-
海绵是具有代表性的海洋生物,其共附生微生物也是近年来研究的热点。在海洋高盐、高压、低温、寡营养的生存环境下,海绵共附生微生物能够产生结构新颖、生物活性良好的次级代谢产物。其中海绵共附生真菌是海绵化学多样性的重要来源[1]。
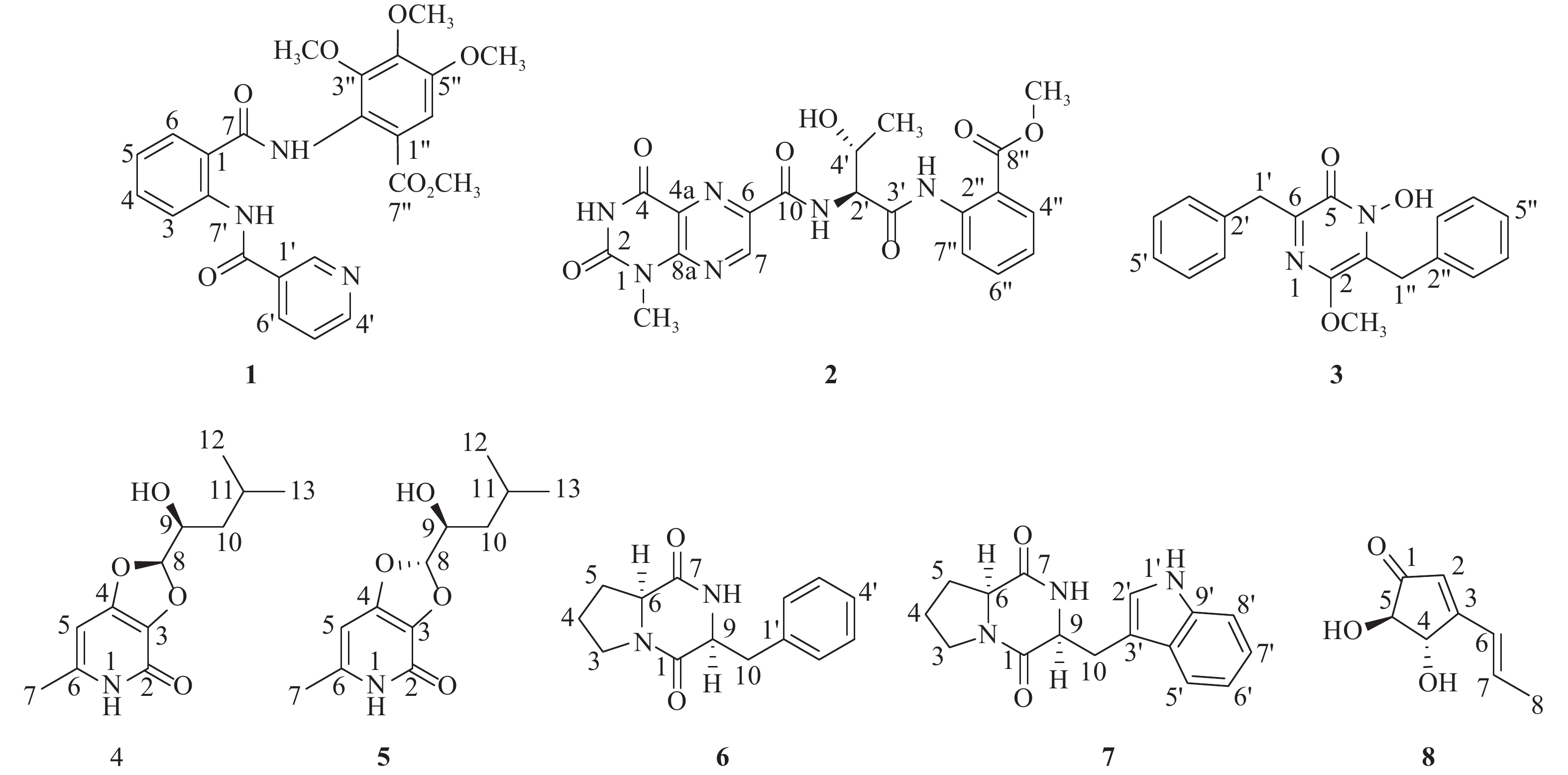
曲霉属 (Aspergillus sp)真菌分布广泛而且研究丰富。海洋曲霉属真菌的次级代谢产物主要包括聚酮类[2]、生物碱类[3]、肽类[4]、萜类[5]等化合物,具有抗肿瘤[6]、抗菌[7]、抗病毒[4]等生物活性。本课题的土曲霉(Aspergillus terreus)是从我国南海西沙永兴岛海域的棕色扁海绵Phakellia fusca中分离得到的,属于散囊菌目(Eurotiales)发菌科(Tri-chocomaceaez)的一种真菌,在海洋动植物和陆地植物中均有分布。该菌的次级代谢产物具有多样性,包括生物碱类化合物[8]、丁烯酸内酯类化合物[9]、萜类化合物[10]、环肽类化合物[11]等。本文采用硅胶柱色谱、Sephadex LH-20凝胶柱色谱、高效液相色谱等多种分离方法从土曲霉Aspergillus terreus中共分离得到8个单体化合物。通过理化常数测定、波谱数据分析等方法确定了化合物的结构。化合物1~8的结构见图1。
-
菌株来源于棕色扁海绵Phakellia fusca,由上海交通大学海洋药物研究中心鉴定为Aspergillus terreus,菌株保存在上海交通大学医学院附属仁济医院药学部海洋药物研究中心(菌株编号152805)。
-
Agilent 600核磁共振波谱仪(美国 Agilent 公司);Waters高效液相色谱仪(美国Waters公司);XBridge C18半制备型液相色谱柱(10 mm×250 mm,5 μm);快速制备色谱仪(法国Interchim公司);OSB-2100旋转蒸发仪(日本EYELA 公司);振荡培养箱(上海知楚)。薄层硅胶、200~300目柱色谱用硅胶(青岛海洋化工厂);Sephadex LH-20凝胶(瑞典GE Healthcare公司);色谱纯试剂(天津康科德科技有限公司);其他分析纯有机试剂(上海化学试剂公司);氘代试剂(剑桥同位素实验室)。
-
取Aspergillus terreus单菌落接种到装有100 ml PDB培养液的250 ml三角瓶中,28 ℃,220 r/min震荡培养3 d,以该发酵液10%的接种量接到装有500 ml的真菌2号培养液(甘露醇20 g,麦芽糖20 g,CaCO3 15 g,葡萄糖10 g,谷氨酸钠10 g,酵母提取物3 g,玉米浆1 g,KH2PO4 0.5 g,MgSO4·7H2O 0.3 g,海盐30 g,蒸馏水1 L)的1 L三角瓶中,28 ℃,220 r/min震荡培养10 d,获得菌株的发酵物。收集发酵液24 L,用等体积的乙酸乙酯萃取3次,浓缩后得到乙酸乙酯相浸膏9.3 g。
-
乙酸乙酯相浸膏首先经Sephadex LH-20凝胶柱色谱分离,以二氯甲烷-甲醇(体积比为1∶1)作为溶剂进行洗脱,得到组分Fr.1~Fr.4。组分Fr.2经硅胶柱色谱(石油醚:丙酮 = 100∶1~0∶100)分离得到组分Fr.2-1~Fr.2-9。组分Fr.2-5经反相中压柱色谱分离得到8个亚组分,其中Fr.2-5d经重结晶得到化合物3 (2.5 mg)。组分Fr.2-6经LH-20凝胶柱色谱和反相半制备HPLC(38%乙腈-水)分离得到化合物1 (3.5 mg, tR = 21.0 min)。化合物2 (3.5 mg, tR = 13.0 min)由组分Fr.2-7经反相半制备HPLC,以33%乙腈-水为流动相等梯度洗脱得到。组分Fr.2-8以乙腈-水 (体积比10∶90~100∶0)为流动相,经反相中压柱色谱和反相半制备HPLC(20%乙腈-水)分离得到化合物4 (2.0 mg, tR=30.0 min)、 化合物5 (4.0 mg, tR=28.0 min)和化合物6 (9.0 mg, tR=14.0 min)。Fr.3经过硅胶柱色谱分离得到7个组分,其中Fr.3-3经反相半制备HPLC进一步纯化得到化合物7 (1.7 mg, tR=12.0 min)。组分Fr.3-4以20%~100%的乙腈-水为流动相,经反相中压柱色谱和反相半制备HPLC(15%乙腈-水)分离得到化合物8 (18.0 mg, tR = 8.0 min)。
-
化合物1为黄色粉末(甲醇),硫酸/香草醛显色为黄色,ESIMS给出的分子离子峰[M+H]+m/z 466.15。1H NMR (600 MHz, CDCl3)中,δH 12.23 (1H, s)为氨基质子信号;一组邻位二取代的苯环质子信号δH 8.82 (1H, dd, J=8.5, 0.8 Hz, H-3), 7.89 (1H, dd, J=7.9, 1.3 Hz, H-6), 7.60 (1H, td, J=8.5, 1.3 Hz, H-4), 7.22 (1H, m, H-5),芳香质子信号δH 9.21 (1H, brs, H-9), 8.70 (1H, d, J=4.5 Hz, H-1′), 8.25 (1H, dt, J=8.0, 2.2 Hz, H-3′), 7.36 (1H, dd, J=8.0, 4.5 Hz, H-2′),提示3-取代吡啶环的存在;1个芳香质子信号δH 7.27 (1H, s, H-10′);4个甲氧基质子信号δH 3.97 (3H, s, 4″-OCH3), 3.91 (3H, s, 3″-OCH3), 3.90 (3H, s, 5″-OCH3), 3.82 (3H, s, 7″-OCH3)。13C NMR (150 MHz, CDCl3)共显示24个碳信号,结合DEPT谱,推断δC 168.2, 167.2, 164.0为羰基碳信号;17个芳香碳信号;δC 61.3, 61.3, 56.5, 52.7为4个甲氧基碳信号。碳信号归属为:δC 168.2 (C-7)、167.2 (C-7′′)、164.0 (C-7′)、152.6 (C-4′)、151.5 (C-5′′)、149.3 (C-2′)、148.8 (C-3′′)、146.9 (C-4′′)、140.4 (C-2)、135.2 (C-6′)、133.6 (C-4)、130.3 (C-1′)、127.9 (C-6)、125.8 (C-2′′)、123.8 (C-5)、123.6 (C-5′)、121.8 (C-3)、120.4 (C-1′′)、119.0 (C-1)、108.8 (C-6′′)、61.3 (3″-OCH3)、61.3 (4″-OCH3)、56.5 (5″-OCH3)、52.7 (7″-OCH3)。该化合物核磁数据与参考文献[11]对照基本一致,确定化合物为methyl-3,4,5-trimethoxy-2-(2-(nicotinamido)benzamido) benzoate。
化合物2为黄色粉末(甲醇),ESIMS给出的分子离子峰[M+H]+m/z 457.14。1H NMR (600 MHz, DMSO-d6)中,δH 12.19 (1H, s, 3-NH), 11.10 (1H, s, 1′′-NH), 8.52 (1H, d, J = 8.1 Hz, 1′-NH)为氨基质子信号;1个芳香质子单峰信号δH 9.29 (1H, s, H-7);一组邻位二取代的苯环质子信号δH 8.44 (1H, d, J = 8.5 Hz, H-7′′), 7.92 (1H, dd, J = 7.9, 1.5 Hz, H-4′′), 7.63 (1H, td, J = 7.9, 1.5 Hz, H-6′′), 7.20 (1H, td, J = 7.6, 1.5 Hz, H-5′′);2个相邻的连接杂原子的次甲基质子信号δH 4.55 (1H, dd, J = 8.1, 2.9 Hz, H-2′), 4.41 (1H, m, H-4′);3个甲基质子信号δH 3.70 (3H, s, H-9′′), 3.52 (3H, s, H-9), 1.19 (3H, d, J = 6.4 Hz, H-5′)。13C NMR (150 MHz, DMSO-d6)共显示20个碳信号,结合DEPT谱,推断δC 168.8, 167.3, 162.7, 159.5, 150.1为羰基碳信号;10个芳香碳信号;δC 65.9, 59.8为2个连杂原子的次甲基碳信号;δC 52.4, 28.6, 20.5为3个甲基碳信号,结合氢谱信号,确定有一个甲氧基和一个氮甲基。碳信号归属为:δC 168.8 (C-3′)、167.3 (C-8″)、162.7 (C-10)、159.5 (C-4)、151.2 (C-8a)、150.1 (C-2)、146.3 (C-7)、139.3 (C-2′′)、138.2 (C-6)、134.2 (C-6′′)、130.7 (C-4′′)、127.2 (C-4a)、123.4 (C-5′′)、120.7 (C-7′′)、117.1 (C-3′′)、65.9 (C-4′)、59.8 (C-2′)、52.4 (C-9″)、28.6 (C-9)、20.5 (C-5′)。该化合物的比旋光值为
$[\alpha]_{\rm{D}}^{20} $ +98 (c 0.1, MeOH)。该核磁数据与参考文献[12]对照基本一致,确定该化合物为terrelumamide A。化合物3为白色结晶(甲醇),ESIMS给出的分子离子峰[M+H]+m/z 323.13。1H-NMR (600 MHz, CDCl3)中,δH 7.2-7.5 (10H, m, H-3′-H-7′, H-3′′-H-7′′)为10个芳香质子信号,提示存在2个单取代苯基;2个亚甲基质子信号δH 4.20 (2H, brs, H-1′′), 3.94 (2H, brs, H-1′);1个甲氧基质子信号δH 3.92 (3H, s, 2-OCH3)。13C-NMR (150 MHz, CDCl3)共显示19个碳信号,结合DEPT谱推断δC 158.2为羰基碳信号;12个芳香碳信号;δC 34.0, 30.4为2个亚甲基碳信号,提示结构中存在两个苄基基团;δC 61.8为甲基碳信号;δC 144.2, 140.6, 129.4为3个烯碳信号。碳信号归属为:δC 158.2 (C-5), 144.2 (C-6), 140.6 (C-2), 136.5 (C-2′′), 135.6 (C-1′), 129.6 (C-3′, 7′), 129.4 (C-3, 3′′, 7′′), 128.6 (C-4′, 6′), 127.8 (C-4′′, 6′′), 126.9 (C-5′, 5′′), 61.8 (2-OCH3), 34.0 (C-1′′), 30.4 (C-1′)。该化合物核磁数据与参考文献[13]对照基本一致,确定化合物为emeheterone。
化合物4为黄色粉末(甲醇),硫酸/香草醛显色为紫色,ESIMS给出的分子离子峰[M+H]+m/z 240.12。1H NMR (600 MHz, CD3OD)中,给出1个芳香质子信号δH 6.13 (1H, d, J = 0.7 Hz, H-5);3个次甲基氢信号δH 6.07 (1H, d, J = 3.0 Hz, H-8), 3.89 (1H, dt, J = 10.5, 3.0 Hz, H-9), 1.90 (1H, m, H-11);1个亚甲基质子信号δH 1.58 (1H, ddd, J = 12.2, 10.5, 4.6 Hz, H-10), 1.36 (1H, ddd, J = 12.2, 10.5, 3.0 Hz, H-10);3个甲基质子信号δH 2.28 (3H, s, H-7), 0.99 (3H, d, J = 6.7 Hz, H-13), 0.96 (3H, d, J = 6.7 Hz, H-12)。13C NMR (150 MHz, CD3OD)共显示12个碳信号,结合DEPT谱推断δC 155.0为羰基碳信号;4个芳香碳信号;δC 115.8, 70.5, 25.2为3个次甲基脂肪碳信号,结合对应的氢信号提示结构中存在1个缩醛碳信号和一个连氧次甲基碳信号;δC 40.4为亚甲基碳信号;δC 24.0, 21.8, 18.8为3个甲基碳信号。碳谱信号归属为:δC 157.9 (C-4)、155.0 (C-2)、143.5 (C-6)、132.7 (C-3)、115.8 (C-8)、95.0 (C-5)、70.5 (C-9)、40.4 (C-10)、25.2 (C-11)、24.0 (C-12)、21.8 (C-13)、18.8 (C-7)。该化合物的ECD曲线显示在217 nm处有负的Cotton 效应(Δε −5.86),其核磁和ECD数据与参考文献[14]对照基本一致,最终确定该化合物为(8R, 9S)-dihydroisoflavipucine。
化合物5为黄色结晶(甲醇),硫酸/香草醛显色为紫色,ESIMS给出的分子离子峰[M+H]+m/z 240.12。1H NMR (600 MHz, CD3OD)中,给出1个芳香质子信号δH 6.13 (1H, d, J = 0.7 Hz, H-5);3个次甲基氢信号δH 6.06 (1H, d, J = 3.0 Hz, H-8), 3.90 (1H, dt, J = 10.5, 3.0 Hz, H-9), 1.90 (1H, m, H-11);1组亚甲基质子信号δH 1.56 (1H, ddd, J = 12.3, 10.5, 4.6 Hz, H-10), 1.36 (1H, ddd, J = 12.3, 10.5, 3.0 Hz, H-10);3个甲基质子信号δH 2.28 (3H, s, H-7), 0.99 (3H, d, J = 6.6 Hz, H-13), 0.95 (3H, d, J = 6.6 Hz, H-12)。13C NMR (150 MHz, CD3OD)共显示12个碳信号,结合DEPT谱推断δC 155.0为羰基碳信号;4个芳香碳信号;δC 115.8, 70.5, 25.2为3个次甲基碳信号,结合对应的氢信号提示结构中存在1个次甲二氧基碳信号和一个连氧次甲基碳信号;δC 40.5为亚甲基碳信号;δC 24.0, 21.8, 18.8为3个甲基碳信号。碳信号归属为:δC 157.8 (C-4)、155.0 (C-2)、143.4 (C-6)、132.8 (C-3)、115.8 (C-8)、95.1 (C-5)、70.5 (C-9)、40.5 (C-10)、25.2 (C-11)、24.0 (C-12)、21.8 (C-13)、18.8 (C-7)。该化合物的核磁数据与化合物4对比基本一致,ECD曲线显示在217 nm处有正的Cotton 效应(Δε +25.34),提示为化合物4的差向异构体。将此化合物的核磁和ECD数据与参考文献[14]对照基本一致,最终确定化合物为(8S, 9S)-dihydroisoflavipucine。
化合物6为黄色粉末(甲醇),硫酸/香草醛溶液无明显显色,ESIMS给出的分子离子峰[M+H]+m/z 245.12。1H NMR (600 MHz, CDCl3)中,给出1组单取代的苯环芳香质子信号δH 7.32 (2H, t, J = 7.5 Hz, H-5′), 7.26 (1H, t, J = 7.5 Hz, H-4′), 7.20 (2H, d, J = 7.5 Hz, H-6′);2个次甲基氢信号δH 4.25 (1H, dd, J=10.5, 2.9 Hz, H-9), 4.04 (1H, t, J = 7.8 Hz, H-6);4组亚甲基质子信号δH 3.65-3.50 (2H, m, H-3); 3.65-3.50 (1H, m, H-10), 2.76 (1H, dd, J=14.5, 10.5 Hz, H-10); 2.30 (1H, m, H-5), 1.88 (1H,m, H-5); 1.98 (2H, m, H-4)。13C NMR (150 MHz, CDCl3)共显示14个碳信号,结合DEPT谱推断δC 169.6, 165.3为酰胺羰基碳信号;6个芳香碳信号;δC 59.3, 56.4为2个连氮次甲基碳信号;δC 45.6, 37.0, 28.5, 22.7为4个亚甲基碳信号,提示结构中存在苯丙氨酸和脯氨酸片段。碳信号归属为:δC 169.6 (C-7)、165.3 (C-1)、136.1 (C-1′)、129.4 (C-2′)、129.4 (C-6′)、129.3 (C-3′)、129.3 (C-5′)、127.7 (C-4′)、59.3 (C-6)、56.4 (C-9)、45.6 (C-3)、37.0 (C-10)、28.5 (C-5)、22.7 (C-4)。该化合物的比旋光值为
$[\alpha]_{\rm{D}}^{20} $ -47 (c 0.1, MeOH),将核磁数据与参考文献[15]对照基本一致,最终确定化合物为cyclo(S-Pro-S-Phe)。化合物7为浅黄色粉末(甲醇),硫酸/香草醛显色不明显,ESIMS给出的分子离子峰[M+H]+m/z 284.13。1H NMR (600 MHz, DMSO-d6)中给出2个氨基质子信号δH 10.83 (1H, s, H-1′), 7.71 (1H, s, H-8);1组邻二取代的苯环芳香质子信号δH 7.54 (1H, d, J = 8.0 Hz, H-5′), 7.30 (1H, d, J = 8.0 Hz, H-8′), 7.03 (1H, t, J = 7.3 Hz, H-7′), 6.94 (1H, t, J = 7.3 Hz, H-6′);1个芳香质子单峰信号δH 7.16 (1H, s, H-2′);2个次甲基氢信号δH 4.28 (1H, t, J = 5.0 Hz, H-9), 4.04 (1H, t, J = 8.5 Hz, H-6);4组亚甲基质子信号δH 3.36 (1H, m, H-3), 3.23 (1H, m, H-10), 3.21(1H, m, H-3), 3.05 (1H, m, H-10), 1.95 (1H, m, H-5), 1.66 (1H, m, H-4), 1.59 (1H, m, H-4), 1.36 (1H, m, H-5)。13C NMR (150 MHz, DMSO-d6)共显示16个碳信号,结合DEPT谱推断δC 169.0, 165.5为酰胺羰基碳信号;8个芳香碳信号;δC 58.4, 55.2为2个连氮次甲基碳信号;δC 44.6, 27.7, 25.8, 21.8为4个亚甲基碳信号。碳信号归属为:δC 169.0 (C-7)、165.5 (C-1)、136.0 (C-9′)、127.3 (C-4′)、124.4 (C-2′)、120.8 (C-7′)、118.6 (C-5′)、118.2 (C-6′)、111.2 (C-8′)、109.3 (C-3′)、58.4 (C-6)、55.2 (C-9)、44.6 (C-3)、27.7 (C-5)、25.8 (C-10)、21.8 (C-4)。将核磁数据与化合物6对比,化合物7中吲哚基取代了化合物6中的苯基。该化合物的比旋光值为
$[\alpha]_{\rm{D}}^{20} $ -90 (c 0.1, MeOH),将该核磁数据与参考文献[16]对照基本一致,最终确定化合物为brevianamide F。化合物8为棕黄色油状(甲醇),ESIMS给出的分子离子峰[M+Na]+m/z 177.06。1H NMR (600 MHz, DMSO-d6)中,给出3个烯氢信号δH 6.72 (1H, m, H-7), 6.37 (1H, d, J = 15.8 Hz, H-6), 6.00 (1H, s, H-2),其中一对为反式烯氢;2个羟基信号δH 5.80 (1H, s, 5-OH), 5.68 (1H, s, 4-OH);2个连氧次甲基质子信号δH 4.50 (1H, m, H-4), 3.89 (1H, m, H-5);1个甲基质子信号δH 1.88 (3H, d, J = 6.3 Hz, H-8)。13C NMR (150 MHz, DMSO-d6)共显示8个碳信号,结合DEPT谱,推断δC 203.7为酮羰基碳信号;4个双键碳信号;δC 80.8, 76.4为2个连氧次甲基碳信号;δC 19.1为甲基碳信号。碳信号归属为:δC 203.7 (C-1)、168.5 (C-3)、139.4 (C-7)、125.5 (C-6)、124.8 (C-2)、80.8 (C-5)、76.4 (C-4)、19.1 (C-8)。该化合物的比旋光值为
$[\alpha]_{\rm{D}}^{20} $ +78 (c 0.1, MeOH),将该化合物核磁数据与参考文献[17]对照基本一致,确定化合物为terrein。 -
对分离得到的化合物进行α-葡萄糖苷酶抑制活性的测试。采用PBS缓冲液为反应体系,利用α-葡萄糖苷酶,以4-硝基苯基-α-D吡喃葡萄糖苷(PNPG)为特异性底物,以阿卡波糖作为阳性药,分别设立空白对照组、α-葡萄糖苷酶空白组和PNPG空白组,评价化合物的α-葡萄糖苷酶的抑制活性。结果表明,化合物3具有较强的α-葡萄糖苷酶的抑制活性,IC50值为14.28 µmol/L。其他化合物没有明显的α-葡萄糖苷酶的抑制活性。另外,还对化合物的抗氧化活性进行测试。采用DPPH的方法,以抗氧化剂N-乙酰半胱氨酸作为阳性药对分离得到的化合物进行了体外抗氧化活性测试。结果显示这些化合物抗氧化活性不明显。
-
本研究从棕色扁海绵共附生真菌土曲霉中分离得到了8个化合物,其中化合物3、4、5、7为首次从该菌中分离得到,丰富了土曲霉次级代谢产物的多样性,为进一步探索该属真菌的化学成分和生源途径提供了理论依据。
根据文献报道,化合物2可以提高胰岛素的敏感性[13],化合物4和5测试了多个肿瘤细胞系,均显示细胞毒活性不明显[15],化合物6对大肠杆菌、金黄色葡萄球菌、黄体微球菌、白色念珠菌和隐球菌等具有很好的抗菌活性[16],化合物7对PaCa-2胰腺细胞的抗癌活性和抗菌活性都不明显[17],化合物8能够抑制雄激素依赖性前列腺癌细胞LNCaP-CR的血管生成素分泌,能够抑制人脐静脉内皮细胞的血管形成[18]。为了更好的探究该真菌代谢产物的活性,对分离得到的化合物进行了α-葡萄糖苷酶抑制活性和抗氧化活性测试。其中化合物3显示了较强的α-葡萄糖苷酶的抑制活性,IC50值为14.28 µmol/L,其α-葡萄糖苷酶抑制活性的机制有待于进一步研究。
Study on chemical constituents of sponge-associated Aspergillus terreus
-
摘要:
目的 对海绵共附生真菌土曲霉Aspergillus terreus中的化学成分进行研究。 方法 采用葡聚糖凝胶柱色谱、硅胶柱色谱和高效液相色谱等手段分离纯化;通过波谱数据鉴定化合物结构;采用PNPG法和DPPH法分别对分离得到的化合物进行α-葡萄糖苷酶抑制活性和抗氧化活性测试。 结果 从土曲霉Aspergillus terreus中分离得到8个化合物,分别鉴定为methyl-3,4,5-trimethoxy-2-(2-(nicotinamido) benzamido) benzoate ( 1 )、terrelumamide A ( 2 )、emeheterone ( 3 )、(8R,9S)-dihydroisoflavipucine ( 4 )、(8S,9S)-dihydroisoflavipucine ( 5 )、cyclo(S-Pro-S-Phe) ( 6 )、brevianamide F ( 7 )、terrein ( 8 )。活性测试结果表明,化合物 3 具有较强的α-葡萄糖苷酶的抑制活性,IC50值为14.28 µmol/L。 结论 化合物 3 、 4 、 5 、 7 为首次从土曲霉Aspergillus terreus中分离得到。 -
关键词:
- 土曲霉 /
- 化学成分 /
- α-葡萄糖苷酶抑制活性
Abstract:Objective To study the chemical constituents of Aspergillus terreus from sponge epiphytic fungal. Methods Sephadex LH-20 column chromatography, silica gel column chromatography and high performance liquid chroma-tography were used to separate and purify the compounds. The structures of compounds were identified by spectroscopic data. The α-glucosidase inhibitory activity and antioxidant activity of the compounds were tested by PNPG and DPPH methods, respectively. Results Eight compounds were isolated from Aspergillus terreus and identified as methyl-3,4,5-trimethoxy-2-(2-(nicotinamido) benzamido) benzoate ( 1 ), terrelumamide A ( 2) , emeheterone ( 3 ), (8R,9S)-dihydroisoflavipucine ( 4 ), (8S,9S)-dihydroisoflavipucine ( 5 ), cyclo(S-Pro-S-Phe) ( 6 ), brevianamide F ( 7 ), terrein ( 8 ). Compound 3 showed strong inhibitory activity against α-glucosidase and the IC50 value was 14.28 μmol/L. Conclusion Compounds 3 , 4 , 5 , and 7 were obtained from Aspergillus terreus for the first time. -
药物利用研究(DUR)是促进用药安全、有效和经济的重要手段[1]。随机对照临床试验(RCT)作为评价药物安全性、有效性的金标准,在外推至日常诊疗环境时往往面临挑战。作为RCT的重要补充,真实世界研究(RWS)考察日常诊疗环境中产生的真实世界数据(RWD),注重评价药物使用的“安全性”和“有效性”,已经成为药物利用研究的热点[2]。RWS着眼于应用到医疗实践环境中,大大缩短了试验周期、降低了成本,真实世界实效性临床研究更加易于获取全面的病例数据,使其结果更具有可靠性及可行性[3]。应当正确认识两者关系,将两者作为互补且相辅相成的研究方法和手段来为药物利用评价和监管评价等方面提供循证支持[4-7]。
随着医院管理信息系统(HIS)的高速发展以及高新传感器技术在生物医疗领域探索运用,使得逐步精准化、数字化患者的各项检查及健康诊疗数据成为现实,并进一步完善患者诊疗、实验室检查以及用药信息等全生命周期的医疗记录,且便于追溯及交互关联[2]。尽管数据库技术及大数据挖掘服务于药物安全性、有效性等方面研究成为现实,但应用于DUR尚缺乏具体的技术指导方案、自然流程等。本研究借鉴国内外RWS在药品器械上市后适应证开发及安全性评价方面的做法,梳理DUR中RWS有效技术手段和方法路径,为RWS更好的服务于DUR提供借鉴和参考。
1. 相关概念
DUR是按照预定的标准,评价、分析和解释一个给定的医疗卫生制度下药物利用的模式、质量、影响因素和结果,着重于药物的市场销售、分配、处方和使用情况,强调由此产生的医疗、社会和经济方面的结果。广泛应用于药物流行病学、抗菌药物管理、药物监测、药物警戒等方面的研究。2020年4月,国家药监局发布《真实世界证据支持药物研发与审评的指导原则(试行)》[8],对RWD、真实世界证据(RWE)以及RWS等概念做了相关阐述[8-9]。RWS作为实现从RWD到RWE的有效手段,是连接两者的桥梁[10]。然而,大规模的数据并不一定就能产生有价值的证据,只有通过适用性评估的RWD、分析得出医疗产品的使用和潜在收益或风险的临床证据时才有可能使数据转变为证据[11-12]。数据适用性即从数据使用者角度出发,评价数据满足使用者需求的程度[13],强调数据质量在开展相应RWS方面的可应用程度[14]。
2. 真实世界药物利用研究现状
2.1 真实世界研究与药物利用研究结合现状
国家药品监督管理局自2014年起就陆续出台多项措施,支持RWS用于医疗器械评价、药物审评、研发及监管决策,完善医疗器械不良事件监测和再评价制度,并联合高校、医联体推进多项试点工作的开展,出台了《真实世界研究支持儿童药物研发与审评的技术指导原则(试行)》[15]等法规文件以推进RWS。目前RWS主要集中在以下3个方面:①药物治疗效果[16-17],RWS在药物疗效、不良事件、安全可靠方面的结果研究,以满足药物对人类临床应答的解释以及推广方面应用;②指南或临床实践[18-20],国内外权威的指南是临床实践的重要参考依据,RWS也可用于协助制定和修订患者治疗方案,而RWE有利于指南更加科学性和具有实践性。此外,RWS还可以用于协助政府部门管理的指导性文件的制定;③经济效益[21-23],RWS应用于卫生经济学中筛选研究和治疗选择等方面,帮助医师制定最优的药物治疗方案,并提供合理的经济成本。此外,RWS在帮助制定个性化医疗政策方面也具有很大的潜力。
2.2 真实世界研究体系现状
国际上,随着药品审评和监管标准的不断提高,越来越多的研究者重视RWS,目前已经形成了良好的研究体系,比如美国的以患者为中心的结局研究所(PCORI)和欧盟临床试验公共注册和结果数据库(EUPAS)。RWS主要集中于:①在研究用药的随机对照试验、观察性研究及实践指南中的应用;②在疾病的发病风险评估、医疗健康保险的应用。目前国内的RWS主要涉及:①在中医方面的应用研究[24-26],包括中药的药物疗效及不良反应的研究;②基于医院信息数据库疾病及其合并疾病的用药特征的分析;③在医疗大数据及循证医学方面的应用[27-29]。
RWS的研究设计和方法学也不断完善,近年来不少国家或国际组织都陆续出台关于RWS的指南以及指导原则,提高了RWS的质量和可靠性,比如美国 FDA[30-31]、欧洲EMA[32],英国NICE[33]。
3. 真实世界研究在药物利用研究中的应用
3.1 真实世界药物利用研究的方法
3.1.1 数据来源及研究问题
RWS收集真实诊疗数据或者基于已经存在的研究型数据库或数据研究平台,建立登记数据库,针对具体研究问题,运用循证医学方法,开展数据分析,从而回答验证假设[34-36]。RWD通常来自于以上一个或多个数据库,包含需要主动收集的数据以及常规诊疗行为产生的临床数据。随着医学大数据的快速发展,一些研究型数据库或数据研究平台也逐步拓展,目前,国内外利用公共数据库如SEER、MIMIC等进行相关研究已成为RWS的重要发展方向。
RWS通常基于研究目的建立研究数据库或数据集,研究要素一般包含目标患者人口学特征、用药信息、门诊、住院信息、实验室检查、治疗转归与结局,以及其他研究目的所涉及到的临床治疗、护理、手术处置等信息。如果数据来自多个不同数据库,还必须通过如患者身份证号码、住院号/检查号、姓名等患者唯一标识码进行辨识和数据关联。如果研究的资料内容涉及到患者的个人信息等情况,还要注意取得伦理学审核以及保护患者隐私[37-41]。真实世界DUR的药品数据通常通过ATC编码来规范,采用用药依从性,限定日剂量(DDD)、平均治疗天数(ATD)、总DDD数、处方年费用等DUR指标[42]。基于药物效果和安全性研究、经济学和药物政策、多个疾病和多个治疗方案的复杂病情分析是当前真实世界药物利用研究的一个热点。
RWS作为一项非随机、开放性、不使用安慰剂的研究。为了挖掘真实的临床医疗环境产生的诊疗数据,应把质量控制作为全局指标来进行把握,并从研究伊始就建立起全面的数据质量控制方案并严格遵守。同时,在研究中详细记录异常情况[43],还要注意数据清洗以及混杂因素的控制,如此才能保证研究证据的质量及等级[44]。
3.1.2 研究人群及纳排标准
RWS人群纳入条件较为宽松,但仍在研究中需要明确与研究目的相关或可能影响研究的因素,以及纳入及排除标准的研究时间段和制定日期。研究通常通过WHO国际疾病分类(ICD-9/10)筛选研究人群。研究人群通常为患有特定疾病的患者、药物使用者(罕见病、孕妇、儿童等)以及患有多种疾病的复杂病例或有多种伴随症状的人群。有时候为解决研究对象以往接受过某种治疗措施可能导致的选择偏倚,还需要考虑遵照首次用药人群的设计[45]。最后,研究者需谨慎纳入和排除标准,以免直接影响研究结果的外推。
研究者应当严格参照PICOTS原则明确的6个关键点(总体、干预、比较对象、结果、时间和场所),同利益相关者一同提出针对研究问题可利用的科学方法[46]。
3.1.3 真实世界药物利用研究设计
观察性研究设计是RWS中广泛使用的设计类型之一[44],常见的有前瞻性观察研究[47]、回顾性队列研究[18,48]、Meta分析[49]等。根据不同的研究目的和研究对象,可以选择适合的研究方法和样本来源,通常需要开展大规模、跨学科的合作,以确保研究结果的可靠性和科学性。RWS设计时要结合研究目的来具体确定研究要素,综合考虑年龄、混杂偏倚和特殊人群、药品ATC编码,以及病历等非结构化数据。设计通常包括以下几个阶段:①定义问题:在研究开始之前明确研究的目的和问题,确定研究的对象、变量和数据采集方式。需要考虑研究的可行性、科学性和意义。②研究设计:根据定义的问题,制定设计方案。明确设计类型、样本容量、数据采集方式、结局指标以及数据分析方法等。③招募研究对象:确定研究对象的选择标准,并依照这些标准进行样本招募。④数据收集和管理:采集所需的研究数据,将收集到的数据进行规范化、清洗、质量控制和审查等处理。⑤数据分析:使用统计学方法进行数据分析,包括描述性统计、回归分析、生存分析和成本效益分析等。⑥结果解释和推广:将研究结果进行整合、解释和推广,发表研究报告和文章,向目标受众,如医师、政策制定者、患者和公众等,传达研究结论和建议。
3.1.4 特征变量及评价指标
RWS设计阶段应该充分了解现有数据的优缺点,并恰当合理的定义并描述暴露因素,尽可能的收集与暴露相关的特征指标。RWS结局指标是评估一种治疗或干预措施在真实医疗实践中的效果和安全性以及相关临床和经济结果的指标。
通常有以下几种结局指标:①主要疗效结局:主要的成果、结果或效果指标。例如,治疗效果、复发率、临床终点事件;②次要疗效结局:主要疗效结局之外的其他疗效结果或事件。如总体存活率(OS)、无进展生存期(PFS)、无病生存期(DFS)、疾病进展时间(TTP)、治疗失败时间(TTF)、死亡率,住院时间等[50];③安全性结局[12,51]:一般采用药物不良反应(ADR)、不良反应发生率(IRs,通常以1000人/年表示)[12]、危险性信号、药物相互作用等;④经济学结局[52-53]:包括成本效益和成本效用评估、日均费用、医疗保险、社会资源利用及患者的自付费用等。
选取结局指标时需要根据研究目的和研究对象,进行目标导向和可行性评估。常规首选应该是临床意义明确和易于全面评估的主要疗效结局,同时可以考虑次要疗效结局和安全性结局作为辅助评估。为综合评估治疗效果和成本并获得系统的经济评估结果,相关经济学方面的评价指标也应该考虑进来。
3.2 统计及敏感性分析
通常对目标患者群体和治疗模式进行描述性统计分析,分析各分类变量的频率、百分比,以及在连续区间尺度上测量的变量平均值、标准差、中位数及范围,有学者应用Kaplan-Meier(KM)生存函数进行相关生存分析[54]。针对研究目标确定分析要素,选用合适的统计分析方法,如卡方检验、logistic回归和多元线性回归等,对治疗结局、暴露因素、协变量数据类型及分布情况进行校正分析。由于所有研究结果基于假设提出,而这些假设往往是推论真实性的依据。研究者需对数据的局限性和问题本质有清晰认识,研究过程中对假设进行调整,评价观察结果对特定假设的敏感度或方向大小上的一致性。
3.3 混杂因素及偏倚控制
3.3.1 混杂因素
RWD来源包含电子病历、医保数据库、生命体征记录、医学图像等,存在许多复杂的混杂因素。混杂因素可能的类型包括个体基线特征、随时间变化的特征、医疗诊断和治疗、环境因素。常见的混杂控制策略包括随机对照、匹配分析、协变量校正、倾向值和剂量反应模型等。除此之外,在实施RWS时,还要注意有代表性的样本选择,对数据质量和分析偏倚进行评估和控制,以获得准确和可靠的研究结论。
3.3.2 偏倚控制
RWS是在真实临床环境下进行的研究,目标人群的治疗措施因非随机分配影响内部真实性,虚弱个体治疗措施与结局之间的关联性等,使得其研究结果可能存在一定的偏倚,这些偏倚可能影响研究的可靠性和有效性。常见的偏倚类型包括选择性偏倚、信息偏倚[55]、报告偏倚、记忆偏倚等,常见的偏倚控制方法有模拟试验、设计分层、倾向值匹配、重复量表、级联分析等。
4. 展望
真实世界DUR作为一种新兴的药物评价方法,可将从真实世界环境下收集和分析的大量数据利用起来,通过实效性、回顾性研究使得过往产生的既有诊疗数据进一步提炼成RWE而二次利用。通过研究分析获得的循证医学证据,可以为DUR提供有价值的依据,帮助优化药物使用、个体化医疗、提高患者结局、降低医疗费用、促进医学的健康发展;也可采用前瞻性研究大样本或特殊人群,为其更好、更安全有效用药提供证据。
同时,RWS也是评价药物滥用的有效手段。随着大数据和医疗技术的不断发展,RWS将会成为药物治疗效果、患者治疗策略和临床实践方面重要的研究领域,并将不断地推动医药的创新、优化和进步。
值得注意的是,RWS是一项复杂的研究工作,需要具备较强的统计、数据挖掘和医学知识背景,同时也面临着数据质量、缺失值、样本匹配和结果影响因素多等问题。因此,在实施RWS时,需要有效的科学设计、广泛报告,同时进行敏感度分析和可能存在的偏差分析,为制定更加科学和有效的药物治疗方案提供科学支持。
-
[1] 朱伟明, 王俊锋. 海洋真菌生物活性物质研究之管见[J]. 菌物学报, 2011, 30(2):218-228. [2] LI D H, HAN T, GUAN L P, et al. New naphthopyrones from marine-derived fungus Aspergillus niger 2HL-M-8 and their in vitro antiproliferative activity[J]. Nat Prod Res,2016,30(10):1116-1122. doi: 10.1080/14786419.2015.1043553 [3] GU B B, JIAO F R, WU W, et al. Preussins with inhibition of IL-6 expression from Aspergillus flocculosus 16D-1, a fungus isolated from the marine sponge Phakellia fusca[J]. J Nat Prod,2018,81(10):2275-2281. doi: 10.1021/acs.jnatprod.8b00662 [4] MA X, NONG X H, REN Z, et al. Antiviral peptides from marine Gorgonian-derived fungus Aspergillus sp. SCSIO 41501[J]. Tetrahedron Lett,2017,58(12):1151-1155. doi: 10.1016/j.tetlet.2017.02.005 [5] MIAO F P, LIANG X R, LIU X H, et al. Aspewentins A-C, norditerpenes from a cryptic pathway in an algicolous strain of Aspergillus wentii[J]. J Nat Prod,2014,77(2):429-432. doi: 10.1021/np401047w [6] 王宇, 李占林, 白皎, 等. 海洋真菌烟曲霉Aspergillus fumigatus YK-7中生物碱类代谢产物及其抗肿瘤活性研究[J]. 中国药学杂志, 2017, 52(15):1308-1312. [7] BUTTACHON S, RAMOS A A, INÁCIO Â, et al. Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA0062[J]. Mar Drugs,2018,16(4):E119. doi: 10.3390/md16040119 [8] AN X, FENG B M, CHEN G, et al. Two new asterriquinols from Aspergillus sp. CBS-P-2 with anti-inflammatory activity[J]. J Asian Nat Prod Res,2016,18(8):737-743. doi: 10.1080/10286020.2016.1161613 [9] GUO F, LI Z L, XU X W, et al. Butenolide derivatives from the plant endophytic fungus Aspergillus terreus[J]. Fitoterapia,2016,113:44-50. doi: 10.1016/j.fitote.2016.06.014 [10] LIU Z M, CHEN Y, CHEN S H, et al. Aspterpenacids A and B, two sesterterpenoids from a mangrove endophytic fungus Aspergillus terreus H010[J]. Org Lett,2016,18(6):1406-1409. doi: 10.1021/acs.orglett.6b00336 [11] CAPON R J, RATNAYAKE R, STEWART M, et al. Aspergillazines A-E: novel heterocyclic dipeptides from an Australian strain of Aspergillus unilateralis[J]. Org Biomol Chem,2005,3(1):123-129. [12] HE F, BAO J, ZHANG X Y, et al. Asperterrestide A, a cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162[J]. J Nat Prod,2013,76(6):1182-1186. doi: 10.1021/np300897v [13] YOU M, LIAO L J, HONG S H, et al. Lumazine peptides from the marine-derived fungus Aspergillus terreus[J]. Mar Drugs,2015,13(3):1290-1303. doi: 10.3390/md13031290 [14] KAWAHARA N, NOZAWA K, NAKAJIMA S, et al. Emeheterone, a pyrazinone derivative from Emericella heterothallica[J]. Phytochemistry,1988,27(9):3022-3024. doi: 10.1016/0031-9422(88)80722-2 [15] CHEN T, LAM C K, CHEN W D, et al. NMR screening approach for discovery of new 6-methylpyridinone derivatives from the marine-derived fungus Leptosphaerulina sp[J]. Arab J Chem,2017,10(2):288-294. doi: 10.1016/j.arabjc.2015.06.015 [16] WANG G H, DAI S K, CHEN M J, et al. Two diketopiperazine cyclo(pro-phe) isomers from marine bacteria Bacillus subtilis sp. 13-2[J]. Chem Nat Compd,2010,46(4):583-585. doi: 10.1007/s10600-010-9680-8 [17] WANG B, PARK E M, KING J B, et al. Transferring fungi to a deuterium-enriched medium results in assorted, conditional changes in secondary metabolite production[J]. J Nat Prod,2015,78(6):1415-1421. doi: 10.1021/acs.jnatprod.5b00337 [18] ARAKAWA M, SOMENO T, KAWADA M, et al. A new terrein glucoside, a novel inhibitor of angiogenin secretion in tumor angiogenesis[J]. J Antibiot (Tokyo),2008,61(7):442-448. doi: 10.1038/ja.2008.60 期刊类型引用(0)
其他类型引用(1)
-





 下载:
下载:






