-
针对非小细胞肺癌(NSCLC)的靶向药EGFR-TKIs,自第一代问世以来发展迅速,目前第四代已在临床研发阶段。但由于新的耐药突变的不断产生,导致其应用受限。因此如何克服EGFR-TKIs耐药问题和寻找可改善肺癌靶向治疗耐药的药物已成当务之急。植物来源的单体成分种类繁多,应用前景巨大。毒胡萝卜(Thapsia garganica L.,伞形科毒胡萝卜属)是于地中海西部沿海地区发现的一种开花植物,一直被作为止痛剂应用于传统医学。毒胡萝卜素是从该植物中分离的单体化合物,作为内质网应激(ERS)的诱导剂,近年也被用于抗肿瘤和抗病毒研究。吉非替尼作为第一代EGFR-TKIs,副作用小,有效率高。本研究旨在探讨毒胡萝卜素和吉非替尼联合用药对人肺腺癌耐药细胞株PC9/GR耐药性的影响,并探讨其可能的机制。
-
吉非替尼(纯度99.94%,HY-50895)、毒胡萝卜素(纯度99.95%,HY-13433)、CCK8试剂(HY-K0301-500T)均购自美国MCE公司;胎牛血清(F8318-500ML)和DMEM培养基(D6429-500ML)购自美国Sigam Aldrich公司;胰酶(25200-072)购自美国Gibco公司;凋亡试剂盒(559763)购自美国BD公司; ATF-6抗体(#65880)、IRE1α抗体(#3294)均购自美国CST公司;羊抗兔二抗(AS003)购自abclonal;PVDF膜购自Millipore;其余试剂均为国产分析纯试剂。人肺腺癌细胞(PC9)由同济医院惠赠,PC9/GR细胞购自湖南丰晖生物科技有限公司。
-
PC9细胞使用DMEM培养基培养,其中含10%的胎牛血清、100 U/ml的青霉素和100 μg/ml的链霉素,于37 ℃、5% CO2的恒温培养箱中孵育。PC9/GR细胞使用上述培养基维持培养并额外含800 ng/ml的吉非替尼。
-
检测细胞对吉非替尼的耐药性:分别取对数生长期的PC9和PC9/GR细胞制成单细胞悬液,调整细胞浓度为40 000/ml,接种于96孔板,每孔100 μl细胞悬液,在培养箱中孵育过夜。吸弃96孔板上清液,加入含药培养基(PC9细胞中吉非替尼浓度分别为0、1、5、10、20、40、80、250、500、1 000 nmol/L;PC9/GR细胞中吉非替尼的浓度分别为0、1、10、100、1 000、2 500、5 000、10 000、20 000、40 000 nmol/L),同时设对照组,每组设4个复孔。72 h时吸弃上清液,再加入含CCK8的DMEM,0.5 h后用酶标仪检测A450。
检测毒胡萝卜素对细胞增殖的影响:分别取对数生长期的PC9和PC9/GR细胞同上述处理铺96孔板,贴壁后加入浓度为0.1、1、10、100、1 000、10 000 nmol/L的毒胡萝卜素,同时设对照组,每组设4个复孔。72 h时使用CCK8法检测A450。
检测毒胡萝卜素对PC9/GR细胞耐药的逆转:取PC9/GR对数生长期的细胞同上述处理铺96孔板,设12组,加入吉非替尼的浓度分别为0、4.5、4.5、4.5、4.5、4.5、0、9、9、9、9、9 μmol/L,毒胡萝卜素的浓度分别为0、0、0.1、1、10、100、 0、0、0.1、1、10、100 nmol/L。72 h时用CCK8法检测A450。
检测毒胡萝卜素对PC9/GR细胞耐药的逆转:取PC9/GR对数生长期的细胞同上述处理铺96孔板,设两组,为吉非替尼单药组和联合用药组:其中单药组设7个小组,加入吉非替尼的浓度分别为0、1、10、102、103、104、105 μmol/L;联合用药组设7个小组,每小组加入10 nmol/L的毒胡萝卜素,同时加入浓度分别为0、1、10、102、103、104、105 nmol/L的吉非替尼。72 h时用CCK8法检测A450。逆转倍数(RF)=逆转前的IC50值/逆转后的IC50值。
-
取对数生长期的PC9/GR铺6孔板,每孔3×105个细胞,孵育过夜,吸弃上清液,加入含药培养基(对照组为9 μmol/L的吉非替尼组,实验组为9 μmol/L的吉非替尼组+10 nmol/L的毒胡萝卜素联合用药组),孵育72 h后,收集上清液及贴壁细胞,按说明书加入膜联蛋白(Annexin V-PE),室温孵育20 min后,再加入7-AAD,5 min后,收集细胞,流式细胞仪上机检测。
-
取对数生长期的PC9/GR铺6孔板,每孔3×105个细胞,孵育过夜,吸弃上清液,加入含药培养基(每孔吉非替尼浓度依次为0、0、4.5、4.5、9、9 μmol/L,毒胡萝卜素浓度依次为0、10、0、10、0、10 nmol/L),培养72 h,去上清液,收集细胞蛋白,BCA法测量样品中总蛋白浓度。蛋白变性后取蛋白样品,使用蛋白印迹法检测IRE1α、ATF-6蛋白表达。
-
数据采用GraphPad Prism 9软件进行统计分析,计量资料用(
$ \bar x $ ±s)表示,符合正态分布和方差齐性者采用单因素方差分析的单向分类方差分析(ANOVA),对取得的数据两两比较使用 LSD 检验。以P<0.05 为有统计学意义。 -
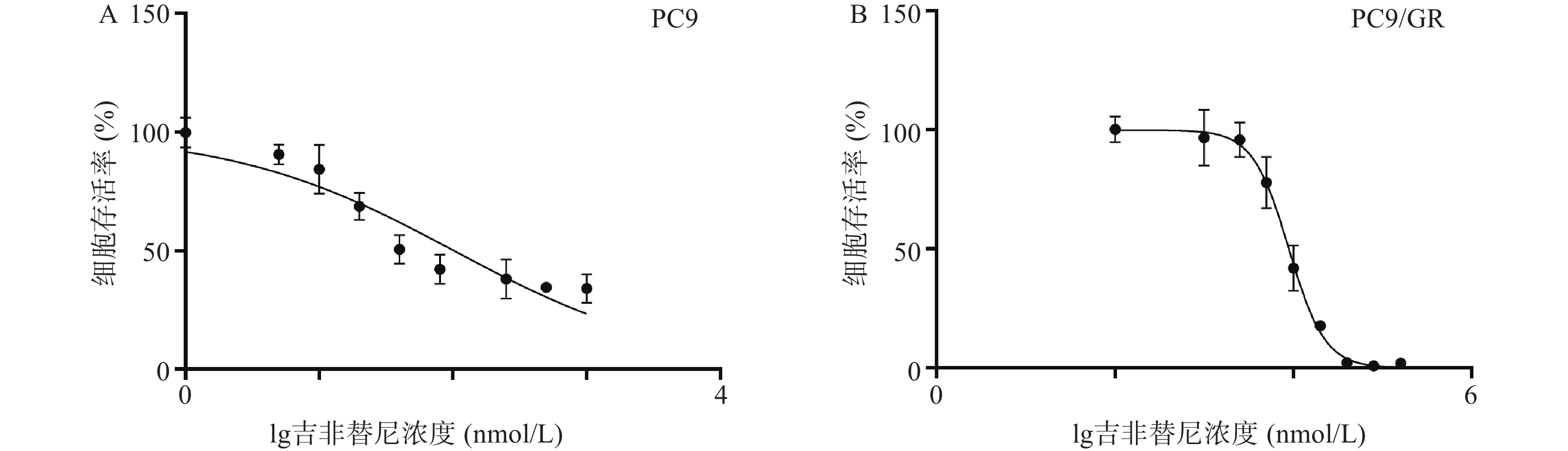
吉非替尼对人肺腺癌细胞株PC9和PC9/GR的IC50值经计算分别为0.1019 μmol/L(图1A)和8.912 μmol/L(图1B),按公式计算耐药倍数为87.5(本课题中PC9和PC9/GR细胞株的IC50值分别以0.1 μmol/L和9 μmol/L进行实验)。
-
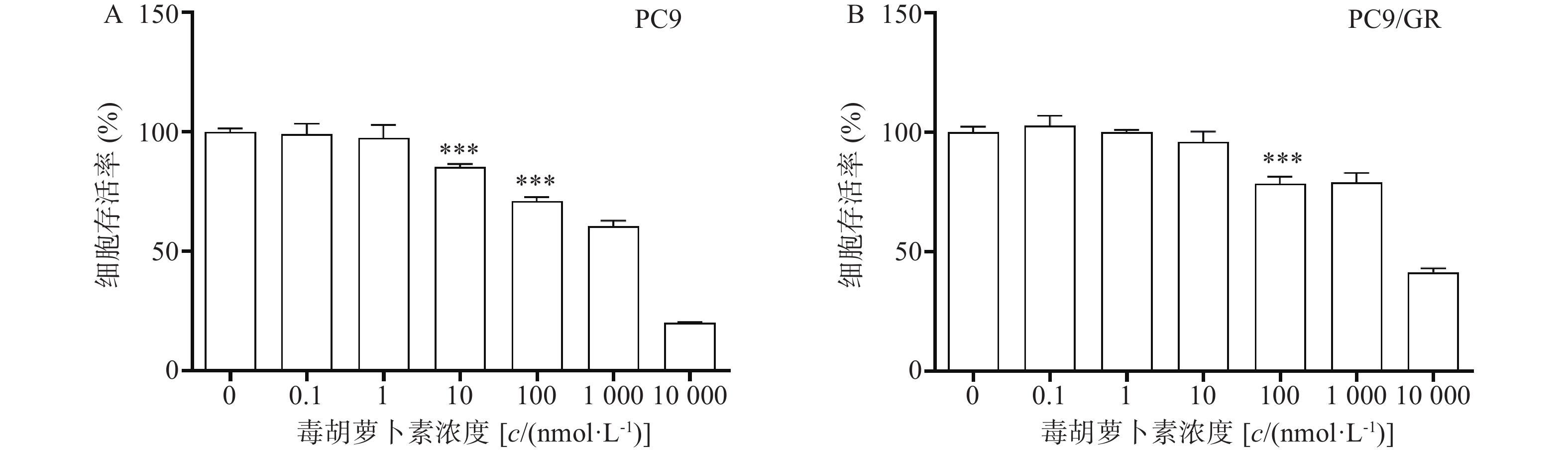
毒胡萝卜素对人肺腺癌细胞株PC9和PC9/GR的增殖均有抑制作用,呈剂量依赖性。当毒胡萝卜素浓度为10 nmol/L时,对PC9和PC9/GR的抑制率分别为14.7%(图2A)和4.11%(图2B)。毒胡萝卜素浓度为10 nmol/L时对PC9细胞的增殖显示较弱的抑制作用,而对PCR/GR细胞增殖的作用与对照组相比无统计学差异。抑制率(IR)=(1−药物组A值/对照组A值)×100%。本课题中,毒胡萝卜素的工作浓度选择10 nmol/L或者围绕10 nmol/L进行设计。
-
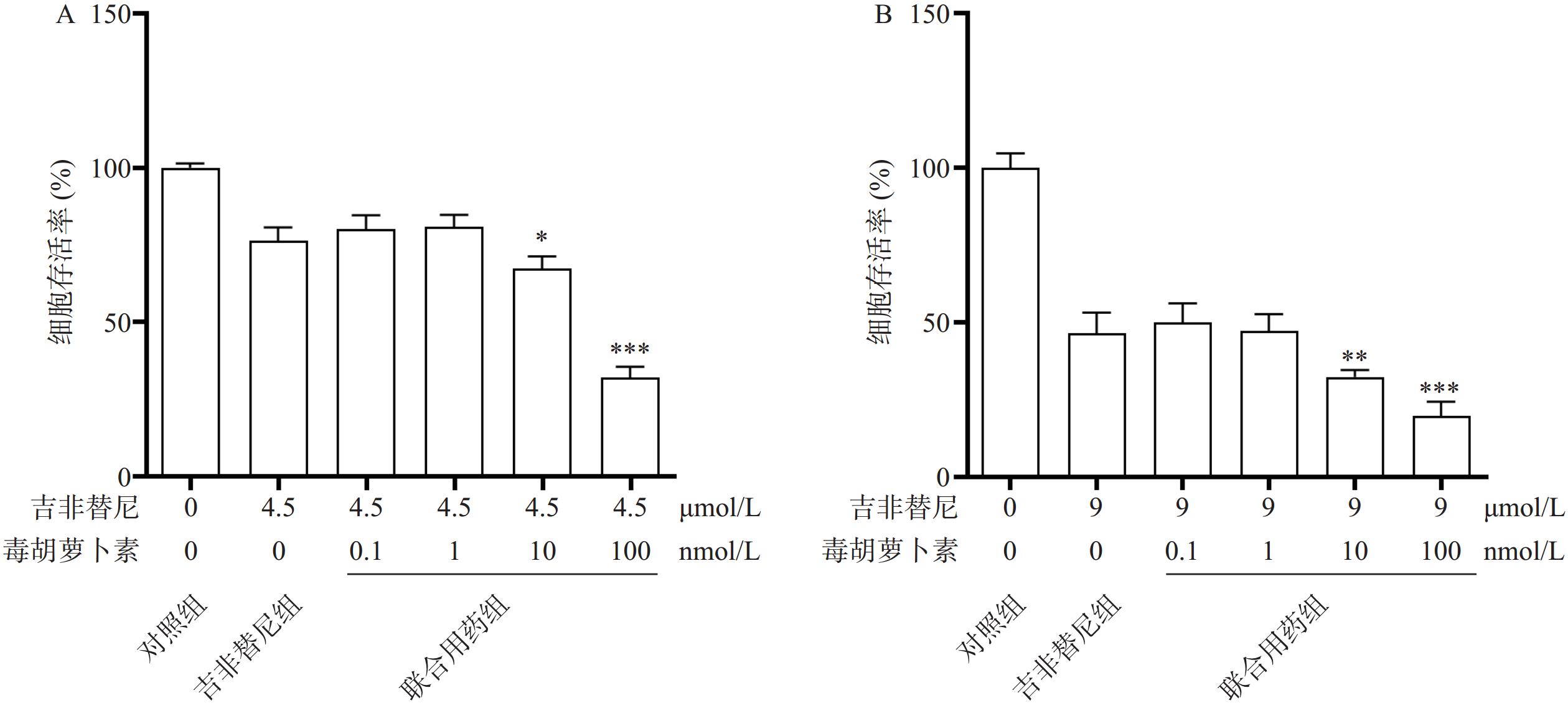
相比于吉非替尼单独用药,毒胡萝卜素(浓度依次为0、0.1、1、10、100 nmol/L)和吉非替尼(4.5 μmol/L或9 μmol/L)联合用药72 h时,PC9/GR的细胞增殖率在10 nmol/L和100 nmol/L时显著下降,并且具有统计学差异性(图3A、B)。
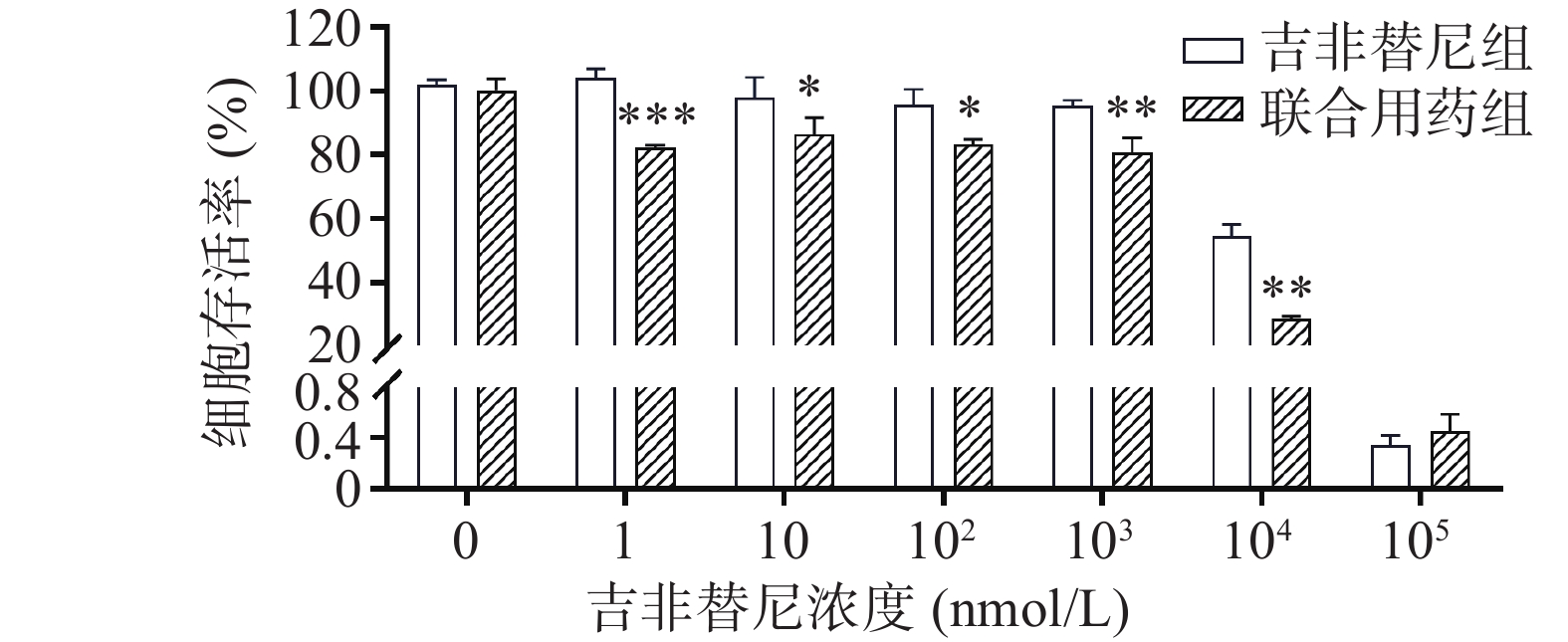
在不同浓度(0、1、10、102、103、104、105 nmol/L)的吉非替尼联合10 nmol/L的毒胡萝卜素作用后,PC9/GR的细胞增殖率相比吉非替尼单独用药(吉非替尼组浓度依次为0、1、10、102、103、104、105 nmol/L)也出现了下降,具有统计学差异(图4)。计算得出吉非替尼单独用药的IC50值为11.039 μmol/L,联合用药后其IC50值为3.584 μmol/L,其RF为3.15。
-
与对照组(图5A)、吉非替尼单独作用(图5B)、毒胡萝卜素单独作用(图5)相比,联合用药(图5)后PC9/GR细胞早期凋亡和晚期凋亡的比例均相应增加,并且具有统计学差异(图5)。说明毒胡萝卜素在一定程度上可以改善PC9/GR细胞的耐药性。
-
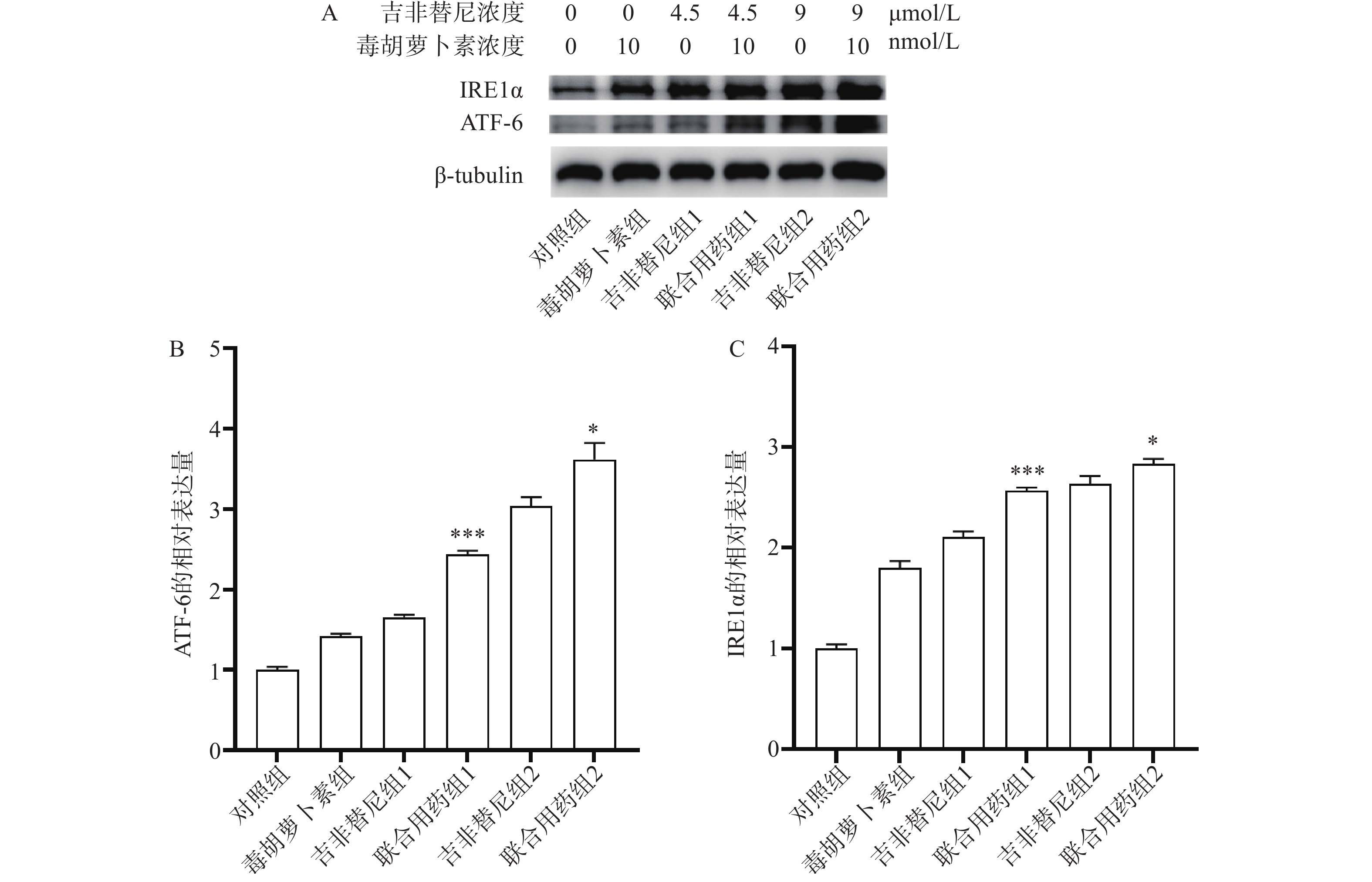
结果显示,与吉非替尼组1或2相比,药物作用后联合用药组1或联合用药组2中PC9/GR细胞的ATF-6及IRE1α蛋白表达均上调(图6A),并且有统计学差异(图6B、C)。ATF-6和IRE1α均是ERS的指标蛋白,说明PC9/GR细胞在药物联合作用后发生了ERS。
-
肺癌是当今世界最常见、致死率最高的恶性肿瘤之一,严重危害人类健康[1]。NSCLC是最常见的病理类型,约占所有比例的85%[2]。约70%的NSCLC患者在确诊时已处于晚期,失去了手术的机会。近年来,NSCLC的分子靶向治疗发展迅速,靶向药EGFR-TKIs不断更新,成为放化疗和免疫治疗之外的重要治疗手段之一。
截止目前,EGFR-TKIs已从第1代发展至第3代[3],且有多款第4代靶向药已进入临床实验阶段。然而由于抗肿瘤耐药性,患者使用靶向药一定时间后不可避免的会出现耐药[4]。于是寻找能够克服肿瘤耐药的药物及方法已经成为肿瘤治疗领域中亟待解决的问题。由于中草药植物的毒副作用小且疗效明确,故从中寻找能够克服肿瘤耐药的成分已经成为一种趋势。毒胡萝卜素是从地中海菊属植物中提取的单体成分[5],作为常用的ERS的诱导剂,毒胡萝卜素在科研中有着广泛的应用[6]。也有文献报道其有抗肿瘤活性[7],同时对冠状病毒、合胞病毒、甲型流感病毒等有较强的抑制作用[8]。本研究中使用的吉非替尼耐药细胞株PC9/GR经CCK8实验证明其对吉非替尼具有耐药性。实验结果显示,吉非替尼对PC9的IC50值为0.1019 μmol/L(图1A),对PC9/GR的IC50值为8.912 μmol/L(图1B),耐药倍数为87.6。当毒胡萝卜素浓度为 10 nmol/L时,对PCR/GR细胞增殖的作用与对照组相比无统计学差异,故该浓度的毒胡萝卜素未显示出对PCR/GR的细胞增殖的抑制作用(图2B),故本课题中毒胡萝卜素选择10 nmol/L作为实验浓度。而提高毒胡萝卜素的浓度,其对PC9/GR细胞抑制率也增加,表明毒胡萝卜素本身对于肺腺癌细胞PC9/GR具有抑制作用。实验发现联合用药中当毒胡萝卜素浓度为大于或等于10 nmol/L时,PCR/GR细胞的增殖相比吉非替尼单独用药受到了更强的抑制,并具有显著性差异(图3A、B)。本课题同时使用不同浓度的吉非替尼联合10 nmol/L的毒胡萝卜素共同作用于PC9/GR细胞,结果发现所有联合用药组相比于吉非替尼单独用药组,均对PC9/GR细胞的生长产生抑制作用。分别计算吉非替尼单药和联合用药对PC9/GR细胞的IC50值,发现联合用药的IC50值降低(图4),逆转倍数为3.15。说明10 nmol/L的毒胡萝卜素可以有效改善PC9/GR对吉非替尼的耐药。
肿瘤细胞的耐药性严重影响着患者的预后。耐药突变、自噬、肿瘤免疫微环境都是肿瘤细胞产生耐药的因素[9]。由于耐药突变,EGFR-TKIs治疗已经陷入了研究人员开发新药物与肿瘤细胞产生新耐药突变的循环之中。近年来,因自噬异常与肿瘤发生和进展、细胞死亡及抗肿瘤治疗疗效相联系而受到广泛关注[10]。但是抑制自噬只能延缓EGFR-TKI耐药的产生,并不能有效解决耐药的问题[11]。因此,耐药问题亟待找到新的解决方法。体内低氧、低糖等恶劣环境会导致肿瘤细胞发生ERS,并启动未折叠蛋白反应从而让细胞重新恢复稳态。ERS过度可启动细胞凋亡。因此,ERS也与肺癌治疗息息相关[12]。毒胡萝卜素作为研究ERS常用的激动剂,是一种特异性的肌浆/内质网Ca2+-ATP酶抑制剂,能够使内质网中Ca2+量减少、未折叠蛋白增多,引起ERS[13]。本研究中通过细胞凋亡实验发现,和单独用药相比,毒胡萝卜素联合吉非替尼联合用药可以进一步促进PC9/GR细胞的凋亡(图5B、D和E)。通过蛋白印迹实验证实,联合用药相比吉非替尼单独用药,PC9/GR细胞中ATF-6(图6A、B)和IRE1α蛋白(图6A、C)的表达均上调,表明联合用药后PC9/GR细胞处于ERS状态。这是毒胡萝卜素有效改善PC9/GR对吉非替尼耐药的可能原因之一。
综上所述,体外实验证实毒胡萝卜素低浓度下可以有效促进PC9/GR的ERS,高浓度下对肺癌细胞具有直接杀伤作用,说明毒胡萝卜素本身具有抗肿瘤活性,本课题同时证实,毒胡萝卜素能增强吉非替尼对PC9/GR的杀伤作用。其可能的机制是,在抗肿瘤药物和ERS状态的双重压力下,肺腺癌耐药细胞株PC9/GR对靶向药吉非替尼的敏感性增加。因此,毒胡萝卜素有望成为解决非小细胞肺癌靶向治疗耐药的潜在药物之一,但其中的机制仍需进一步的探索。
Improvement of gefitinib-resistance of PC9/GR by thapsigargin combined with gefitinib
-
摘要:
目的 研究毒胡萝卜素(thapsigargin)联合吉非替尼(gefitinib)对人肺腺癌耐药细胞株PC9/GR增殖的影响并探讨可能的机制。 方法 吉非替尼单独用药或吉非替尼与毒胡萝卜素联合应用,通过CCK8实验检测上述两组药物对PC9/GR细胞增殖的影响;应用流式细胞术鉴定两组药物对PC9/GR细胞凋亡的影响;应用Western blotting法检测两组药物对PC9/GR细胞蛋白ATF-6和IRE1α表达的影响。 结果 细胞增殖实验显示,与吉非替尼单独用药相比,联合用药组中PC9/GR的增殖受到更强的抑制作用;凋亡实验显示,相较于吉非替尼单独用药,联合用药能够进一步促进细胞的凋亡;Western blotting法显示,与吉非替尼单独用药相比,联合用药后PC9/GR中ATF-6和IRE1α蛋白(内质网应激标志物)表达上调,其差异具有统计学意义。 结论 在毒胡萝卜素诱导下,PC9/GR细胞对吉非替尼的敏感性增加,其中机制可能与内质网应激有关。 Abstract:Objective To study the effect and mechanism of the thapsigargin combined with gefitinib on the proliferation of human lung adenocarcinoma gefitinib resistance cell line PC9/GR. Methods The cell viability of PC9/GR treated with gefitinib alone or gefitinib combined with thapsigargin was evaluated by CCK8 assay. The flow cytometry was used to analyze the PC9/GR cell apoptosis indued by the two group drugs. The ATF-6 and IRE1α protein expression of PC9/GR cells treated with the two group drugs were detected by Western blotting. Results The group of drug combination exhibited enhanced ability to inhibit cell proliferation, promote cell apoptosis and upregulate the ATF-6 and IRE1α protein expression of the PC9/GR compared with the group gefitinib used alone. Conclusion The sensitivity of PC9/GR to gefitinib was increased when the cells were treated by thapsigargin, which may be related with the state of endoplasmic reticulum stress(ERS) induced by thapsigargin. -
Key words:
- gefitinib /
- thapsigargin /
- tumor drug resisdance /
- apoptosis /
- endoplasmic reticulum stress
-
-
[1] SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3):209-249. doi: 10.3322/caac.21660 [2] ZHANG S L, BAI X L, SHAN F P. The progress and confusion of anti-PD1/PD-L1 immunotherapy for patients with advanced non-small cell lung cancer[J]. Int Immunopharmacol, 2020, 80:106247. doi: 10.1016/j.intimp.2020.106247 [3] 陆文清, 孟周文理, 虞永峰, 等. 非小细胞肺癌第三代表皮生长因子受体-酪氨酸激酶抑制剂的耐药机制及治疗策略[J]. 上海交通大学学报(医学版), 2022, 42(4):535-544. [4] WU S G, SHIH J Y. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer[J]. Mol Cancer, 2018, 17(1):38. [5] JASKULSKA A, JANECKA A E, GACH-JANCZAK K. Thapsigargin-from traditional medicine to anticancer drug[J]. Int J Mol Sci, 2020, 22(1):4. doi: 10.3390/ijms22010004 [6] KATSIOUGIANNIS S, TENTA R, SKOPOULI F N. Autoimmune epithelitis (Sjögren’s syndrome); the impact of metabolic status of glandular epithelial cells on auto-immunogenicity[J]. J Autoimmun, 2019, 104:102335. [7] KÖRBEL C, LINXWEILER M, BOCHEN F, et al. Treatment of SEC62 over-expressing tumors by thapsigargin and triflu-operazine[J]. Biomol Concepts, 2018, 9(1):53-63. [8] AL-BELTAGI S, PREDA C A, GOULDING L V, et al. Thapsigargin is a broad-spectrum inhibitor of major human respiratory viruses: coronavirus, respiratory syncytial virus and influenza A virus[J]. Viruses, 2021, 13(2):234. doi: 10.3390/v13020234 [9] O'DONNELL J S, TENG M W L, SMYTH M J. Cancer immunoediting and resistance to T cell-based immunotherapy[J]. Nat Rev Clin Oncol, 2019, 16(3):151-167. doi: 10.1038/s41571-018-0142-8 [10] WU M, ZHANG P H. EGFR-mediated autophagy in tumourigenesis and therapeutic resistance[J]. Cancer Lett, 2020, 469:207-216. doi: 10.1016/j.canlet.2019.10.030 [11] SMYTH M J, NGIOW S F, RIBAS A, et al. Combination cancer immunotherapies tailored to the tumour microenvironment[J]. Nat Rev Clin Oncol, 2016, 13(3):143-158. doi: 10.1038/nrclinonc.2015.209 [12] 王安琪, 孙国平. 内质网应激在抗肿瘤治疗中的作用及研究进展[J]. 中国药理学通报, 2021, 37(12):1643-1647. [13] LINDNER P, CHRISTENSEN S B, NISSEN P, et al. Cell death induced by the ER stressor thapsigargin involves death receptor 5, a non-autophagic function of MAP1LC3B, and distinct contributions from unfolded protein response components[J]. Cell Commun Signal, 2020, 18(1):12. doi: 10.1186/s12964-019-0499-z -





 下载:
下载: