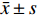-
在全球范围内,乳腺癌是女性癌症死亡的主要原因,2018年约有210万女性疑似患有乳腺癌,占女性癌症的25%[1]。在我国,乳腺癌在女性肿瘤中发病率居首位,死亡率居第5位,近几十年来乳腺癌负担迅速增长[2]。目前,雌激素敏感的乳腺癌患者主要以内分泌治疗为主,然而,缺乏激素受体的乳腺癌细胞通常使用化疗药物,如紫杉醇和阿霉素[3]。但是,化疗药物对肿瘤细胞和正常细胞均表现出毒性反应,这限制了其临床应用。此外,细胞毒等抗肿瘤药物表现出的细胞耐药性进一步限制其应用。因此,迫切需要寻找毒性较小、效果较好的治疗药物。
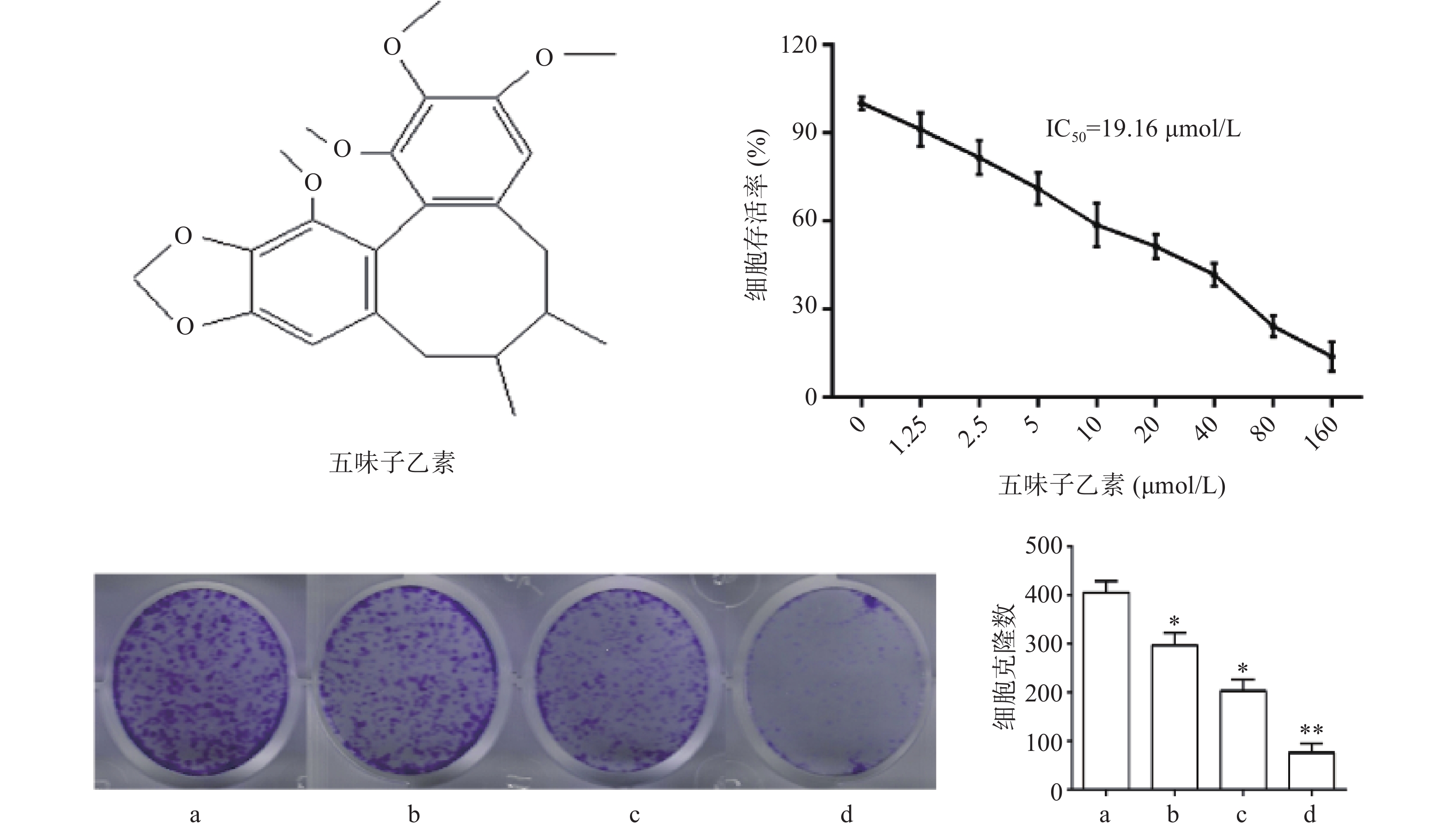
五味子乙素(schisandrin B, Sch B)是从五味子中提取的主要活性成分[4]。据报道[5],Sch B可通过抑制NF-κB激活及MAPK/ Erk/p38/c-Jnk信号通路激活而有效减轻炎症反应,NASSER等[6]发现,Sch B可通过抑制PI3K/AKT和STA3/JAK2信号通路磷酸化,从而降低细胞内ROS的产生发挥抗前列腺癌作用,Wang等[7]发现,Sch B可通过TGF-b信号通路靶向miR-101-5p抑制大鼠肝纤维化。Dai等[8]发现,Sch B可通过STAT3的磷酸化和核转位发挥抗乳腺癌活性,但是Sch B 如何影响乳腺癌细胞增殖凋亡能力及具体机制尚不清楚。本研究以Sch B为研究对象,探讨其对人乳腺癌MDA-MB-231细胞凋亡的影响及其作用机制。
-
人乳腺癌细胞株MDA-MB-231(中科院细胞库,目录号:SCSP-5043);甘草查尔酮A(纯度≥98%,批号76296-75-6,上海麦克林生化科技有限公司);CCK-8试剂盒、Annexin V-FITC/PI凋亡试剂盒、JC-1荧光探针、DCFH-DA荧光探针、Hoechst 33342 染色剂、PBS(大连美仑生物);1640培养基、胎牛血清( FBS) (美国HyClone公司);ECL发光液、BCA蛋白定量试剂盒(美国Thermo公司);Bcl-2、Bax、CHOP、GPR78、ATF4、PERK、p-PERK、p-eIF2α、eIF2α、β-actin、二抗(美国 Cell Signaling Technology公司)。
-
人乳腺癌细胞系MDA-MB-231细胞培养于含10%胎牛血清、100 μg/ml链霉素及100 U/ml青霉素的L-15培养基中,置于37 ℃、5% CO2的饱和湿度培养箱培养,细胞2~3 d可传代培养。Sch B药物处理时,首先取生长状态良好的对数期细胞进行实验,细胞分为对照组和药物组,根据细胞存活率检测结果计算IC50,药物组分为Sch B低剂量组剂量(1/2倍IC50),Sch B中剂量组(1倍IC50),Sch B高剂量组(2倍IC50)。
-
取状态良好的对数期MDA-MB-231细胞,以8×103/100 μl细胞密度接种于96孔板,于37 ℃,5% CO2培养箱培养过夜。将细胞分为空白组(含培养基,不含细胞)、对照组(含培养基,含细胞,不含药物)和药物处理组,药物处理组分别以终浓度为1.25、2.5、5、10、20、40、80、160 μmol/L的Sch B处理细胞24 h,每组3个复孔。24 h后每孔加入CCK-8溶液(10 μl), 于培养箱继续培养1 h,用酶标仪在450 nm处测定吸光度(OD)值,计算细胞存活率,并计算药物IC50值。
细胞存活率=[(OD药物−OD空白)/(OD对照−OD空白)]×100%
细胞克隆形成分析:取生长良好的对数期MDA-MB-231细胞,消化成单细胞悬液,以每孔3×103个细胞接种于6孔板,轻轻摇动使细胞分散均匀,过夜贴壁。分为对照组和药物组,对照组加等体积的培养基,药物组分别加以终浓度为(10、20、40 μmol/L)Sch B培养基,置培养箱中培养14 d。然后用PBS清洗2次,室温下用4%多聚甲醛固定15 min,1%结晶紫染色10 min。显微镜观察克隆形成情况,观察3个视野中克隆数量。
-
将生长良好的对数期MDA-MB-231细胞,以每孔2.5×105个/ml细胞密度接种于6孔板,置培养箱过夜培养。待细胞贴壁后,分别将终浓度为10、20、40 μmol/L的Sch B处理24 h。用不含EDTA胰酶消化细胞,PBS洗涤2次,结合缓冲液(100 μl)重悬细胞,转入流式管,然后再分别加入Annexin V-FITC(5 μl)和PI染液(5 μl),室温孵育15 min(避光),最后加入结合缓冲液(400 μl)混匀,流式仪上机检测,Flow Jo软件处理数据。
-
将生长良好的对数期MDA-MB-231细胞,以1×105个/ml细胞密度接种于12孔板,置培养箱过夜培养。待细胞贴壁后,分别将终浓度为10、20、40 μmol/L的Sch B处理24 h。DCFH-DA染液用PBS 1∶1000稀释成工作液,每孔加入200 μl工作液,37 ℃培养箱中避光孵育30 min,PBS洗涤2次,倒置荧光显微镜拍照,Image J软件检测荧光强度。
-
将生长良好的对数期MDA-MB-231细胞,以2.5×105个/ml细胞密度接种于6孔板,于培养箱过夜培养。待细胞贴壁后,将细胞依次分为对照组和药物组,对照组细胞加等体积培养基,药物组分别加终浓度为10、20、40 μmol/L的Sch B及加或者不加活性氧(ROS)清除剂(NAC)5 mmol/L, 内质网应激抑制剂4-PBA 2 mmol/L处理24 h。用RIPA(含PMSF蛋白酶抑制剂)裂解液提取蛋白,BCA法测定蛋白浓度,加入5×上样缓冲液金属浴(100 ℃)使蛋白变性。20 μg蛋白样品经PAGE凝胶电泳后转至PVDF膜,5%脱脂牛奶封闭1 h,孵育一抗(4 ℃冰箱过夜),1×TBST洗涤3次,二抗室温孵育2 h,采用ECL显影液显色,凝胶成像系统拍照,Image J软件分析。
-
数据采用GraphPad软件进行分析,以
$ \bar{x}\pm s $ 表示。组间比较采用t检验和单因素方差分析,P<0.05表示差异有统计学意义。 -
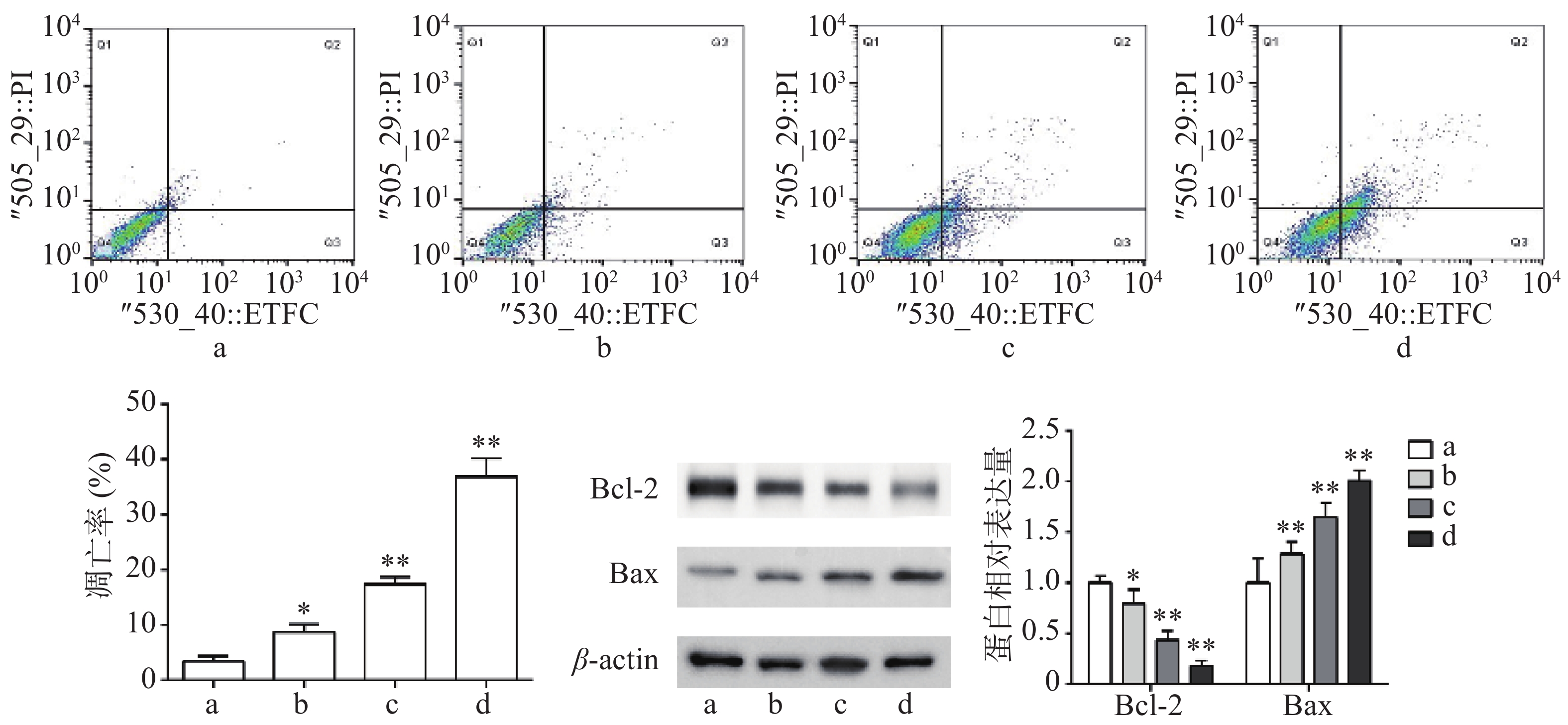
图1结果显示,与对照组比较,随着药物浓度增大,细胞存活率下降,其IC50为19.16 μmol/L,故选择1/2倍IC50(10 μmol/L),1倍IC50(20 μmol/L),2倍IC50(40 μmol/L)3个剂量进行后续实验。细胞克隆实验结果显示,随着药物浓度增大,细胞克隆形成显著被抑制,且呈现剂量依赖关系,差异具有统计学意义(P<0.05)。
-
流式细胞术检测结果显示,与对照组比较,Sch B(10、20、40 μmol/L)均可诱导细胞凋亡,且呈剂量依赖,具有统计意义(P<0.05)。Western blot法检测结果显示,与对照组比较,Sch B(10、20、40 μmol/L)使抗凋亡蛋白Bcl-2的表达显著降低,促凋亡蛋白Bax的表达显著升高( P<0.05),结果见图2。
-
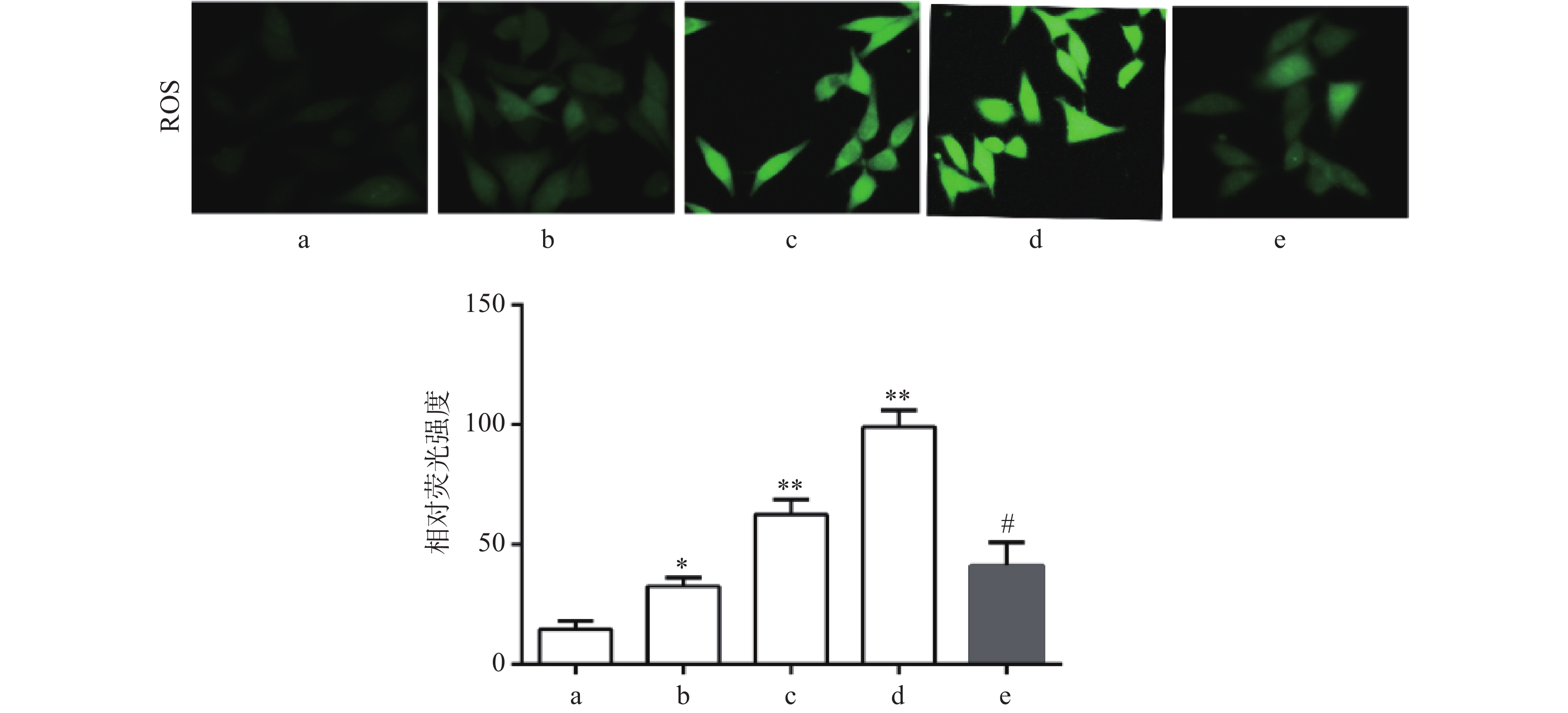
DCF-DA染色结果显示,与对照组比较,Sch B(10、20、40 μmol/L)组绿色荧光逐渐增强,显示细胞内ROS水平增高,且呈剂量依赖,差异有统计学意义(P<0.05),活性氧清除剂NAC(5 mmol/L)预处理2 h可显著抑制由Sch B导致的细胞内ROS增多(P<0.05),结果见图3。
-
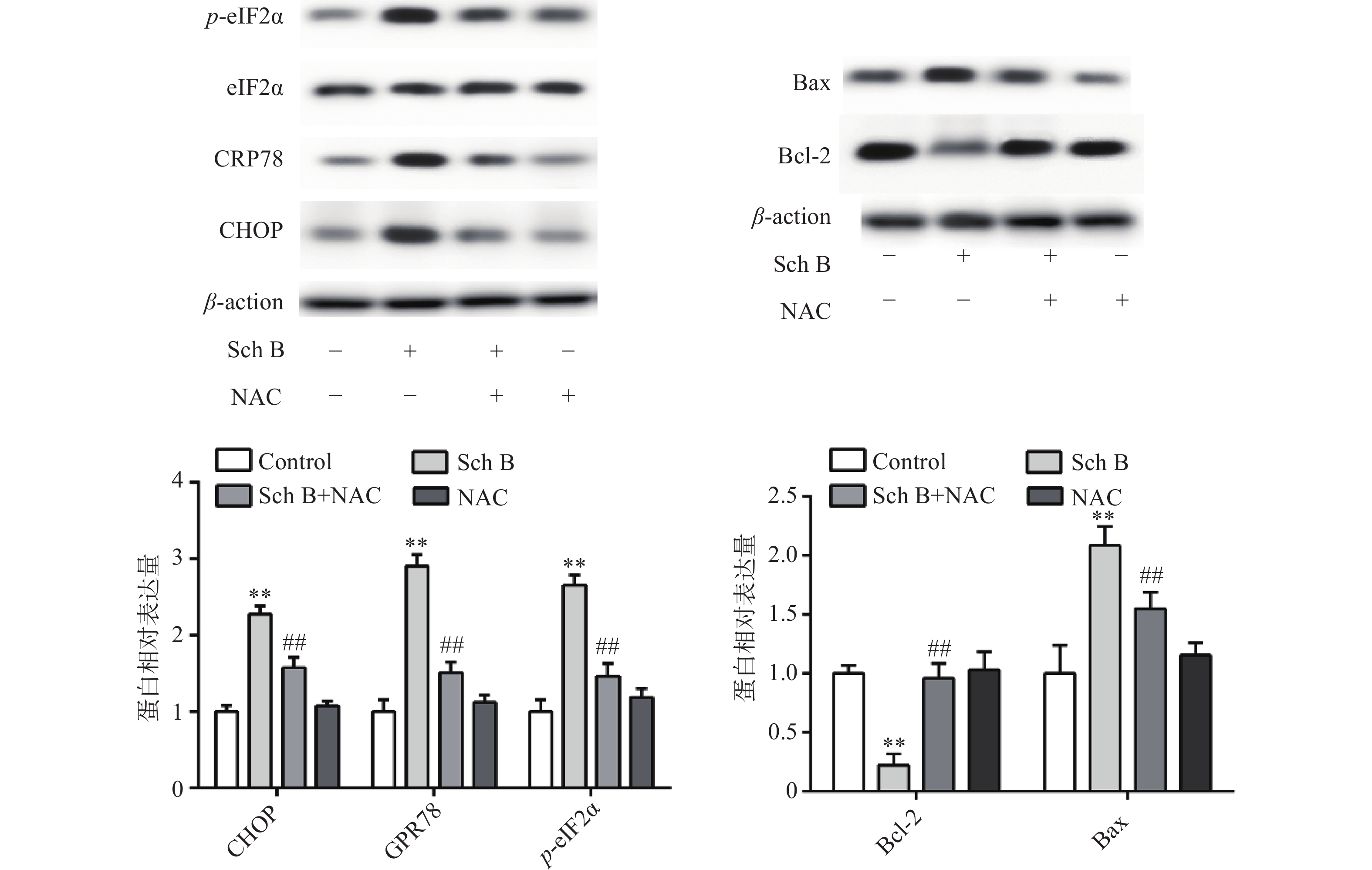
异常内质网应激可引起细胞凋亡,Western blot 法检测结果显示,与对照组比较,Sch B(10、20、40 μmol/L)分别使内质网应激相关蛋白CHOP,GPR78,p-eIF2α表达增多且呈剂量依赖,差异有统计学意义(P<0.05),内质网应激抑制剂4-PBA(2 mmol/L)预处理2 h,可显著抑制由Sch B引起的内质网应激,使内质网应激相关蛋白CHOP、GPR78、p-eIF2α表达降低(P<0.05),结果见图4。
-
上述结果表明Sch B可以使细胞内ROS升高,Sch B也可以使内质网应激诱导细胞凋亡。为了验证ROS升高与内质网应激之间的联系,MDA-MB-231用ROS清除剂NAC(5 mmol/L)预处理2 h,结果显示,NAC可逆转由Sch B引起的内质网应激,使内质网应激相关蛋白CHOP,GPR78,p-eIF2α显著降低(P<0.05),进一步实验证明, 5 mmol/L NAC可逆转由Sch B引起的细胞凋亡,使抗凋亡蛋白Bcl-2的表达显著升高,促凋亡蛋白Bax的表达显著降低(P<0.05),结果见图5。
-
乳腺癌是临床上特别常见的恶性肿瘤,每年的发病率和死亡率都在增加,其发生发展是一个复杂的病理过程,是不受控制的细胞增殖和抵抗凋亡的结果[9]。尽管癌症研究领域取得了相当大的进展,但由于对化疗耐药和高复发率,乳腺癌的总体生存率仍然不令人满意,因此,大量的研究都集中在发现新的有效的治疗乳腺癌的候选药物中[10]。从植物中提取的天然化合物具有更有效和更少的副作用被认为是抗癌药物的重要来源。
ROS在细胞存活和死亡中起着关键作用。在正常生理条件下,适当水平的ROS有助于细胞存活。然而,过量的ROS会导致细胞损伤和凋亡细胞死亡[11]。ROS作为不良刺激之一,可引起内质网功能障碍,诱导内质网应激,称为ROS介导的内质网应激[12]。在本研究中,MDA-MB-231细胞在与Sch B孵育前先经ROS清除剂NAC预处理。凋亡减轻,ROS生成减少,CHOP,GPR78和p-eIF2a表达下调,内质网应激减轻。这说明Sch B诱导的MDA-MB-231细胞内质网应激依赖性凋亡中,ROS是不可或缺的。
内质网是真核细胞中传导和提供蛋白质折叠环境的细胞器,因为它对刺激的敏感性会导致某种情况发生成为内质网应激,内质网应激被认为是应对内质网稳态失衡的各种细胞生物学生理和病理事件的一种高度保守的细胞防御机制,如抗癌药物诱导的细胞凋亡通路[13]。GPR78是调节内质网应激的关键因子,GPR78的激活增加了真核启动子2 alpha (eIF2α) 51位丝氨酸的磷酸化,从而抑制蛋白的合成,磷酸化的eIF2α促进活化转录因子4 (ATF4)和C/EBP同源蛋白(CHOP) 的表达,CHOP是调节Bcl-2家族蛋白表达的关键促凋亡转录因子[14]。我们发现,Sch B可引起乳腺癌MDA-MB-231细胞内质网应激,CHOP,GPR78和 p-eIF2α蛋白水平呈剂量依赖性增加,当使用内质网抑制剂4-PBA后,内质网应激减轻。
综述所述,Sch B可诱导乳腺癌MDA-MB-231细胞凋亡,其机制可能与增加细胞内ROS水平,使p-eIF2α蛋白激活,GPR78和CHOP表达增多,引发细胞内质网应激有关。本研究可为乳腺癌的辅助治疗提供一定的实验依据。
Schisandrin B induces apoptosis of human breast cancer MDA-MB-231 cells through ROS mediated endoplasmic reticulum stress
-
摘要:
目的 研究五味子乙素对人乳腺癌MDA-MB-231细胞凋亡的影响及其作用机制。 方法 用细胞计数试剂(CCK-8)检测不同浓度五味子乙素对MDA-MB-231细胞存活率的影响;五味子乙素(10、20、40 μmol/L)作用 MDA-MB-231 细胞 24 h,分别用Annexin V-FITC/PI检测细胞凋亡情况;用DCFA-DA荧光探针检测细胞内活性氧(ROS)水平;用Western blot法检测细胞凋亡及内质网应激相关蛋白(Bcl-2、Bax、CHOP、GPR78、PERK、p-PERK、p-eIF2α、eIF2)的表达。 结果 与空白组比较,随着五味子乙素浓度增大,细胞存活率明显降低,其IC50为19.16 μmol/L;与对照组比较,五味子乙素(10、20、40 μmol/L)均能抑制细胞克隆形成(P<0.05),且呈剂量依赖;五味子乙素(10、20、40 μmol/L)均可诱导细胞凋亡(P<0.05),使抗凋亡蛋白BCL-2的表达显著降低,促凋亡蛋白Bax的表达显著升高(P<0.05);五味子乙素(10、20、40 μmol/L)显著升高细胞内ROS水平(P<0.05),且呈剂量依赖;五味子乙素(10、20、40 μmol/L)能够激发内质网应激,使内质网应激相关蛋白CHOP、GPR78、p-eIF2α表达增多(P<0.05),且呈剂量依赖。 结论 五味子乙素可能通过ROS介导内质网应激诱导MDA-MB-231细胞凋亡。 Abstract:Objective To study the effects of schisandrin B (Sch B) on the apoptosis of human breast cancer MDA-MB-231 cells and its mechanism. Methods Cell counting reagent (CCK-8) was used to detect the effect of Sch B on the survival rate of MDA-MB-231 cells. MDA-MB-231 cells were treated with Sch B (10, 20, 40 μmol/L) for 24 hours. The cell death was detected by Annexin V-FITC/PI. The levels of intracellular reactive oxygen species (ROS) were detected by DCFA-DA fluorescent probe. Apoptosis and the expression of endoplasmic reticulum stress related proteins (Bcl-2、Bax、CHOP、GPR78、PERK、p-PERK、p-eIF2α、eIF2) were detected by Western blot. Results Compared with the blank group, the cell survival rate decreased significantly (P<0.01) with the increase of Sch B concentration, and its IC50 was 19.16 μmol/L. Compared with the control group, Sch B groups (10, 20, 40 μmol/L) inhibited cell clone formation in a dose-dependent manner (P<0.05). Sch B groups (10, 20, 40 μmol/L) induced apoptosis (P<0.05), significantly reduced the expression of anti-apoptotic protein Bcl-2 and significantly increased the expression of pro-apoptotic protein Bax (P<0.05). Sch B groups (10, 20, 40 μmol/L) significantly increased the level of intracellular ROS in a dose-dependent manner (P<0.05). Sch B groups (10, 20, 40 μmol/L) stimulated endoplasmic reticulum stress and increased the expressions of endoplasmic reticulum stress-related proteins CHOP, GPR78 and p-eIF2α in a dose-dependent manner (P<0.05). Conclusion Sch B induces apoptosis of MDA-MB-231 cells through ROS mediated endoplasmic reticulum stress. -
Key words:
- schisandrin B /
- breast cancer /
- reactive oxygen species /
- endoplasmic reticulum stress /
- apoptosis
-
骨质疏松症(OP)是一种由骨吸收和骨形成之间的关系失衡造成,以低骨量和骨组织微结构破坏为特征,导致骨质脆性增加和易于骨折的全身性骨代谢疾病。其中,成骨细胞是骨形成的功能细胞,在维持骨稳态中起到关键作用[1]。目前,高氧化应激相关的骨丢失已成为骨质疏松研究领域的热点。有研究表明,细胞保护酶是机体对抗氧化应激状态下活性氧(ROS)损伤的主要机制,其活性主要由转录因子Nrf2和FoxO调控,而二者所介导的氧化应激通路同样被证实具有调节成骨细胞氧化还原平衡以及促进骨形成分化的功能[2]。与此同时,β-淀粉样蛋白(amyloid β-protein,Aβ)的沉积可使机体ROS生成增多,进而抑制成骨细胞的增殖、成骨基质的产生及矿化[3]。由此可见,Aβ沉积偶联的氧化损伤是破坏成骨细胞骨形成,进而引发骨丢失的一大诱因。
啤酒花(Hops, Humulus lupulus L.)为桑科葎草属多年生草质蔓生藤本植物,其雌性球穗花序不仅作为啤酒酿造的添加原料,也是全球广泛应用的植物药,在欧洲广泛用于缓解更年期潮热及绝经后骨质疏松症[4]。我们前期研究发现啤酒花能够促进成骨细胞骨矿化结节的形成,降低活性氧水平,并显著改善APP/PS1转基因小鼠的骨丢失[5-6],但其对外源性Aβ损伤成骨细胞的氧化应激水平及骨形成的影响尚不明确。此外,我们首次确认了氧化应激和Aβ沉积之间的双向关联,及其在老年性骨质疏松症发病中的重要作用[7-8]。故本文拟以Aβ损伤的成骨细胞为模型,以Nrf2和FoxO1两条经典氧化应激相关通路为核心,对啤酒花的抗氧化能力及对骨形成干预作用进行探究。
1. 材料与方法
1.1 药材及试剂
啤酒花药材购于昌吉市山水啤酒花有限公司(产地:新疆昌吉;批号:PJH-01),经海军军医大学药学系生药学教研室辛海量副教授鉴定为啤酒花 Humulus lupulus L.的雌性球穗花序。称取啤酒花药材粉末70 g,加入料液比为1∶15的75%乙醇,回流提取3次,减压浓缩干燥成浸膏,HPLC测定得浸膏中主要成分黄腐酚含量为0.55%[9],使用前配制成相应浓度(生药量/ml)的提取液。
其他试剂及厂家:Aβ1-42寡聚体(上海吉尔);N-乙酰-L-半胱氨酸(NAC,上海碧云天);胎牛血清(Gibco,美国);α-MEM培养基等细胞培养试剂(天津灏洋);碱性磷酸酶(ALP)染色试剂盒(南京建成);I型胶原酶(COL-I)、骨桥蛋白(OPN)、核因子-E2-相关因子(Nrf2)、血红素加氧酶-1(HO-1)、NAD(P)H:醌氧化还原酶(NQO1)、细胞叉头框蛋白O1(FoxO1)、超氧化物歧化酶(SOD-2)抗体(Abcam,英国)。
1.2 实验动物及原代成骨细胞分离培养
新生24 h Wistar大鼠,购自上海斯莱克实验动物有限公司[合格证号:2013001831722;许可证号:SYXK(沪)2017-0004]。所有动物实验均符合实验动物伦理学要求。采用二次消化法从新生大鼠颅盖骨分离得到原代成骨细胞,用含10%胎牛血清的α-MEM培养液进行培养,取3~4代成骨细胞进行后续实验分析。
1.3 成骨细胞增殖及碱性磷酸酶检测
取3~4代成骨细胞计算其数目,配制成细胞浓度为1×104个/ml细胞悬液接种于96孔板,根据前期实验结果[9]设置分组:空白对照组,模型组(40 μmol/L Aβ),阳性对照组(NAC, 2.5 mmol/L),啤酒花提取物低剂量组(HLE, 4 μg/ml)、中剂量组(HLE, 20 μg/ml)、高剂量组(HLE, 100 μg/ml),每组设置4个复孔。24 h后按照上述分组更换为含药培养液。给药48 h后采用MTT法检测成骨细胞的增殖情况。
取3~4代成骨细胞计算其数目,配制成细胞浓度为5×104个/ml细胞悬液接种于24孔板。24 h后分别更换为含药培养液(给药浓度同上)。培养过程中每3 d更换1次含药培养液。第8天裂解细胞,收集细胞裂解液,于4 ℃、13 800×g 离心5 min。用对硝基苯磷酸二钠法测定细胞ALP活性[10]。参照ALP染色试剂盒说明书对成骨细胞进行染色。
1.4 成骨细胞凋亡检测
取3~4代成骨细胞计算其数目,配制成细胞浓度为2×105个/ml细胞悬液接种于6孔板。24 h后分别更换为含药培养液(给药浓度同上)。给药48 h后收集各孔中培养基上清液,加入200 μl 0.25%胰蛋白酶消化30 s,离心并重悬,参照凋亡检测试剂盒装载探针,室温避光孵育5 min后,用流式细胞仪进行凋亡率检测。
1.5 蛋白质印迹检测
取3~4代成骨细胞接种于6孔板,24 h后分别更换为含药培养液(给药浓度同上)。给药48 h后进行细胞裂解,提取细胞总蛋白,根据BCA试剂盒进行蛋白定量。蛋白变性后进行十二烷基硫酸钠-聚丙烯酰胺凝胶电泳分离,转膜、封闭后,分别加入COL-I、OPN、Nrf2、HO-1、NQO1、FoxO1及SOD-2抗体,4 ℃孵育过夜。用洗膜缓冲液(Tris-buffered saline/Tween-20,TBST)洗膜3×10 min,加入二抗,室温孵育30 min。用TBST再次洗膜3×10 min,采用ECL试剂进行检测。采用Tanon Image软件对蛋白印迹进行半定量分析。
1.6 免疫荧光检测
取3~4代成骨细胞配制成浓度为2×104个/ml细胞悬液接种于无菌激光共聚焦皿中。24 h后分别更换为含药培养液(Aβ, 40 μmol/L HLE, 100 μg/ml)。给药48 h后用4%的多聚甲醛固定细胞30 min,PBS浸洗后用0.5% Triton X-100(PBS配制)通透20 min,并用5% BSA封闭液室温封闭1 h。先后加入FoxO1抗体及荧光二抗(TRITC标记山羊抗兔抗体)进行孵育,加入DAPI染液避光孵育10 min进行染核。最后洗去多余的DAPI染液,加入少量PBS使细胞保持湿润,并置于荧光显微镜下观察采集图像。
1.7 统计学分析
实验结果以均值±标准差(
$ \bar x$ ±s)表示。采用SPSS 22.0软件进行数据分析,选用单因素方差分析(One-Way ANOVA)进行组间变量的比较分析。2. 结果
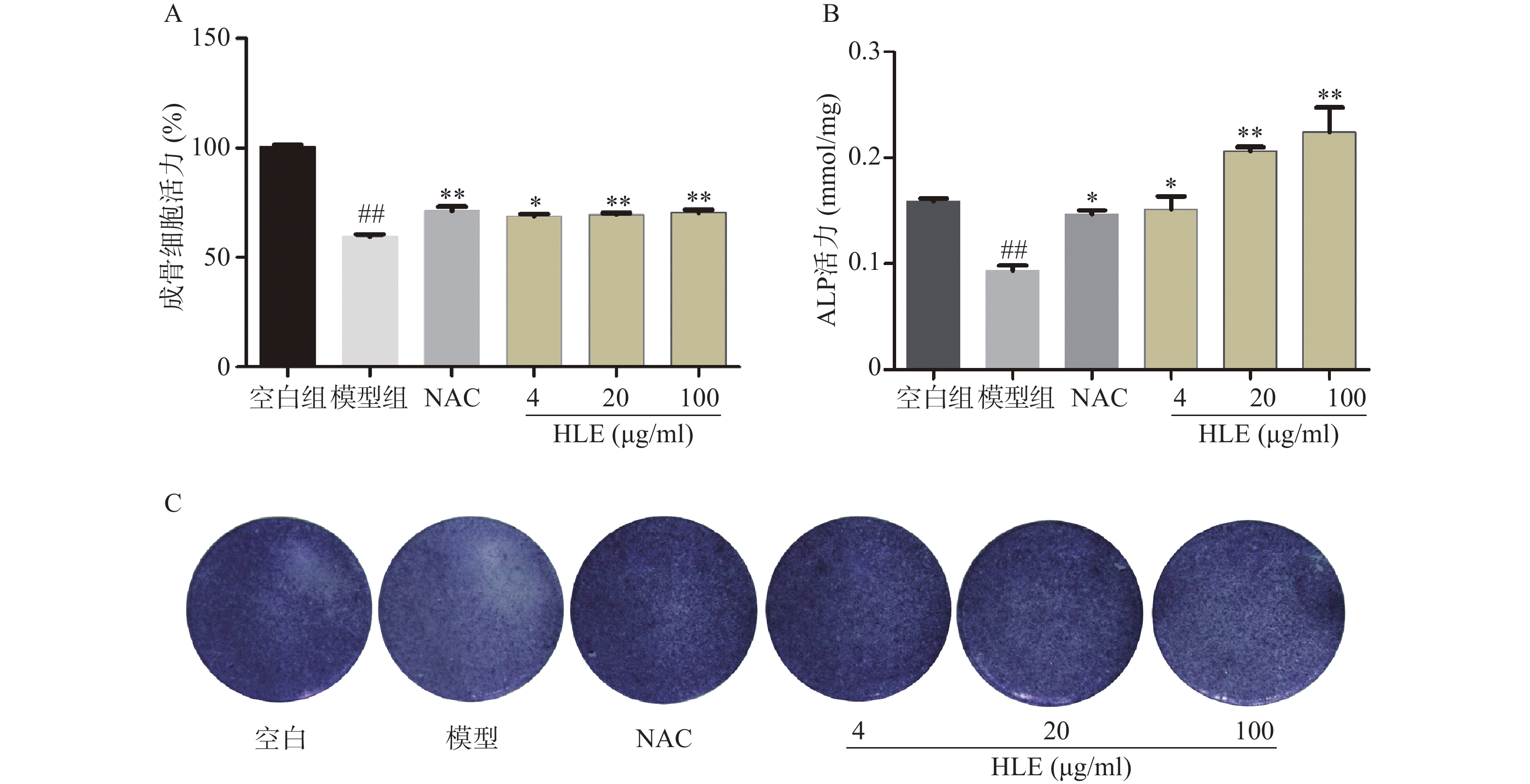
2.1 啤酒花提取物促进Aβ损伤成骨细胞增殖,提高ALP活性
如图1A所示,与空白组相比,Aβ损伤成骨细胞后,其增殖能力显著降低。药物治疗后,低、中、高剂量的啤酒花提取物均可显著促进Aβ损伤成骨细胞的增殖。另一方面,与空白组比,Aβ显著降低了成骨细胞的ALP活性,而啤酒花提取物显著逆转了Aβ损伤成骨细胞的ALP活性,促进成骨细胞的分化(图1B-C)。
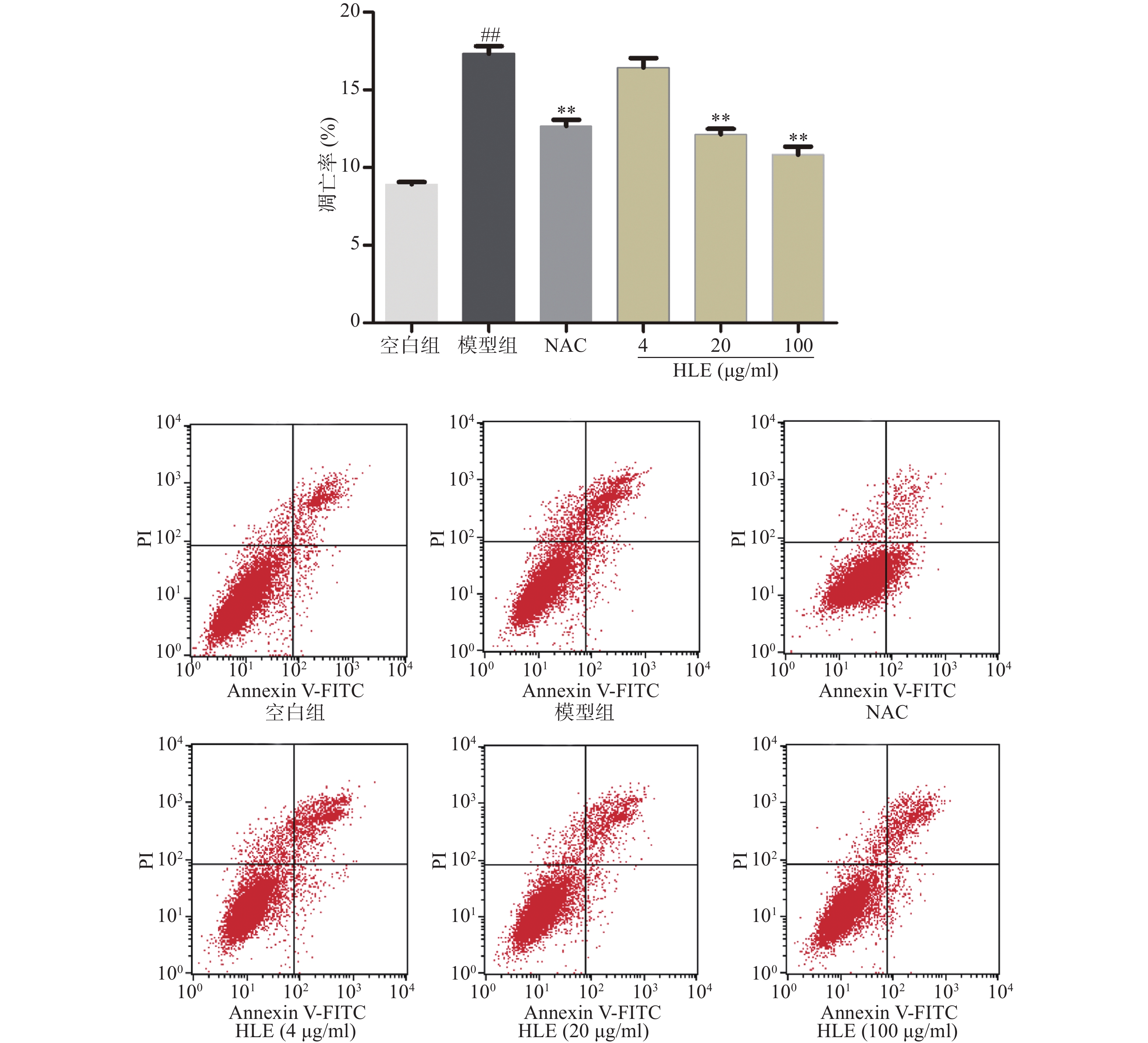
2.2 啤酒花提取物抑制Aβ损伤成骨细胞凋亡
如图2所示,与空白组相比,Aβ损伤提高了成骨细胞的凋亡率,而啤酒花提取物(20,100 μg/ml)可显著抑制Aβ损伤成骨细胞的凋亡。提示啤酒花提取物对Aβ诱导的成骨细胞具有较强的抗凋亡作用。
2.3 啤酒花提取物促进Aβ损伤成骨细胞中骨形成相关蛋白表达
如图3所示,Aβ损伤成骨细胞后,细胞中骨形成相关蛋白COL-I和OPN的表达显著降低。给予啤酒花提取物(20,100 μg/ml)干预后,COL-I及OPN的表达显著增加,提示啤酒花可显著促进Aβ损伤成骨细胞的骨形成。
2.4 啤酒花提取物调节Nrf2氧化应激通路
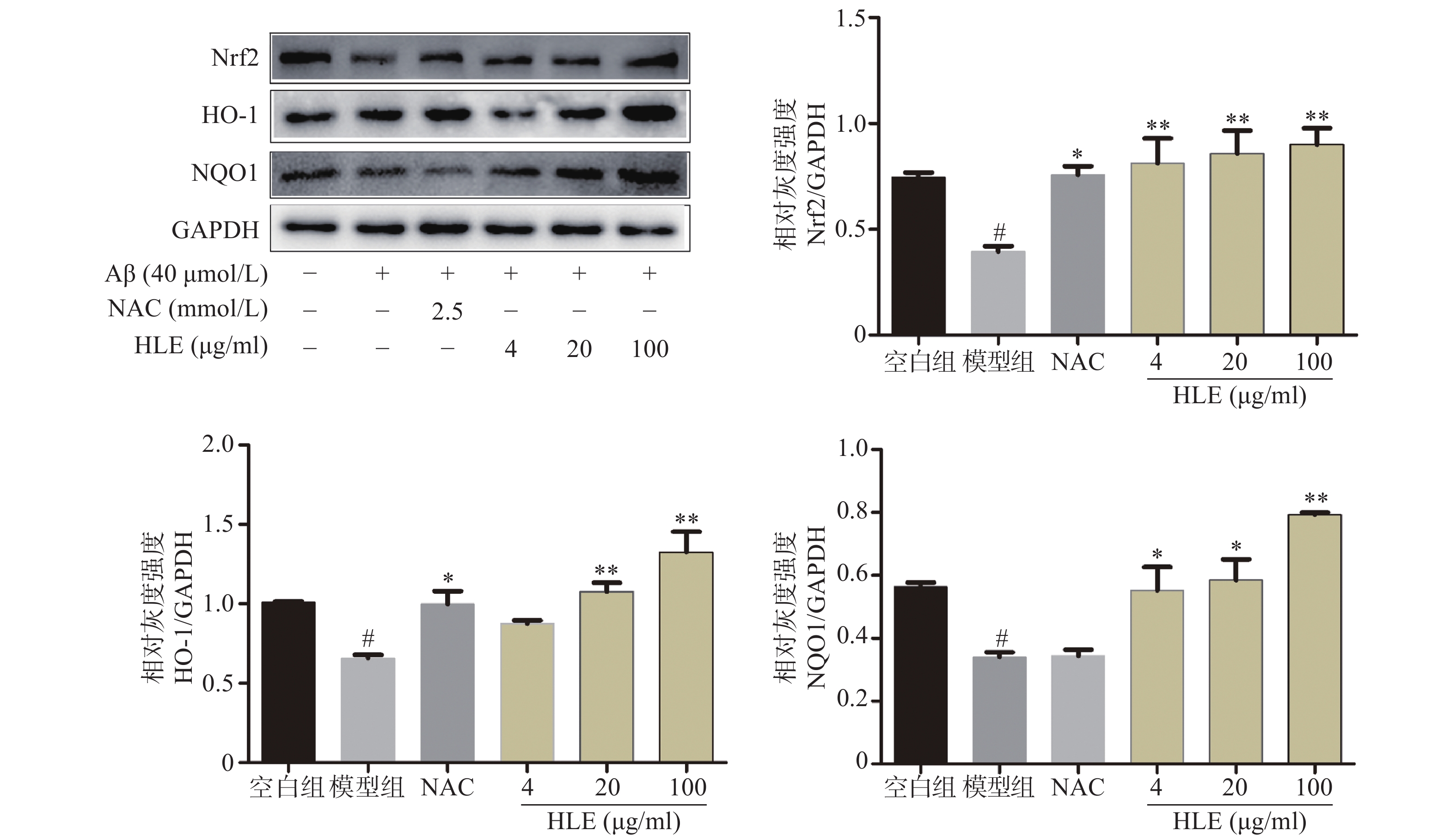
采用Western blot分析Aβ和啤酒花提取物对成骨细胞中Nrf2及其下游抗氧化酶HO-1和NQO1的影响。结果显示,Aβ显著抑制了成骨细胞中Nrf2及其下游蛋白HO-1和NQO1的表达;而低、中、高剂量的啤酒花提取物均可显著促进Aβ损伤成骨细胞中Nrf2、HO-1及NQO1的表达(图4),提示其能够通过介导Nrf2信号通路发挥抗氧化作用。
2.5 啤酒花提取物调节FoxO1抗氧化通路
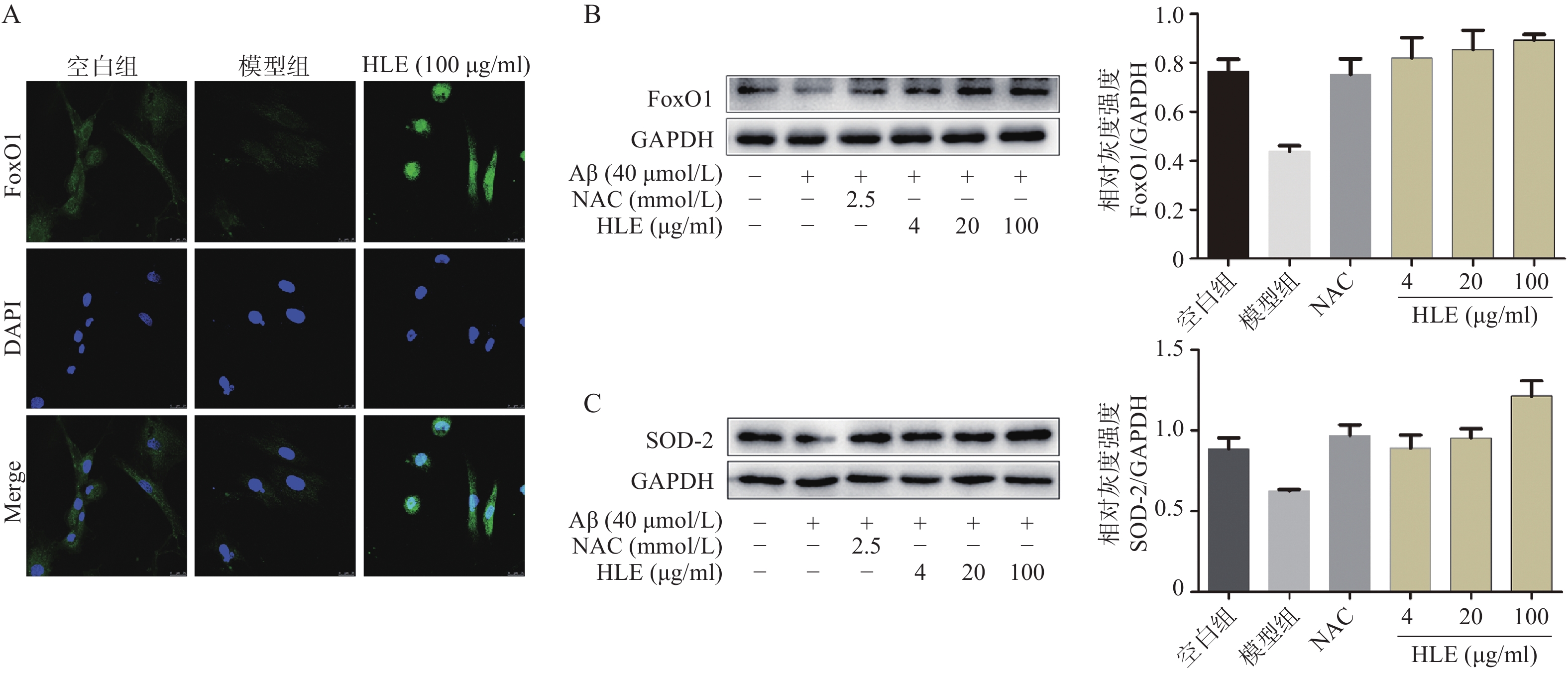
采用免疫荧光及Western blot分析Aβ和啤酒花提取物对成骨细胞中FoxO1信号通路的影响。结果显示,与空白组相比,Aβ显著降低了成骨细胞中的FoxO1含量,而啤酒花提取物(100 μg/ml)可显著下调FoxO1的表达,且使其更多聚集在细胞核内(图5A)。此外,啤酒花提取物(4、20、100 μg/ml)还可显著促进Aβ损伤成骨细胞中FoxO1及其下游蛋白SOD-2的表达,提示啤酒花能够通过介导FoxO1信号通路发挥抗氧化作用。
3. 讨论
成骨细胞是骨形成的主要功能细胞,在骨形成过程中经历增殖、分化、矿化和凋亡四个阶段。其中,成骨细胞的增殖水平反映骨形成的强弱,其分泌的ALP是成骨细胞分化阶段的关键酶[11],可介导骨组织矿化。在骨形成相关蛋白中,COL-I是骨细胞外基质的主要成分之一,约占骨总蛋白的80%[12],而OPN是一种骨基质糖蛋白,能够促进成骨细胞的黏附和分化[13],二者均为成骨细胞分化成熟的标志。此外,Aβ沉积会引起机体的氧化损伤,在骨代谢中可降低骨髓间充质干细胞向成骨细胞的分化,破坏成骨细胞的活性和功能,抑制骨形成[14]。我们前期研究同样发现过量的Aβ在聚集过程中会产生大量的活性氧,使机体处于高氧化应激状态,反过来又刺激Aβ产生和聚集,形成Aβ与氧化损伤相偶联[15]。该机制在老年性骨质疏松症发病中处于特别重要的位置,并受到学界关注[16]。本研究中,啤酒花提取物可显著逆转Aβ损伤所致的成骨细胞增殖水平下降、ALP活性降低,以及COL-I和OPN的低表达,表明其可显著促进成骨细胞的成熟分化;并抑制Aβ损伤成骨细胞的凋亡,表明其可显著改善Aβ沉积所致成骨细胞的活性损伤,促进骨形成,在维持骨稳态中发挥重要作用。
Nrf-2信号通路是典型的抗氧化通路,能够拮抗各种原因引起的氧化应激。激活Nrf2信号通路不仅能够抑制氧化损伤成骨细胞的凋亡,促进骨形成[17],而且能够拮抗Aβ诱导的细胞损伤[18-19]。应激状态下,Nrf-2转移入核内,与基因中的抗氧化反应元件结合,启动下游Ⅱ相代谢酶基因的表达和转录,以增加细胞对氧化应激的抵抗作用,使细胞免于凋亡[20]。FoxO1为调节成骨细胞氧化还原平衡和成骨功能的主要转录因子,其入核可激活下游SOD-2抗氧化酶,调控细胞内的氧化还原平衡,并促进成骨细胞的增殖与分化,在骨代谢中同样发挥着重要作用[20]。本研究结果表明,啤酒花可激活Aβ损伤成骨细胞的Nrf-2和FoxO1信号通路,促进该氧化应激信号通路中相关蛋白的表达,增加成骨细胞对氧化应激的抵抗作用,使其免于凋亡,进而促进成骨细胞增殖与分化。提示啤酒花具有通过抗氧化而调控骨代谢、维持骨稳态之应用潜力。
-
-
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2018,68(6):394-424. doi: 10.3322/caac.21492 [2] 李贺, 郑荣寿, 张思维, 等. 2014年中国女性乳腺癌发病与死亡分析[J]. 中华肿瘤杂志, 2018, 40(3):166-171. doi: 10.3760/cma.j.issn.0253-3766.2018.03.002 [3] TANG Y, WANG Y, KIANI M F, et al. Classification, treatment strategy, and associated drug resistance in breast cancer[J]. Clin Breast Cancer,2016,16(5):335-343. doi: 10.1016/j.clbc.2016.05.012 [4] 白文宇, 王厚恩, 王冰瑶, 等. 五味子化学成分及其药理作用研究进展[J]. 中成药, 2019, 41(9):2177-2183. doi: 10.3969/j.issn.1001-1528.2019.09.033 [5] RAN J, MA C, XU K, et al. Schisandrin B ameliorated chondrocytes inflammation and osteoarthritis via suppression of NF-κB and MAPK signal pathways[J]. Drug Des Devel Ther,2018,12:1195-1204. doi: 10.2147/DDDT.S162014 [6] NASSER M I, HAN T Y, ADLAT S, et al. Inhibitory effects of Schisandrin B on human prostate cancer cells[J]. Oncol Rep,2019,41(1):677-685. [7] WANG C Q, XU C, FU X L, et al. Schisandrin B suppresses liver fibrosis in rats by targeting miR-101-5p through the TGF-β signaling pathway[J]. Artif Cells Nanomed Biotechnol,2020,48(1):473-478. doi: 10.1080/21691401.2020.1717507 [8] DAI X, YIN C, GUO G, et al. Schisandrin B exhibits potent anticancer activity in triple negative breast cancer by inhibiting STAT3[J]. Toxicol Appl Pharmacol,2018,358:110-119. doi: 10.1016/j.taap.2018.09.005 [9] SOPIK V. International variation in breast cancer incidence and mortality in young women[J]. Breast Cancer Res Treat,2021,186(2):497-507. doi: 10.1007/s10549-020-06003-8 [10] SHAMSI M, PIRAYESH ISLAMIAN J. Breast cancer: early diagnosis and effective treatment by drug delivery tracing[J]. Nucl Med Rev Cent East Eur,2017,20(1):45-48. doi: 10.5603/NMR.2017.0002 [11] D'AUTRÉAUX B, TOLEDANO M B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis[J]. Nat Rev Mol Cell Biol,2007,8(10):813-824. doi: 10.1038/nrm2256 [12] WU W S. The signaling mechanism of ROS in tumor progression[J]. Cancer Metastasis Rev,2006,25(4):695-705. [13] LI Q, LU X H, WANG C D, et al. Antiproliferative and apoptosis-inducing activity of schisandrin B against human glioma cells[J]. Cancer Cell Int,2015,15(1):12. doi: 10.1186/s12935-015-0160-x [14] JHENG J R, HO J Y, HORNG J T. ER stress, autophagy, and RNA viruses[J]. Front Microbiol,2014,5:388. 期刊类型引用(4)
1. 徐卫凡,徐武牧,丁卢颖,蒋益萍,夏天爽,辛海量. 巴戟天丸防治D-半乳糖损伤成骨细胞骨丢失的作用及机制研究. 药学实践与服务. 2023(03): 155-159 .  本站查看
本站查看2. 欧泽洁,孔凡玉,周月南,杨朝霞,庞雪莉. 基于超临界CO_2萃取和旋转锥体柱法的啤酒花提取物香气特征比较. 食品安全质量检测学报. 2023(12): 46-53 .  百度学术
百度学术3. 赖立勇,徐圣焱,夏天爽,蒋益萍,辛海量. 基于抗氧化机制的中药及其化学成分在骨质疏松中的应用. 海军军医大学学报. 2022(08): 943-950 .  百度学术
百度学术4. 郝建秦,张晶晶,薛洁,张磊,王德良. 啤酒花中多酚类化合物指纹图谱及化学计量学分析. 中外酒业. 2022(09): 1-7 .  百度学术
百度学术其他类型引用(2)
-






 下载:
下载:

 下载:
下载: