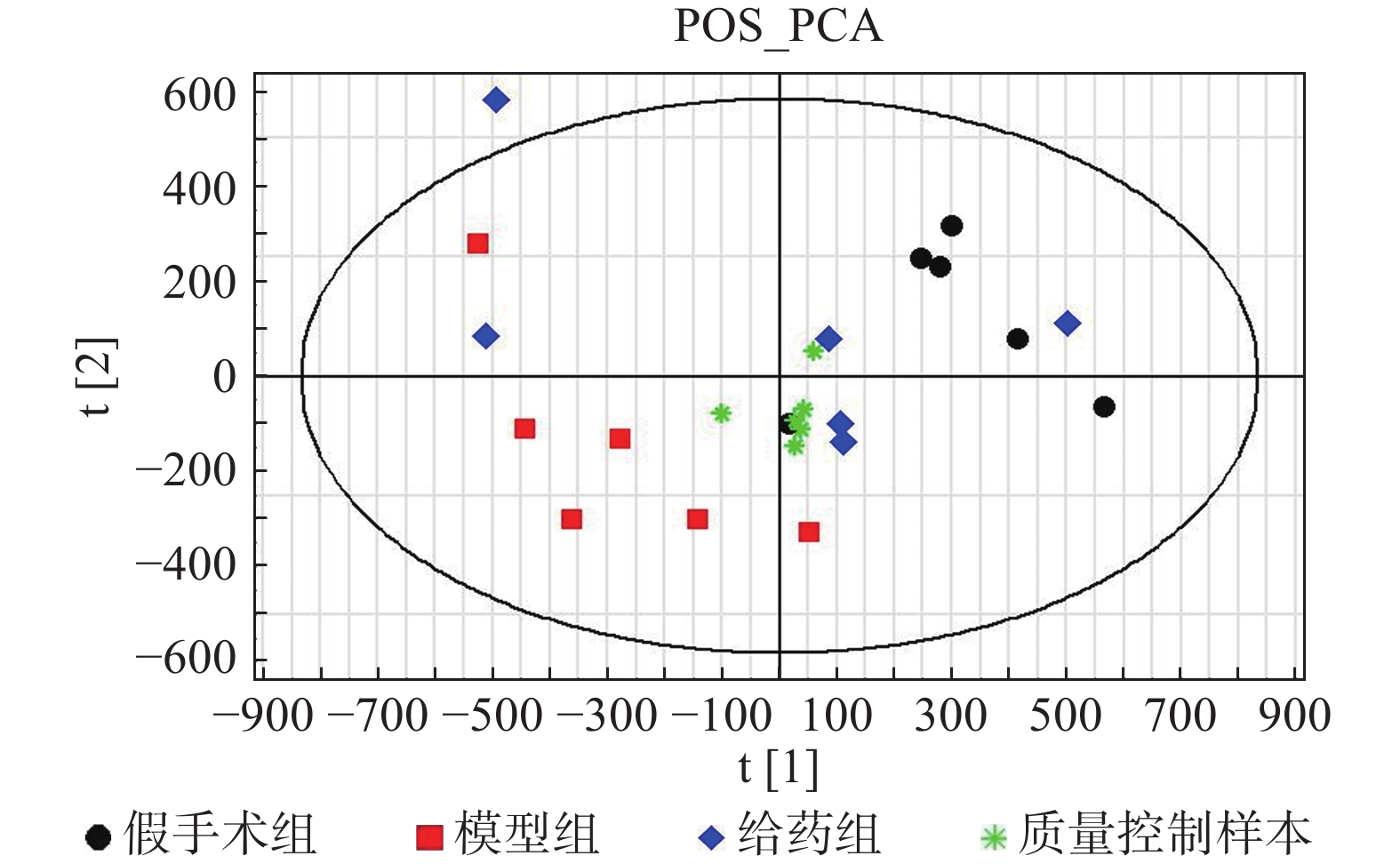
采用PCA方法对各组样本进行整体分析,并通过质量控制样本的聚集程度对系统的稳定性进行考察。根据整体PCA得分图(图2)所示,质量控制样本均聚集良好,其离散度明显低于待分析样本的离散度,表明系统稳定性良好。
-
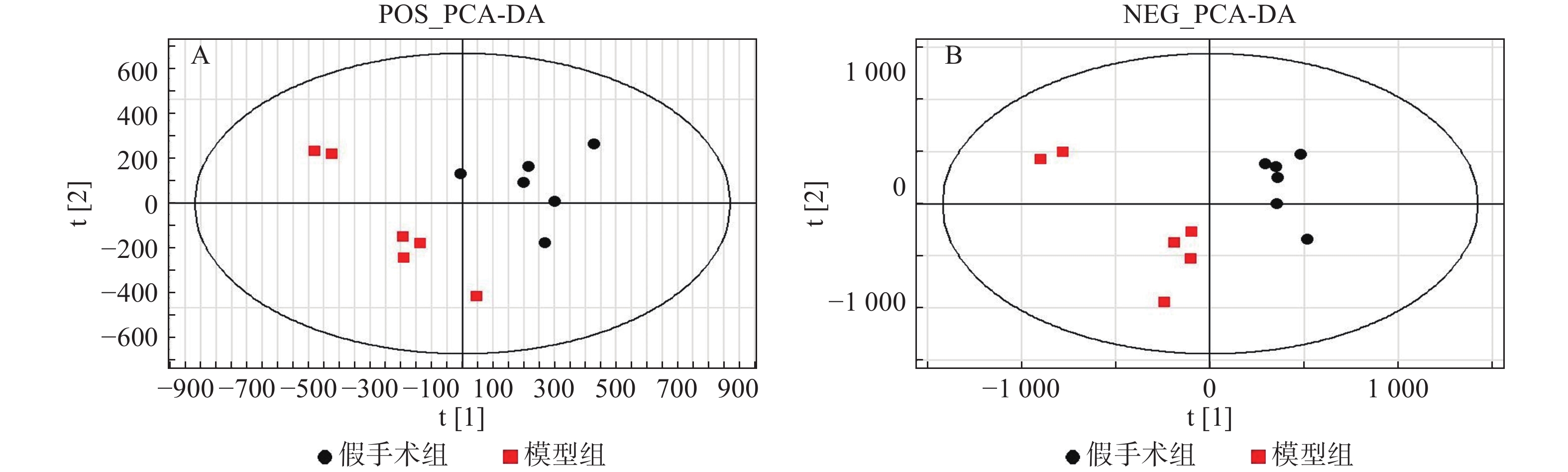
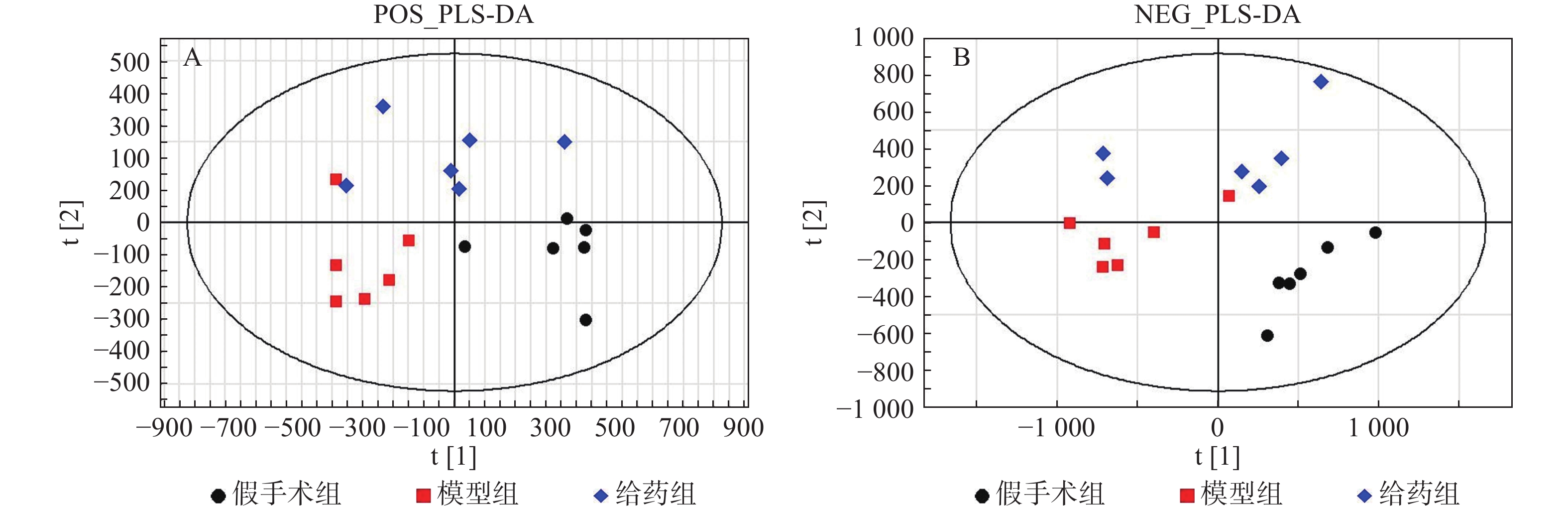
采用PLS-DA方法对假手术组、模型组、给药组样本进行分析。首先对假手术组和模型组单独进行分析,两组在正/负离子模式下的得分图显示,假手术组与模型组区分明显(图3)。进一步对3组样本进行分析,3组在正/负离子模式下的得分图显示,假手术组与模型组有较明显的区分趋势,同时,给药组较模型组有一定程度的回调(图4)。提示脓毒症相关急性肾损伤中,经黄连提取物干预后,代谢物变化发生回调。
-
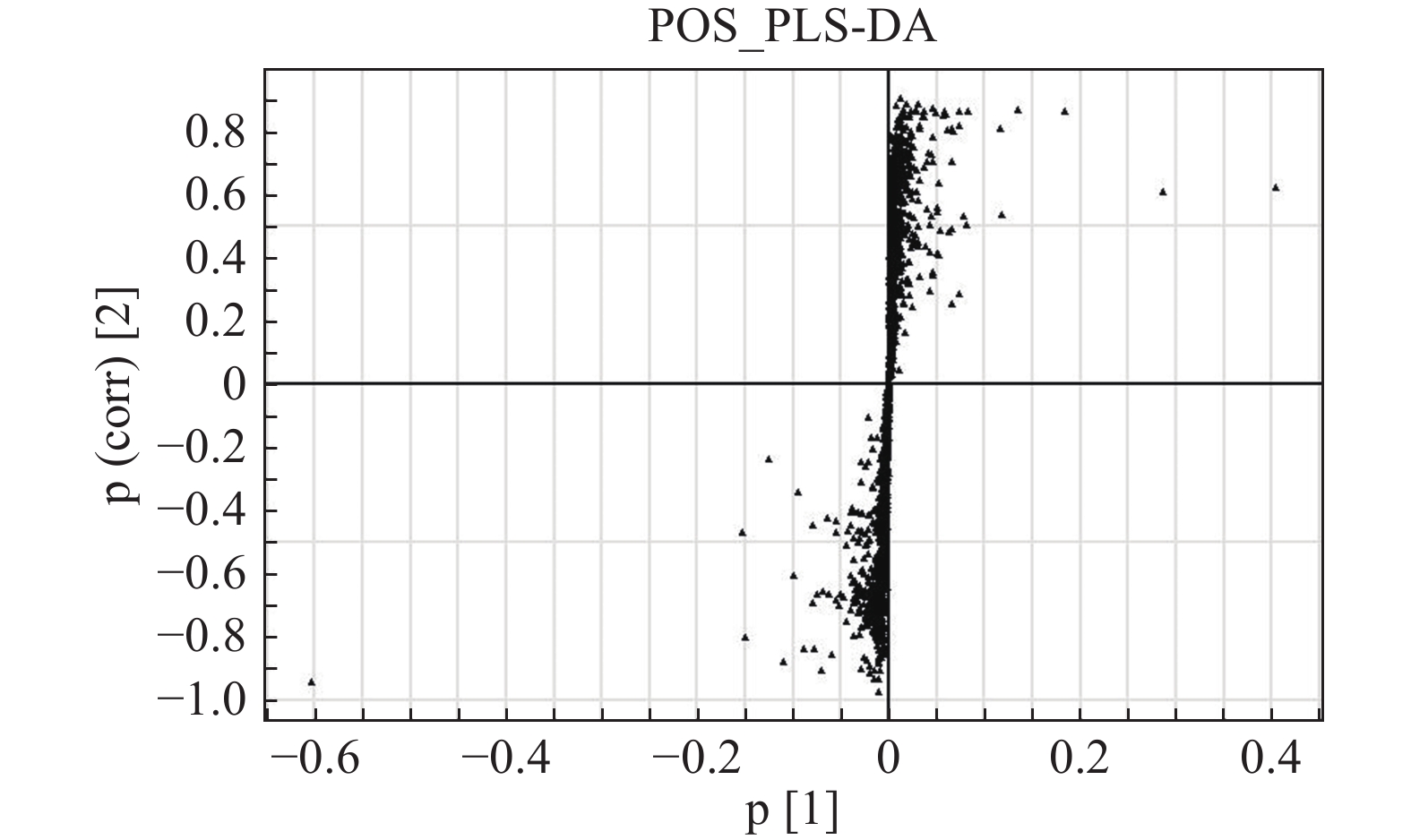
S-plot可以很好地反映各个离子对组间差异的贡献程度,离原点越远的点表明其对组间差异的贡献度越大,其VIP值也越大。正离子模式下对照组和模型组的S-plot显示两组间存在差异代谢物(图5)。
在VIP值>1,且3组ANOVA分析以及Turkey两两分析P值均<0.05的条件下,共筛选并鉴别出16个差异代谢物,包括天冬氨酸、甘氨酸、苏氨酸、脯氨酸、苯丙氨酸、缬氨酸、酪氨酸、色氨酸、磷酸、苹果酸、柠檬酸、果糖、葡萄糖、松二糖、肌醇,主要参与氨基酸代谢和糖代谢(表2)。在这16个代谢物中,其中8个代谢物水平在黄连提取物干预下发生回调,它们是天冬氨酸、甘氨酸、苏氨酸、苯丙氨酸、酪氨酸、柠檬酸、葡萄糖、肌醇。提示黄连提取物可能通过改善脓毒症相关急性肾损伤的代谢物变化从而起到治疗作用。
表 2 脓毒症相关肾损伤差异代谢物及代谢途径
序号 保留时间(t/min) VIP 代谢途径 代谢物 分子式 倍数变化 模型组/假手术组 给药组/模型组 1 7.37 1.37 氨基酸代谢 L-天冬氨酸(L-aspartic acid) C4H7NO4 0.63 1.56 2 10.99 1.36 L-甘氨酸(glycine) C2H5NO2 1.25 0.78 3 12.65 1.49 L-苏氨酸(L-threonine) C4H9NO3 1.18 0.64 4 14.96 2.71 L-脯氨酸(L-proline) C5H9NO2 0.79 0.38 5 15.64 1.48 L-苯丙氨酸(L-phenylalanine) C9H11NO2 1.45 0.18 6 20.52 1.68 L-缬氨酸(L-valine) C5H11NO2 0.84 0.13 7 21.11 1.07 L-酪氨酸(L-tyrosine) C9H11NO3 1.28 0.12 8 25.58 1.93 色氨酸(tryptophan) C11H12N2O2 0.71 0.61 9 10.63 1.69 糖代谢 磷酸(phosphoric acid) H3PO4 0.7 0.99 10 14.13 1.08 L-苹果酸(L-malic acid) C4H6O5 0.9 0.64 11 17.03 1.56 L-苏糖酸(L-threonic acid) C4H8O5 0.81 0.37 12 20.1 1.63 柠檬酸(citric acid) C6H8O7 0.77 1.99 13 20.91 4.44 D-果糖(D-fructose) C7H15NO6 0.82 0.68 14 21.38 1.34 D-葡萄糖(D-glucose) C6H12O6 1.33 0.68 15 22.3 1.91 松二糖(turanose) C12H22O11 0.81 0.66 16 24.27 2.26 肌醇(inositol) C6H12O6 1.34 0.59 -
脓毒症是由机体对感染反应失调所引起的威胁生命的多器官系统功能障碍,而急性肾损伤是最常见的情况,多发生在脓毒症早期。约51%的脓毒症患者会发生急性肾损伤,导致病死率增高41%[6]。与非脓毒症性急性肾损伤比较,脓毒症相关急性肾损伤起病更急、肾功能损害程度更严重、系统性炎症反应更重、器官功能衰竭评分也更高[7]。目前,我们对急性肾损伤发病机制的了解有限,肾血管收缩和肾血流减少导致肾缺血,促炎和抗炎反应的早期激活,以及肾小管细胞的凋亡均可导致肾损伤[8]。由于肾脏是排泄代谢终产物的主要器官,故其损伤必然会引起肾脏代谢状况的改变。
近年来,中草药在世界范围内得到越来越多的认可,而黄连以其泻火解毒、清热燥湿的功效被广泛使用。黄连的主要成分包括小檗碱、巴马汀、药根碱等生物碱类[9]。大量研究表明,黄连具有抗菌、消炎、抗高血压、抗氧化、降糖和降胆固醇的作用,具有很强的临床使用意义[10]。Huang等[11]研究发现黄连解毒汤能通过抑制炎症反应延长脓毒症大鼠的生存时间,有效保护心肌细胞。Zhang等[12]证实黄连对慢性肾功能衰竭大鼠的肾功能有一定的改善作用。代谢组学通过考察生物体受刺激后代谢产物的变化,以研究生物体代谢途径,研究对象包括代谢中间产物或终产物等体内小分子代谢物[13]。黄连对脓毒症相关的急性肾损伤有何影响,是否具有保护作用,本研究利用基于GC-MS的代谢组学对此进一步探讨。
本研究通过代谢组学研究,发现共有16个代谢物与脓毒症相关急性肾损伤有关,主要参与氨基酸代谢和糖代谢。在这些代谢物中,有8个代谢物在黄连提取物干预后发生回调。
在氨基酸代谢中,天冬氨酸水平在脓毒症相关急性肾损伤中降低,甘氨酸、苏氨酸、苯丙氨酸、酪氨酸水平升高,这些代谢物在黄连提取物干预后都发生了回调。既往研究提示,脓毒症相关急性肾损伤的肾组织中,天冬氨酸和苏氨酸水平均降低[8],可能是由于防御和修复过程中的蛋白质合成增加了氨基酸的消耗,这与本课题组的结果存在部分差异,可能需要进一步通过血液及尿液样本进行研究及探讨。甘氨酸作为一种抗炎、抗氧化的保护剂,有研究提示甘氨酸衍生物可以通过减轻脂质过氧化从而改善肾缺血/再灌注损伤[14]。有趣的是,另有研究显示甘氨酸通过激活N-甲基-D-天冬氨酸(N-methyl-D-aspartate,NMDA)受体加重氧化应激以及肾缺血/再灌注损伤[15]。因此,课题组推测脓毒症相关急性肾损伤中,甘氨酸水平升高一方面可能起到自我保护作用,而另一方面可能加重肾损伤;黄连提取物可能通过减少甘氨酸从而抑制NMDA受体的激活,减轻脓毒症相关急性肾损伤。苯丙氨酸通过苯丙氨酸羟化酶生成酪氨酸,在慢性肾脏病患者中,苯丙氨酸水平升高,酪氨酸水平降低,这可能与苯丙氨酸羟化酶活性降低相关[16]。而在脓毒症相关急性肾损伤中,苯丙氨酸及酪氨酸水平均升高,提示该过程中苯丙氨酸羟化酶活性可能未发生明显变化,而可能与其他代谢途径相关。对甲酚硫酸盐作为一种尿毒症毒素,为肠道细菌代谢酪氨酸、苯丙氨酸的产物。在脓毒症情况下,肠道微环境收到干扰[17],我们推测苯丙氨酸及酪氨酸水平的升高可能进一步引起对甲酚硫酸盐水平的升高,加重肾损伤。既往有研究提示,黄连可以调节肠道菌群[18],我们推测黄连可能通过联合改善内源代谢变化和调节肠道环境对脓毒症相关急性肾损伤起到保护作用。
在糖代谢中,柠檬酸水平在脓毒症相关急性肾损伤中降低,葡萄糖和肌醇水平升高,这些代谢物在黄连提取物干预后都发生了回调。曾有研究表明,柠檬酸循环是脓毒症中受影响最大的代谢途径[19]。在脓毒症相关急性肾损伤模型中,三羧酸循环/氧化磷酸化过程减弱,糖酵解过程增强,这一代谢重编程现象称为瓦博格效应[20]。柠檬酸水平的降低也验证了这一现象。葡萄糖水平升高的可能原因是糖异生维持内源性葡萄糖生成,以防止低血糖的发展,从而建立脓毒症耐受性[21]。脓毒症时,体内迅速升高的升糖激素和前炎性因子加重了这一现象。但是,糖异生过程增强可能进一步减弱氧化磷酸化过程,我们推测黄连提取物可能通过抑制糖酵解、增强氧化磷酸化,从而增强机体抗炎能力,减轻脓毒症相关急性肾损伤。
本研究尚有一些不足之处,主要在于分析黄连提取物功效时局限于脓毒症的某一时间点,而不能获知脓毒症相关性急性肾损伤全病程中肾损伤的情况,以及黄连提取物对肾脏的保护作用。其次,收集样本的手段比较简单,难以消除潜在的影响因素。总之,黄连提取物可能通过改善脓毒症相关急性肾损伤的代谢物变化从而起到治疗作用,但其中的机制还有待进一步研究。这一研究结果表明,黄连具有成为脓毒症相关急性肾损伤新的治疗手段的潜质,尽管可行性需要更多验证,但为治疗脓毒症相关急性肾损伤提供了新的思路。
The protective effect of Rhizoma Coptidis extracts against the sepsis associated with acute kidney injury based on metabolic analysis
-
摘要:
目的 探讨黄连提取物(Rhizoma Coptidis extracts,RCE)对脓毒症相关急性肾损伤的影响及潜在机制。 方法 将C57BL/6小鼠分为假手术组、模型组以及治疗组3组;采用试剂盒检测血清肌酐(serum creatinine,Scr)及尿素氮(blood urea nitrogen,BUN)水平;采用气相色谱-质谱进行代谢组学分析。 结果 模型组较假手术组Scr、BUN水平均上调;而治疗组较模型组均下调,差异均有统计学意义(P<0.05)。代谢组学分析共获得16个代谢物可能与脓毒症相关急性肾损伤过程有关,主要参与氨基酸代谢和糖代谢。在这16个代谢物中,8个代谢物水平在黄连提取物干预下发生回调。 结论 黄连提取物可能通过改善脓毒症相关急性肾损伤的代谢物变化从而起到治疗作用。 Abstract:Objective To investigate the potential mechanism of Rhizoma Coptidis extracts (RCE) against sepsis associated with acute kidney injury. Methods C57BL/6 mice were divided into sham group, model group and RCE treatment group. The levels of Scr and BUN were measured by test kits. Gas chromatography-mass spectrometry was used to analyze metabolic changes in kidneys. Results The levels of Scr and BUN were increased in the model group than sham, which were reversed by RCE. 16 metabolites related to the progress of sepsis associated with acute kidney injury were detected, which were involved in amino acid metabolism and carbohydrate metabolism. Among these metabolites, the level of 8 metabolites can be reversed with RCE treatment. Conclusion RCE might exert therapeutic effects in sepsis associated with acute kidney injury by altering multiple metabolic pathways. -
Key words:
- sepsis /
- acute kidney injury /
- Rhizoma Coptidis /
- metabonomics
-
粉-液双室袋是采用特定工艺将药物和注射用溶剂独立封装在不同的两个腔室中的一种静脉注射用产品,在医护人员紧缺或战备、紧急救援等情况下,其优势突出[1]。1996年,日本研制出世界首个粉-液双室袋产品——头孢唑林钠氯化钠注射剂[2]。2015年,原中国食品药品监督管理总局出台首个《粉液双室袋产品技术审评要点》[3],国内首个粉-液双室袋产品(注射用头孢他啶/氯化钠注射液)于2019年获得药品注册批件,正式上市。粉-液双室袋因其结构上的创新,给药预处理步骤简化在使用上具有独特的优势,因此粉-液双室袋产品自上市后就受到了广泛的关注。但对于这样一个新产品,是否真正安全有效,是否具有成本-效益,与市场上正在使用的传统粉针剂相比是否具有明显的优势等问题目前均未得到解答,利益各方大都采取观望的态度。
为促进粉-液双室袋产品临床合理应用,依据国家卫健委2020年发布的《药品临床综合评价管理指南(试行)》[4],通过对文献资料进行调研,提取粉-液双室袋常用评价指标,以传统粉针产品为对照,从安全性、有效性、经济性、适宜性、可及性、创新性6个维度对粉-液双室袋产品进行综合评价。
1. 资料和方法
1.1 文献检索策略
以“双室袋” “双腔袋” “多室袋” “多腔袋” “Multi chamber bag”和“dual chamber bag”等为关键词,在中国知网、万方数据、维普、PubMed 、Web of Science等数据库中进行系统文献检索,对发表年度不设限制。表1为具体的检索式及相应检索结果。
表 1 检索式及检索结果检索条件 各数据库检索结果 中国知网 万方数据 维普 PubMed Web of Science (主题=双室袋 + 粉液双室袋)OR(主题=双腔袋)
OR(主题=多室袋)OR(主题=多腔袋)80 ((((任意字段=双室袋 OR 任意字段=粉液双室袋)OR 任意字段=多室袋)OR 任意字段=双腔袋)OR 任意字段=多腔袋) 189 主题=(双室袋)OR 主题=(粉液双室袋)OR 主题=(多室袋)
OR 主题=(双腔袋)OR 主题=(多腔袋)6740 (Multi chamber bag)OR(dual chamber bag) 42 (Multi chamber bag)OR (dual chamber bag) 166 1.2 纳入与排除标准
纳入标准:①研究对象为粉-液双室袋;②内容为安全性、有效性、经济性、适用性、创新性、可及性的研究;③文献类型为随机对照试验或观察实验;④有参考价值的多室袋研究文献。
排除标准:①研究对象为液-液双室袋;②内容为生产工艺、设备、分装技术和其他无关内容;③文献类型为综述、会议论文、专利、成果;④重复文献、不可下载文献。
1.3 文献筛选和信息提取
文献由2名研究员独立、同步进行筛选。参考中国医药包装协会发布的《基础输液临床使用评估指南(试行)》[5]和国家卫健委发布的《药品临床综合评价管理指南(试行)》[4]提取文献中的可用指标。如产生争议,由课题组成员讨论决定。
2. 结果
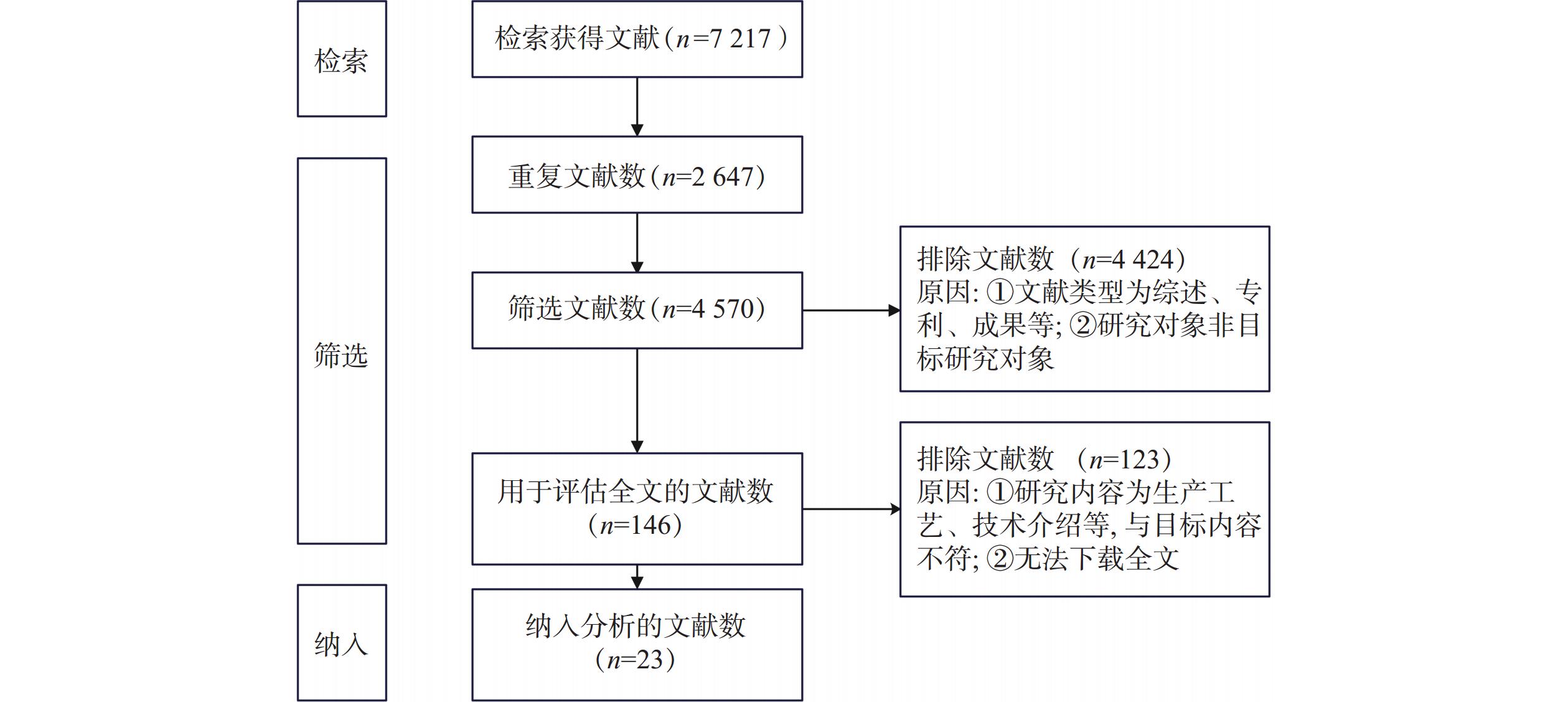
2.1 文献检索与筛选结果
系统检索文献得
7217 篇,排除重复文献2647 篇、文献类型为综述、会议论文等4424 篇、文献内容为生产工艺和技术介绍等123篇,最终纳入分析文献23篇,其中英文文献2篇,中文文献21篇。具体筛选过程如图1。2.2 指标提取及评估结果
2.2.1 评价指标
文献中使用的评价指标涉及5个维度,经过整合、汇总,见表2。由于双室袋产品和传统粉针剂的药物成分、给药途径一致,仅包装和给药预处理存在区别,故有效性、安全性两个维度仅包括与包装和给药预处理相关的指标。其中,虽配制过程刺伤、划伤等为操作失误,但双室袋产品简化了药液配制过程,无需使用注射器辅助配制,可完全避免意外伤害,与传统粉针剂存在差异,故纳入为安全性指标。
表 2 已发表文献使用的评价指标评价维度 判别可用指标 有效性 药液稳定性、配制浓度准确性、药液残留量 安全性 不溶性微粒、刺伤划伤等意外事情发生率 经济性 配制成本a、废弃物重量、住院成本、血液感染发生率 适宜性 配制时间、平均医护人员人力占用、包装重量和
储运体积b、环境适应性、废弃物处理难易程度可及性 生产厂家数量、产品原材料供应能力、患者可负担性 a:为药液成本、配制用品以及配制人工成本的总和;b:包括药液配制过程所需用品储运体积总和,传统粉针剂产品的配制用品包括注射器、西林瓶粉针、配制用溶剂等,粉-液双室袋产品仅包括产品本身。 2.2.2 有效性
于庆坤等[6-12]研究结果显示,与传统粉针剂相比,双室袋产品的药液含量随时间变化小,配制的实际浓度更接近理论浓度,无残留药液,具体见表3和表4。
表 3 药液配置后5 h的稳定性对比[7]产品名称 不同温度时的百分含量(%) 4℃ 25℃ 非PVC粉-液双室袋产品 96.33 96.32 玻璃瓶粉针产品 95.37 95.79 2.2.3 安全性
静脉输液中的不溶性微粒会造成血栓和静脉炎[13],药典规定用于静脉注射、滴注的药品需检查不溶性微粒。双室袋法配制过程不溶性微粒无明显增加[8,10,14-15],详见表5。以18名护士为观察对象,粉针配制过程1名护士被划伤,双室袋配制过程无人员刺伤、划伤[10]。粉-液双室袋以非PVC多层共挤膜为膜材,其强光照射实验表明0.9%氯化钠、5%葡萄糖、葡萄糖氯化钠、复方氯化钠注射液与膜材相容性良好,稳定性试验表明非PVC多层共挤膜的水蒸气渗透、透光率、pH、易氧化物检测均符合规定[14,16]。
表 5 配制过程不溶性微粒比较文献作者 样品 不溶性微粒数(个/ml) ≥5 ≥8 ≥10 ≥12 ≥25 ≥100 李英等[15]a 粉针输液产品 318 66 21 6 0 0 配制增加微粒数b 285 55 15 3 0 0 双室袋输液产品 2 0 0 0 0 0 双室袋增加微粒数c 0 0 0 0 0 0 沈敏娜等[16]a 粉针输液产品 322 68 23 7 0 0 配制增加微粒数 289 55 17 5 0 0 双室袋输液产品 3 1 0 0 0 0 双室袋增加微粒数c 0 0 0 0 0 0 王宇航等[8]a 粉针输液产品 240 326.5 43 24 7 0 双室袋输液产品 240 2 0 0 0 0 罗莉等[10]d 粉针输液产品 219.52±84.73 43.93±21.68 14.93±7.96 4.05±2.60 0.01±0.04 双室袋输液产品 3.49±0.95 0.39±0.19 0.20±0.11 0.13±0.09 0.03±0.03 a:实验重复配制(均≥100份),由于数据资料不服从正态分布,选中位数表征平均水平,表中均为中位数值;b:增加微粒数=溶液微粒数−(粉体+液体),表示溶配方法增加的不溶性微粒;c:双室袋法配置的不溶性微粒增加数计算结果为负值,由于混合前为取出粉末后在非封闭的环境中进行溶解测试,而混合后则是在封闭的袋内进行开通溶解后测试,从而导致粉体检测结果高于混合液的情况出现,因此即配型双室袋法配置的不溶性微粒增加数视为零;d:本实验所取数据为不溶性微粒数范围值。 2.2.4 经济性
在纳入的双室袋产品相关经济性研究[8,17-18]中考虑到的成本指标包括配制成本(包括配制环境、设备以及环境维护费、人工成本、耗材成本)、废弃物重量,效果/效益以血液感染(BSI)为指标。采用双室袋输液,药液配制过程得到简化,一方面可节省储存场所、人工及耗材成本,一方面产生的废弃物减少,废弃物处理费会有所减少。王宇航等[8]通过计算发现按照4.82元/kg的垃圾清理费算,每年可减少废弃物处理费约7万元。此外,双室袋输液配制过程减少与空气的接触,可在一定程度上减少输液后血液感染的发生率。苗雅楠等[17]发现在
1000 例患者中,输液系统由半开放式转换为全密闭输液系统可减少172例血液感染。已上市的粉-液双室袋产品与对应的传统粉针相比,有效期相同,对于储备来说,两者轮换周期一致。2.2.5 适宜性
对于易溶于水但在水溶液中不稳定的药物一般选择制成注射用无菌粉针,此类产品在使用前需要与特定的配制用溶剂临时配制注射用溶液。与粉针剂西林瓶产品运用的药液配制方法相比,双室袋法输液预处理环节有所简化,能明显缩短药液配制时间[8,10,19],具体见表6,在医护资源匮乏(如应急医学救援)时,可减少医护人员人力的占用。此外双室袋为全密闭输液系统,对环境的耐受性强。
2.2.6 可及性
可及性包括可获得和可负担两方面的要求。双室袋产品的药液与传统粉针一致,包装材料有所不同,目前双室袋包材审批通过已登记的有15项(1项为进口),双室袋产品生产厂家超过5家,产品大都为头孢类抗感染药物,在生产供应方面,可以满足可及性的要求。此外,2022年《国家基本医疗保险药品目录》的协议期内谈判药品部分有5个粉-液双室输液产品被纳入,为医保乙类药品,可达到患者可负担的要求。
2.2.7 创新性
截止到2023年4月,与粉-液双室输液袋有关的发明专利有22项,实用新型专利89项,外观专利5项,包括生产、灌装、检漏等方面。粉-液双室袋产品能缩短配液时间,简化输液预处理过程,降低了输液对环境的要求,顺应了突发事件应急医学救援与创伤急救的需求。
3. 讨论
本研究采用系统综述的方法对现有文献信息分析提取,得到的指标可作为评价指标池的一部分,后续可用于建立粉-液双室袋产品综合评价指标体系。
粉-液双室袋产品与传统粉针药效成分、给药途径相同,我国对药品上市后包装变更无临床试验的要求[20],已发表文献中均未报告其有效性、安全性的临床结局指标,且两者仅在包材和给药预处理方面存在差异,故纳入的有效性指标只包括药物学工艺重现性,安全性指标只包括不良事件或风险的比较指标。除列出指标外,安全性评价还需考虑渗漏隐患、破损率、误配、错配发生率等。经济性评价具有时间性,存在偏倚风险,仍需对此类产品进行更为可靠的经济学研究。适用性的考查应注重产品在紧急救援使用时缩短抢救时间,节约医护资源的能力。粉-液双室袋的技术壁垒较高,可及性方面不如传统粉针剂,但符合可及性的基本要求。
粉-液双室袋属于全封闭式输液系统,无空气通路,细菌污染降低,减少了输液反应的发生[21]。药液配制时间明显缩短,提高救援成功率[22];无需临时计算溶剂用量,配制准确度高,无药液残留,且对环境、技术要求不高,降低了人工、设施及耗材成本;无需借助注射器反复穿刺橡胶塞,不溶性微粒显著减少,刺伤、划伤等意外事情可避免。虽单价高于传统粉针产品,但其在废弃物处理成本、人工成本以及输液后静脉炎发生率的减少方面展现出了一定的优势。此外国内双室袋产品的生产企业超过5家,且在原材料上摆脱了对进口的依赖,5种粉-液双室袋产品已纳入医保目录,符合可及性要求。
粉-液双室袋为新兴产品,仅有23篇符合纳入条件。外文文献较少,仅纳入2篇。因多室袋与双室袋原理一致,故将以多室袋为研究对象的文献也纳入。期待随着粉-液双室袋在我国广泛应用,未来可获得高质量、精细化的研究数据,以开展更全面、可靠的综合评价研究。
-
表 1 黄连提取物对小鼠外周血生化指标的影响
组别 Scr (μmol/L) BUN (mmol/L) 假手术组 9.83±1.95 8.08±0.84 模型组 50.83±13.53* 27.67±5.22* 给药组 29.67±4.96# 16.33±2.69# *P<0.05,与假手术组比较;#P<0.05,与给药组比较。 表 2 脓毒症相关肾损伤差异代谢物及代谢途径
序号 保留时间(t/min) VIP 代谢途径 代谢物 分子式 倍数变化 模型组/假手术组 给药组/模型组 1 7.37 1.37 氨基酸代谢 L-天冬氨酸(L-aspartic acid) C4H7NO4 0.63 1.56 2 10.99 1.36 L-甘氨酸(glycine) C2H5NO2 1.25 0.78 3 12.65 1.49 L-苏氨酸(L-threonine) C4H9NO3 1.18 0.64 4 14.96 2.71 L-脯氨酸(L-proline) C5H9NO2 0.79 0.38 5 15.64 1.48 L-苯丙氨酸(L-phenylalanine) C9H11NO2 1.45 0.18 6 20.52 1.68 L-缬氨酸(L-valine) C5H11NO2 0.84 0.13 7 21.11 1.07 L-酪氨酸(L-tyrosine) C9H11NO3 1.28 0.12 8 25.58 1.93 色氨酸(tryptophan) C11H12N2O2 0.71 0.61 9 10.63 1.69 糖代谢 磷酸(phosphoric acid) H3PO4 0.7 0.99 10 14.13 1.08 L-苹果酸(L-malic acid) C4H6O5 0.9 0.64 11 17.03 1.56 L-苏糖酸(L-threonic acid) C4H8O5 0.81 0.37 12 20.1 1.63 柠檬酸(citric acid) C6H8O7 0.77 1.99 13 20.91 4.44 D-果糖(D-fructose) C7H15NO6 0.82 0.68 14 21.38 1.34 D-葡萄糖(D-glucose) C6H12O6 1.33 0.68 15 22.3 1.91 松二糖(turanose) C12H22O11 0.81 0.66 16 24.27 2.26 肌醇(inositol) C6H12O6 1.34 0.59 -
[1] POSTON J T, KOYNER J L. Sepsis associated acute kidney injury[J]. BMJ,2019:k4891. doi: 10.1136/bmj.k4891 [2] UCHINO S, KELLUM J A, BELLOMO R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study[J]. JAMA,2005,294(7):813-818. doi: 10.1001/jama.294.7.813 [3] COCA S G, YUSUF B, SHLIPAK M G, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis[J]. Am J Kidney Dis,2009,53(6):961-973. doi: 10.1053/j.ajkd.2008.11.034 [4] WANG J, RAN Q, ZENG H R, et al. Cellular stress response mechanisms of <italic>Rhizoma Coptidis</italic>: a systematic review[J]. Chin Med,2018,13:27. doi: 10.1186/s13020-018-0184-y [5] CHOI Y Y, KIM M H, CHO I H, et al. Inhibitory effect of <italic>Coptis chinensis</italic> on inflammation in LPS-induced endotoxemia[J]. J Ethnopharmacol,2013,149(2):506-512. doi: 10.1016/j.jep.2013.07.008 [6] WANG S, XIAO C X, LIU C J, et al. Identification of biomarkers of sepsis-associated acute kidney injury in pediatric patients based on UPLC-QTOF/MS[J]. Inflammation,2020,43(2):629-640. doi: 10.1007/s10753-019-01144-5 [7] BAGSHAW S M, UCHINO S, BELLOMO R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes[J]. Clin J Am Soc Nephrol,2007,2(3):431-439. doi: 10.2215/CJN.03681106 [8] IZQUIERDO-GARCIA J L, NIN N, CARDINAL-FERNANDEZ P, et al. Identification of novel metabolomic biomarkers in an experimental model of septic acute kidney injury[J]. Am J Physiol Renal Physiol,2019,316(1):F54-F62. doi: 10.1152/ajprenal.00315.2018 [9] WANG J, CHEN Y, YUAN Z M, et al. Differences in effective mechanisms of <italic>Coptidis Rhizoma</italic> and bile processed <italic>Coptidis Rhizoma</italic> on heat syndrome based on urinary metabonomics[J]. China J Chin Mater Med,2016,41(14):2638-2645. [10] ZHOU Y T, LIAO Q F, LIN M N, et al. Combination of <sup>1</sup>H NMR-and GC-MS-based metabonomics to study on the toxicity of <italic>Coptidis Rhizome</italic> in rats[J]. PLoS One,2014,9(2):e88281. doi: 10.1371/journal.pone.0088281 [11] 黄鑫, 郭力恒, 马世玉, 等. 黄连解毒汤对脓毒症大鼠的心脏保护作用[J]. 中西医结合心脑血管病杂志, 2012(6):710-712. doi: 10.3969/j.issn.1672-1349.2012.06.037 [12] 张玲, 熊维建, 张太君. 黄连碱对慢性肾功能衰竭大鼠的治疗作用及其机制研究[J]. 中国现代应用药学, 2017, 34(1):30-33. [13] 陆荣华. 代谢组学在肾脏疾病中的应用[J]. 国际泌尿系统杂志, 2012, 32(11):847-852. [14] BI W, WANG F G, BI Y, et al. Renal ischemia/reperfusion injury in rats is attenuated by a synthetic glycine derivative[J]. Eur J Pharmacol,2009,616(1-3):256-264. doi: 10.1016/j.ejphar.2009.06.027 [15] ARORA S, KAUR T, KAUR A, et al. Glycine aggravates ischemia reperfusion-induced acute kidney injury through N-Methyl-D-Aspartate receptor activation in rats[J]. Mol Cell Biochem,2014,393(1-2):123-131. doi: 10.1007/s11010-014-2052-0 [16] BOIRIE Y, ALBRIGHT R, BIGELOW M, et al. Impairment of phenylalanine conversion to tyrosine in end-stage renal disease causing tyrosine deficiency[J]. Kidney Int,2004,66(2):591-596. doi: 10.1111/j.1523-1755.2004.00778.x [17] FAY K T, FORD M L, COOPERSMITH C M. The intestinal microenvironment in sepsis[J]. Biochim Biophys Acta Mol Basis Dis,2017,1863(10 Pt B):2574-2583. [18] HE K, HU Y R, MA H, et al. <italic>Rhizoma Coptidis</italic> alkaloids alleviate hyperlipidemia in B6 mice by modulating gut microbiota and bile acid pathways[J]. Biochim Biophys Acta,2016,1862(9):1696-1709. doi: 10.1016/j.bbadis.2016.06.006 [19] XU E Y, PERLINA A, VU H, et al. Integrated pathway analysis of rat urine metabolic profiles and kidney transcriptomic profiles to elucidate the systems toxicology of model nephrotoxicants[J]. Chem Res Toxicol,2008,21(8):1548-1561. doi: 10.1021/tx800061w [20] GÓMEZ H, KELLUM J A, RONCO C. Metabolic reprogramming and tolerance during sepsis-induced AKI[J]. Nat Rev Nephrol,2017,13(3):143-151. doi: 10.1038/nrneph.2016.186 [21] WEIS S, CARLOS A R, MOITA M R, et al. Metabolic adaptation establishes disease tolerance to sepsis[J]. Cell,2017,169(7):1263-1275. doi: 10.1016/j.cell.2017.05.031 -





 下载:
下载:
 下载:
下载: