-
据世界卫生组织最新数据,截至2023年3月7日,全球已报告新型冠状病毒肺炎(COVID-19)确诊病例约7.6亿例,死亡超过686万例[1]。目前,抗病毒治疗可以抑制病毒复制,加快病毒清除,减轻炎症及免疫反应等,被认为是降低COVID-19重症率、住院率及死亡率的重要手段之一[2]。2021年12月,美国食品药品监督管理局批准奈玛特韦片/利托那韦片组合包装(Paxlovid)的紧急使用授权申请,使其成为治疗COVID-19的新型口服药物。2022年2月,国家药品监督管理局附条件批准Paxlovid进口注册[3],用于成人伴有进展为重症高风险因素的轻至中度COVID-19患者。国外多项研究已经证实,Paxlovid能显著降低COVID-19患者的病毒载量、全因住院率、重症率和死亡率等[4-5]。然而,目前罕见报道中国人群使用Paxlovid的疗效等相关数据。鉴于人种差异及病毒变异,开展此类研究十分必要。本研究旨在探索中国人群中Paxlovid对COVID-19患者早期预后不良的危险因素,并构建预测模型,以期为提高该类患者的救治效果提供参考。
-
回顾性分析2023年1月至2023年3月于闽南地区3家军队三甲医院(第九〇九医院、第九一〇医院和陆军第七十三集团军医院)使用Paxlovid的COVID-19住院患者。纳入标准:①COVID-19患者,诊断及临床分型符合《新型冠状病毒感染诊疗方案(试行第10版)》的标准[6];②年龄≥18岁;③用药时间≥3 d。排除标准:①未使用Paxlovid的患者;②Paxlovid停药28 d内失访的患者;③合并心、肺、肝、肾等重要器官严重损害者;④联合与Paxlovid存在严重相互作用的药物;⑤资料不全者等。本研究经牵头单位第九〇九医院伦理委员会批准通过。
-
收集患者的一般资料(性别、年龄、体重)、发病天数、Paxlovid疗程,合用药及辅助治疗情况、用药前的检查指标[血氧饱和度、核酸检测阈值循环数(CT值)、淋巴细胞计数、估算的肾小球滤过率(eGFR)、丙氨酸氨基转移酶(ALT)、天门冬氨酸氨基转移酶(AST)、三酰甘油(TG)、胆固醇(TC)、低密度脂蛋白(LDL-C)、高密度脂蛋白(HDL-C)、肌酸激酶(CK)、C反应蛋白(CRP)、降钙素原(PCT)、D-二聚体等]、合并疾病[高血压、动脉粥样硬化性心血管疾病(ASCVD)、慢性肺病等];结局指标[6]:Paxlovid停药28 d内若出现死亡或进展为重型、危重型COVID-19者则定义为早期预后不良;若Paxlovid停药28 d内出现COVID-19中型、轻型或痊愈者以及停药超过28 d出现死亡或进展为重型、危重型COVID-19者则定义为非早期预后不良。发病天数定义为患者首次出现临床症状或获得核酸检测阳性结果至第一次使用Paxlovid的日期。
-
运用SPSS 21.0软件进行统计分析,连续变量符合正态性分布的数据,采用(
$ \bar{x} $ ±s)表示,组间采用独立Student’s t检验;不符合正态性分布的数据采用中位数(四分位数间距)表示,组间采用Man-Whitney U检验;二分类变量以例数表示,采用χ2检验。单因素分析中P<0.05的变量,以向后LR法进入二元Logistic回归分析。采用受试者工作特征曲线(ROC)计算曲线下面积(AUC)评估模型的预测效能。P<0.05表示差异具有统计学意义。 -
2023年1月至2023年3月于闽南地区3家军队三甲医院使用Paxlovid治疗的COVID-19住院患者共129例,经筛选最终纳入92例进行分析。其中,男69例(75.00%),女23例(25.00%),平均年龄(76.26±15.81)岁,体重(62.86±10.69)kg,发病天数(8.60±5.94)d,核酸检测CT值(27.84±5.52)。
-
92例Paxlovid治疗的COVID-19患者中,早期预后不良者有31例(33.70%),其中,死亡11例(35.48%),危重型17例(54.84%),重型3例(9.68%)。
-
比较早期预后不良组与非早期预后不良组的患者一般资料、发病天数、检查指标、合并疾病、合用药及辅助治疗等情况,结果显示两组在发病天数、淋巴细胞计数、AST、CRP、PCT、D-二聚体等12项临床指标有相关性(P<0.05),见表1。
表 1 两组患者临床资料的单因素分析
项目 非早期预后不良组(n=61) 早期预后不良组(n=31) 统计量 P值 年龄(岁,$ \bar{x} $±s) 77.16±16.47 74.42±14.52 0.785 0.434 性别[女,n(%)] 16(26.23) 7(22.58) 0.146 0.702 体重(m/kg,$ \bar{x} $±s) 63.99±10.09 60.63±11.63 1.430 0.156 发病天数(t/d,$ \bar{x} $±s) 7.72±5.46 12.48±6.56 −3.693 <0.001 血氧饱和度(%,$ \bar{x} $±s) 94.01±4.97 90.91±10.76 1.894 0.061 核酸检测CT值($ \bar{x} $±s) 28.31±5.64 26.90±5.24 1.158 0.250 淋巴细胞计数(×109/L,$ \bar{x} $±s) 0.98±0.59 0.62±0.44 2.992 0.040 eGFR[ml/(min·1.73 m2),$ \bar{x} $±s] 68.29±30.56 57.20±38.44 1.505 0.136 ALT[U/L,M(P25,P75)] 22.90(9.00,121.00) 23.00(6.00,237.20) −0.450 0.653a AST[U/L,M(P25,P75)] 31.00(13.50,88.00) 37.40(21.00,306.20) 2.747 0.006a TG(mmol/L,$ \bar{x} $±s) 1.36±0.87 1.50±0.76 −0.782 0.436 TC(mmol/L,$ \bar{x} $±s) 3.95±0.94 3.59±1.19 1.555 0.123 LDL-C(mmol/L,$ \bar{x} $±s) 2.19±0.69 2.01±0.97 0.998 0.321 HDL-C(mmol/L,$ \bar{x} $±s) 1.14±0.32 1.00±0.44 1.664 0.100 CK(U/L,$ \bar{x} $±s) 145.23±253.51 148.21±107.09 −0.062 0.950 CRP(mg/L,$ \bar{x} $±s) 52.41±46.71 118.72±82.74 −4.918 <0.001 PCT[ng/ml,M(P25,P75)] 0.07(0.01,27.60) 1.40(0.01,42.95) 4.366 <0.001a D−二聚体[μg/ml,M(P25,P75)] 0.79(0.19,11.39) 2.17(0.36,41.83) 4.254 <0.001a 用药前已使用其他抗新冠病毒药物治疗[n(%)] 1(1.64) 5(16.13) 7.079 0.008 用药前已行呼吸机辅助通气[n(%)] 10(16.39) 19(61.29) 19.194 <0.001 合并疾病 高血压[n(%)] 39(63.93) 20(64.52) 0.003 0.956 糖尿病[n(%)] 16(26.23) 14(45.16) 3.352 0.067 ASCVD[n(%)] 29(47.54) 15(48.39) 0.006 0.939 慢性肺病[n(%)] 19(31.15) 5(16.13) 2.404 0.121 治疗方案 Paxlovid疗程(t/d,$ \bar{x} $±s) 4.74±0.68 5.26±2.05 −1.805 0.074 联合免疫抑制剂[n(%)] 42(68.85) 29(93.55) 7.116 0.008 联合抗凝药[n(%)] 36(59.02) 29(93.55) 11.821 0.001 联合抗菌药物[n(%)] 46(75.41) 30(96.77) 6.530 0.011 联合俯卧位治疗[n(%)] 8(13.11) 9(29.03) 3.457 0.088 联合呼吸机辅助通气[n(%)] 11(18.03) 25(80.65) 33.831 <0.001 注:a表示Mann-Whitney U检验。 -
采用二元Logistic回归分析,结果显示其中发病天数、淋巴细胞计数、AST、CRP和联合呼吸机辅助通气等5项临床指标是Paxlovid早期预后不良的独立危险因素,见表2。上述5项独立危险因素构建Logistic模型方程,Logit(P)=−8.371+0.126X发病天数+2.019X淋巴细胞计数+0.023XAST+0.016XCRP+3.528X联合呼吸机辅助通气。采用H-L法(Hosmerand-Lemeshow test)对模型的拟合度进行检验,结果显示模型拟合良好(χ2值=10.480,P=0.233),模型的理论准确度为89.10%。
表 2 Paxlovid早期预后不良多因素logistic分析
项目 回归系数B 标准误S.E 卡方值Waldχ2 自由度df 比值比OR 95%CI置信区间 P值 发病天数(t/d) 0.126 0.061 4.237 1 1.135 1.006~1.279 0.040 淋巴细胞计数 2.019 0.892 5.126 1 7.527 1.311~43.208 0.024 AST 0.023 0.009 6.578 1 1.023 1.005~1.041 0.010 CRP 0.016 0.007 5.744 1 1.016 1.003~1.029 0.017 联合呼吸机辅助通气 3.528 1.054 11.194 1 34.051 4.311~268.936 0.001 常量 −8.371 2.080 16.195 1 <0.001 -
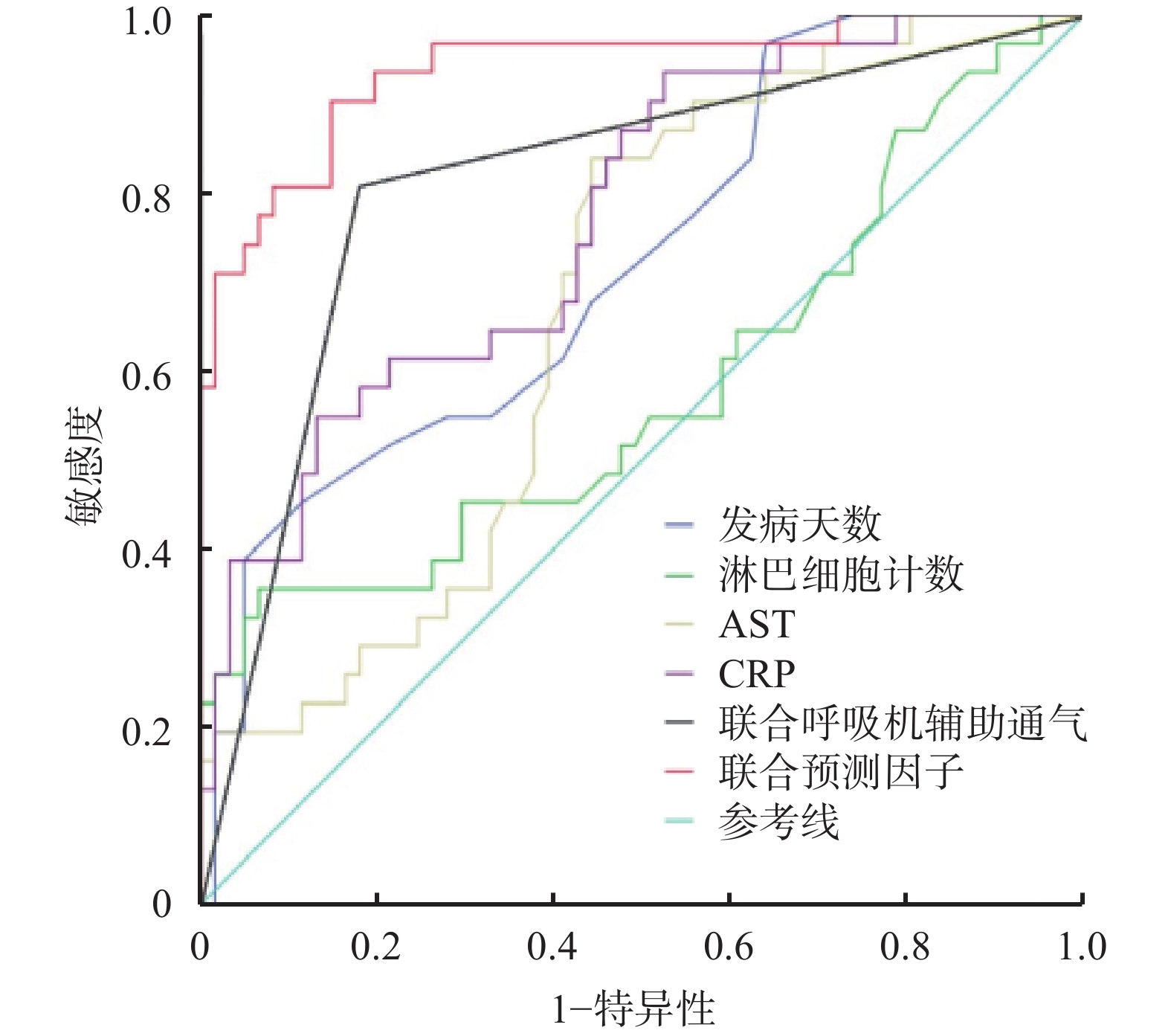
将上述回归方程转换后得出联合预测因子的计算公式,Y联合预测因子=7.875X发病天数+126.188X淋巴细胞计数+1.438XAST+XCRP+220.500X联合呼吸机辅助通气,计算92例患者的Y联合预测因子值,绘制ROC曲线,见图1。分别计算发病天数、淋巴细胞计数、AST、CRP和联合呼吸机辅助通气和联合预测因子AUC,其中联合预测因子AUC最大为0.939(P<0.001),预测价值最优。取约登(Youden)指数最大时即0.756,最佳临界值447.920,敏感度0.903,特异性0.852,见表3。
表 3 各危险因素对Paxlovid早期预后不良的预测价值
项目 最佳临界值 敏感度 特异性 约登指数 AUC(95%CI) P值 发病天数(t/d) 14.500 0.387 0.951 0.338 0.722(0.614~0.831) 0.001 淋巴细胞计数 1.685 0.355 0.934 0.289 0.582(0.449~0.715) 0.202 AST 31.950 0.839 0.557 0.396 0.676(0.566~0.786) 0.006 CRP 104.500 0.548 0.869 0.417 0.772(0.672~0.871) <0.001 联合呼吸机辅助通气 0.500 0.806 0.820 0.626 0.813(0.715~0.911) <0.001 联合预测因子 447.920 0.903 0.852 0.756 0.939(0.885~0.993) <0.001 -
Paxlovid是奈玛特韦片150 mg和利托那韦片100 mg组成的组合包装。奈玛特韦是一种SARS-CoV-2主要蛋白酶Mpro3C-样蛋白酶(3CLpro)的拟肽类抑制剂,能抑制病毒复制。利托那韦是HIV-1蛋白酶抑制剂,对新冠病毒SARS-CoV-2 Mpro无活性,但可以通过抑制肝药酶CYP3A介导的奈玛特韦代谢,升高奈玛特韦血药浓度,而发挥协同作用[7]。本研究中,33.70%使用Paxlovid治疗的COVID-19患者出现早期预后不良,其原因可能与药物特点和患者因素有关。据文献报道[8],大量Mpro突变可能诱导对Paxlovid的抗药性,从而导致治疗失败影响患者预后,但另有研究表明,在对731份样本进行分析后,并未发现Mpro突变和Paxlovid治疗失败之间存在显著的联系[9]。同时,有文献报道Paxlovid治疗后7 d和30 d的COVID-19复发率分别为3.53%和5.40%[10],通常患者的病情较轻,但仍有1.35%的患者出现呼吸衰竭需入住ICU[11]。
患者的发病天数是影响Paxlovid早期预后不良的独立危险因素之一。《新型冠状病毒感染诊疗方案(试行第10版)》推荐Paxlovid用于发病5 d以内的轻、中型且伴有进展为重症高风险因素的成年患者[6]。有文献报道发病时间超过5 d,核酸检测CT值<30者也可使用Paxlovid[12]。但在变异毒株奥密克戎感染的情况下,绝大多数二次传播发生在症状出现后5 d之内,在症状出现后8 d未检测到传染性病毒[13-14]。因此,Paxlovid治疗发病5 d后患者的有效性和安全性目前尚不明确。本研究结果显示,患者发病时间越长,Paxlovid治疗的预后越差。
一项针对国内125例COVID-19住院患者临床特征的研究显示,1/3的患者出现淋巴细胞计数降低,约20%患者存在不同程度的ALT和AST升高,70.4%患者的CRP高于正常范围,且与非危重患者相比,危重患者的淋巴细胞水平更低,CRP、IL-6等炎症指标更高,差异具有统计学意义(P<0.05)[15]。从本研究结果同样可以看出,较低的淋巴细胞计数和较高的AST、CRP均提示COVID-19患者进展为危重型的风险高,故使用Paxlovid治疗的预后效果较差。
联合呼吸机辅助通气仅适用于出现呼吸衰竭的危重型COVID-19患者。本研究结果显示,Paxlovid联合呼吸机辅助通气治疗时早期预后不良的风险高,推测可能与重型、危重型COVID-19患者使用Paxlovid抗病毒治疗效果不佳有关。Paxlovid说明书及第10版诊疗方案仅推荐Paxlovid用于有重症高风险因素的轻至中度COVID-19患者。Paxlovid在国外获批上市主要基于一项EPIC-HR研究,该研究纳入的人群同样是重症高风险因素、轻至中度成年的COVID-19患者[9]。因此,对于重型、危重型COVID-19患者使用Paxlovid的循证依据不足。
目前仍缺乏构建预测模型以评估Paxlovid治疗后早期预后不良的风险。根据本研究所构建方程发现,当Y联合预测因子值大于447.920时,提示Paxlovid治疗后早期预后不良的风险高,建议采取更积极的治疗措施包括联合其他抗COVID-19药物等。本研究可能存在不足之处,未来可通过大规模的临床应用和更多临床研究数据提供足够的循证依据。
Risk factors of poor early prognosis in the treatment of COVID-19 with nematevir and ritonavir tablets and the establishment of prediction model
-
摘要:
目的 探讨奈玛特韦片/利托那韦片(Paxlovid)对新型冠状病毒肺炎(COVID-19)患者早期预后不良的危险因素并构建预测模型,以期为提高该类患者的救治效果提供参考。 方法 回顾性分析2023年1月至2023年3月于闽南地区3家军队三甲医院使用Paxlovid治疗的COVID-19住院患者92例,收集临床指标进行单因素和多因素分析,筛选出Paxlovid早期预后不良的独立危险因素,对Logistic模型方程进行转换建立联合预测因子,采用ROC曲线确定联合预测因子的曲线下面积(AUC)及最佳临界值。 结果 92例患者中,早期预后不良者31例(33.70%),其中,死亡11例(35.48%),危重型17例(54.84%),重型3例(9.68%)。多因素Logistic回归分析结果显示,发病天数、淋巴细胞计数、天门冬氨酸氨基转移酶(AST)、C反应蛋白(CRP)和联合呼吸机辅助通气是使用Paxlovid早期预后不良的独立危险因素。以上述独立危险因素构建联合预测因子(Y)的计算公式,Y联合预测因子=7.875X发病天数+126.188X淋巴细胞计数+1.438XAST+XCRP+220.500X联合呼吸机辅助通气,绘制ROC曲线,联合预测因子的ROC曲线下面积最大为0.939,预测价值最优,约登指数(Youden)最大时(0.756)对应ROC曲线最佳临界值为447.920,模型的理论准确度为89.10%。 结论 发病天数、淋巴细胞计数、AST、CRP和联合呼吸机辅助通气是使用Paxlovid早期预后不良的独立危险因素,用药前可通过上述各危险因素计算联合预测因子,当预测结果大于447.920时,应采取更积极的治疗措施包括联合其他抗COVID-19药物等,以提高患者的救治效果。 Abstract:Objective To explore risk factors of poor early prognosis in the treatment of COVID-19 by nematevir and ritonavir tablets Paxlovid and establish the prediction model to provide reference for improving the effect of such patients. Methods 92 inpatients of COVID-19 treated with Paxlovid in three military tertiary hospital in southern Fujian from January 2023 to March 2023 were retrospectively analyzed. The clinical indicators of 92 inpatients were collected for univariate and multivariate analysis by single factor and multiple factors and the independent risk factors of poor early prognosis in Paxlovid were screened out. Logistic model equation was transformed to construct the combined predictors, and ROC curve was used to determine the area under the curve (AUC) and the optimal critical value of the combined predictors. Results Among 92 patients, 31 (33.70%) developed poor early prognosis, including 11 deaths (35.48%), 17 critical cases (54.84%) and 3 severe cases (9.68%). Multi-factor Logistic regression analysis showed that the disease days, lymphocyte count, aspartate aminotransferase(AST), C reactive protein(CRP) and ventilator-assisted ventilation were independent risk factors for poor early prognosis in Paxlovid. A formula for calculating the combined predictors (Y) was established as Ycombinedpredictors=7.875Xdisease days+126.188Xlymphocyte count+1.438XAST+XCRP+220.500Xventilator-assisted ventilation based on the above independent risk factors, and the ROC curve was drawn. With the maximum area under the ROC curve of the combined predictors being 0.939, the prediction value was best, and the optimal critical value of the ROC curve corresponding to the maximum Youden index (0.756) was 447.920.Theoretical accuracy of the model was 89.10%. Conclusion The disease days, lymphocyte count, AST, CRP and ventilator-assisted ventilation were independent risk factors for poor early prognosis in Paxlovid. Combined predictors could be calculated by the above risk factors before medication. The efficiency should be improved by taking more active treatment, including combining with other anti-COVID-19 drugs when the prediction result exceeds 447.920. -
Key words:
- Naimatwe/Litonavir /
- COVID-19 /
- poor prognosis /
- risk factor /
- prediction model
-
METRNL(Metrn-like)蛋白是近年来发现和证实的新的分泌蛋白[1-2],其与METRN构成了一个两蛋白的新蛋白家族。虽然最初的研究表明,该家族蛋白均可促进神经细胞轴突的生长[2-4],但两者表达差异很大,METRN在中枢神经系统中高特异性表达,而METRNL则在全身较为广泛地表达,提示其可能具有更广泛的生理功能[1-2, 5-6]。
最近的研究发现,METRNL对代谢具有重要的调节作用。其在脂肪组织中表达较高,特别是皮下脂肪,被认为是一种新的脂肪因子[1]。研究发现,该蛋白可以调节脂肪细胞的分化,脂肪细胞中METRNL过表达可提高全身胰岛素敏感性,减少脂肪炎症扩大脂肪细胞的体积等[7]。也有研究发现,METRNL可以在运动后由肌肉组织增加分泌,促进脂肪组织棕色化,从而提高代谢率,减轻体重和改善胰岛素敏感性[8]。这些研究提示,METRNL可能与提高胰岛素敏感性相关。
噻唑烷二酮类药物,如罗格列酮,可以通过激动PPARγ受体,提高胰岛素增敏性,被称为胰岛素增敏剂。但是这类药物与METRNL蛋白之间的关系,至今尚不清楚。我们前期的研究发现,白色脂肪组织中METRNL过表达可以提高PPARγ的表达,促进脂肪重构,降低白色脂肪炎症,但是激动PPARγ对METRNL表达的影响尚未有报道。
本研究拟通过高脂饮食(HFD)诱导的胰岛素抵抗小鼠模型,检测血液METRNL的浓度变化;通过给予胰岛素增敏剂罗格列酮治疗,构建胰岛素增敏动物模型,检测血液中METRNL的水平变化,从而明确激动PPARγ对血液METRNL水平影响,通过实时定量PCR检测不同组织中METRNL的表达,明确PPARγ通过何种组织调控METRNL的表达与血液浓度。
1. 材料和方法
1.1 动物处理
12周龄的雄性C57BL/6小鼠与小鼠饲料,均购自上海斯莱克实验动物有限公司。为检测胰岛素抵抗对METRNL表达的影响,分两组小鼠,每组8只,分别给予正常饮食(NCD)和HFD,均饲养4个月;为了检测胰岛素增敏对于METRNL表达的影响,分两组小鼠,每组8只,两组均先HFD饲养3个月,而后实验组的饲料中加入药物罗格列酮(胰岛素增敏组),剂量为10 mg/kg·d,治疗1个月,对照组继续HFD饲养1个月。
1.2 糖耐量实验
小鼠禁食18 h,腹腔注射30%葡萄糖溶液(2 g/kg),分别在0、30、60、90、120 min取尾静脉血,采用强生血糖仪(OneTouch Ultra)检测小鼠血糖水平。
1.3 酶联免疫吸附实验(ELISA)
戊巴比妥钠麻醉小鼠后(80 mg/kg),心脏取血收集血液,室温静置2 h,3000 r/min离心20 min,取上清液。采用小鼠METRNL ELISA试剂盒(购自美国R&D biosystem公司)检测血清中METRNL浓度。操作步骤参照试剂盒说明书。
1.4 荧光实时定量PCR实验
取小鼠附睾周围白色脂肪、肩胛骨间棕色脂肪、肝脏、腓肠肌、脑组织、肾脏、脾脏组织,采用TRIzol试剂(购自美国Invitrogen公司),按照说明书抽提组织总RNA,用RT-PCR逆转录试剂盒(购自中国TARARA公司)逆转录为cDNA。1 μg的cDNA用于检测METRNL的表达,GAPDH作为内参。采用2−ΔΔCt法与SYBR® Green PCR Master Mix(Applied Biosystems)试剂,反应条件为,95 ℃,5 min, 1个循环;95 ℃, 15 s,60 ℃,30 s,72 ℃,30 s,40个循环。相关引物序列见表1。
表 1 相关引物序列基因 上游序列(5'—3') 下游序列(5'—3') METRNL CTGGAGCAGGGAGGCTTATTT GGACAACAAAGTCACTGGTACA GAPDH GTATGACTCCACTCACGGCAAA GGTCTCGCTCCTGGAAGATG ERRα GCCG CGATGTCCTTTTGTG CTGTACTCGATGCTCCCTGC UCP-1 CACGGGGACCTACAATGCTT ACAGTAAATGGCAGGGGACG clec10a TGGTGTCTTGGTTTCCGTCC AGCTCCTAGCTCTCCTTGGC Mrc-1 CTCTGTTCAGCTATTGGACGC TGGCACTCCCAAACATAATTTGA Lipe GTTATGAGTGCGCTCCGAGA GAGCAAAGCTAGAGTCGGGG LPL GGTTGCGCGTAGAGAGGATG CTCACGCTCTGACATGCCTTC FABP4 AAGGTGAAGAGCATCATAACCCT TCACGCCTTTCATAACACATTCC CD36 ATGGGCTGTGATCGGAACTG TTTGCCACGTCATCTGGGTTT 1.5 统计学处理
所有数据均用(
$ \bar x \pm s $ )表示,用SPSS 10.0处理。两样本均数比较采用t检验。2. 结果
2.1 胰岛素增敏剂罗格列酮治疗改善了高脂饮食诱导的胰岛素抵抗
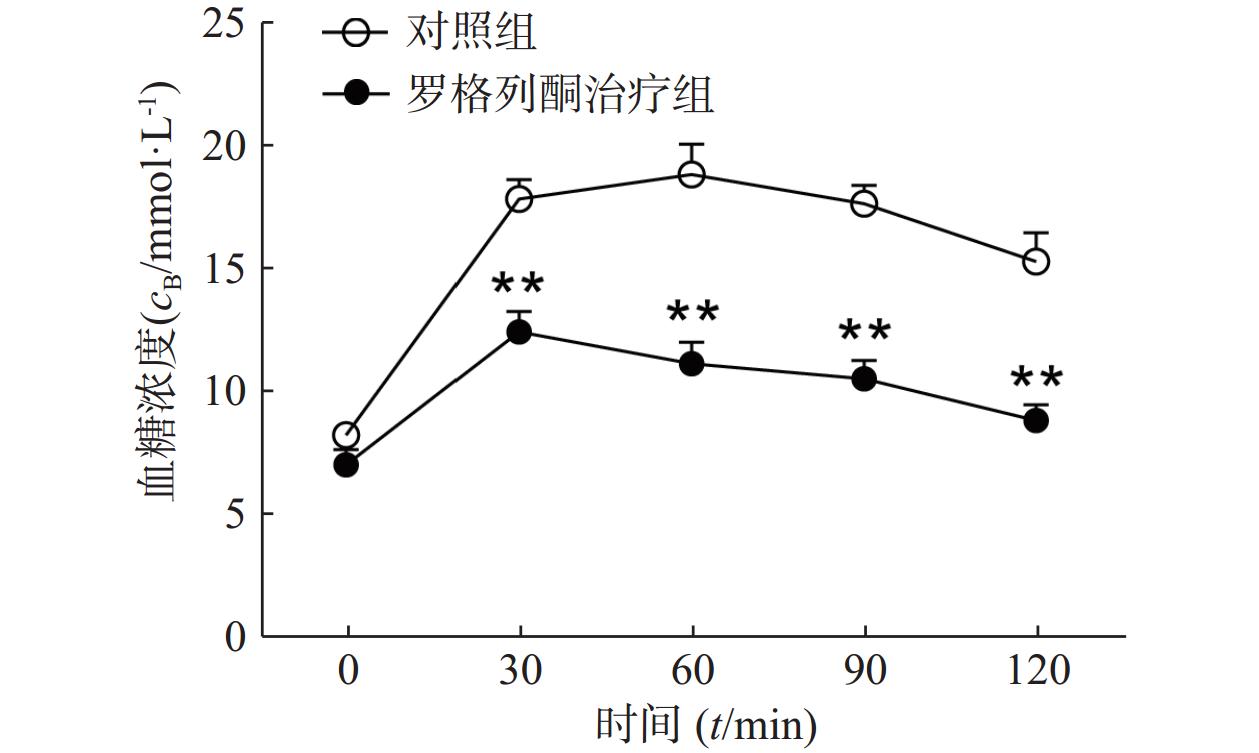
对HFD 3个月的小鼠采用罗格列酮治疗1个月后,葡萄糖耐量实验检测其糖耐量情况如图1所示,罗格列酮治疗组给予葡萄糖后30、60、90、120 min的血糖浓度均明显低于单纯HFD组(P<0.01),说明罗格列酮治疗明显改善了小鼠的糖耐量,提高了机体胰岛素敏感性。
2.2 胰岛素增敏剂罗格列酮治疗升高了胰岛素抵抗小鼠血液中METRNL的水平
取正常对照组、HFD组、HFD罗格列酮治疗组小鼠的血清,ELISA检测METRNL的水平,结果如图2所示,罗格列酮组血清中METRNL的浓度为(6 632±358) pg/ml,是单纯HFD组(4 271±310) pg/ml的1.6倍(P<0.05)。
2.3 胰岛素增敏剂罗格列酮治疗增加棕色脂肪组织和肾脏METRNL的表达
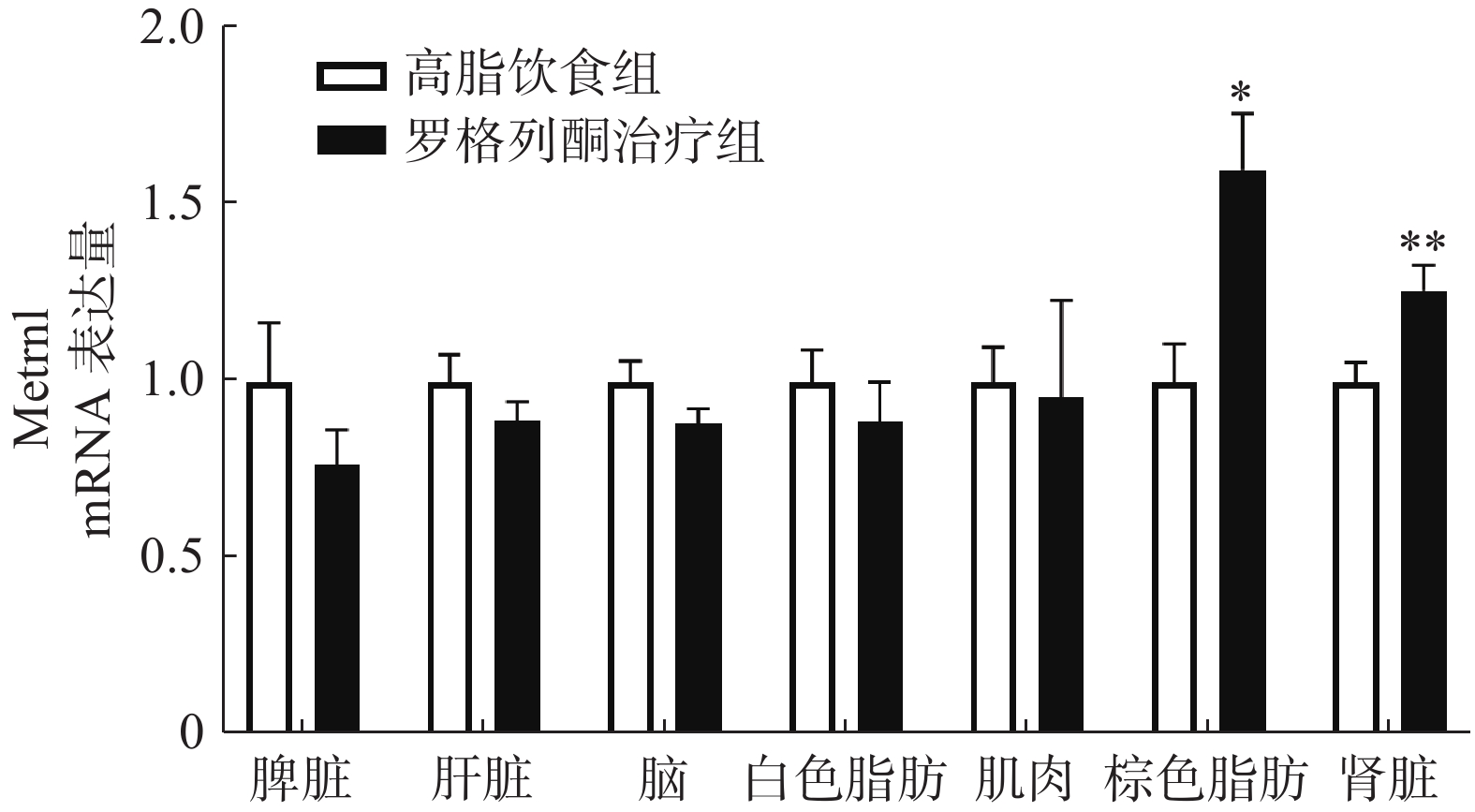
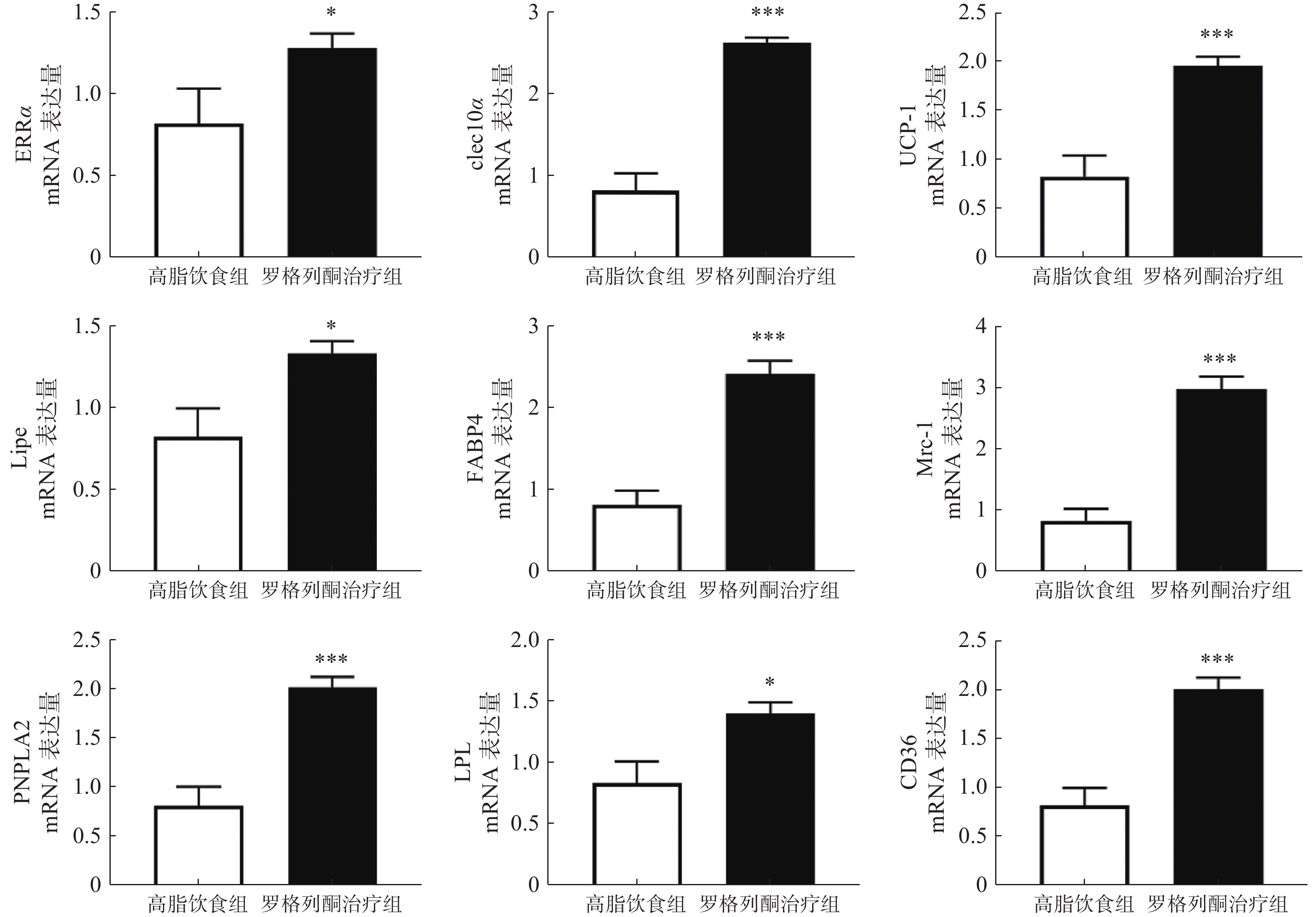
实时荧光定量PCR检测肌肉、肝脏、白色脂肪、棕色脂肪、脑、脾脏、肾脏等组织中METRNL的表达,结果如图3所示,与单纯HFD组相比,罗格列酮治疗组棕色脂肪组织METRNL表达升高1.6倍,肾脏组织METRNL表达升高1.3倍。
2.4 罗格列酮治疗促进了棕色脂肪代谢与棕色脂肪标记蛋白的表达
实时荧光定量PCR检测棕色脂肪组织代谢与棕色脂肪标记蛋白等mRNA表达情况,结果如图4所示,与单纯HFD组相比,罗格列酮治疗组ERRα、UCP-1、clec10a、Mrc-1、Lipe、LPL、FABP4、CD36、PNPLA2等因子表达显著升高。
3. 讨论
本研究通过HFD诱导胰岛素抵抗的肥胖小鼠,发现HFD可以导致METRNL血液水平升高。对肥胖小鼠不同组织METRNL表达的检测显示,脂肪组织METRNL表达显著升高。HFD诱导的胰岛素抵抗小鼠,给予胰岛素增敏剂罗格列酮治疗后,小鼠糖耐量改善,同时,血清METRNL的浓度也升高。这些结果说明,METRNL并非胰岛素敏感性的特异性指标,脂肪可能是使血液METRNL水平改变的主要组织之一。
Li等研究发现,肥胖小鼠的脂肪组织METRNL表达增加[7]。本研究也表明,METRNL的血液浓度在高脂诱导肥胖后升高。Löffler等研究发现,METRNL与脂肪细胞的肥大相关,而脂肪细胞肥大被认为与PPARγ活性的降低相关,是胰岛素抵抗的重要标志之一,进而认为METRNL是机体胰岛素抵抗的标志[9]。然而,在本研究中,胰岛素增敏剂罗格列酮治疗后小鼠胰岛素敏感性提高,同时METRNL表达也显著升高,可见METRNL血液浓度的升高并不能代表胰岛素抵抗的增加。
罗格列酮可以显著提高胰岛素的敏感性,故也称为胰岛素增敏剂。本研究表明,其可以显著提高METRNL的表达,而METRNL又具有促进白色脂肪棕色化和提高胰岛素敏感性的作用,所以METRNL可能参与介导了罗格列酮的胰岛素增敏作用。
我们前期的研究表明,增加白色脂肪表达可以提高血液中METRNL的水平。本研究发现,罗格列酮未促进高脂条件下白色脂肪METRNL的表达,在检测的7种组织中,罗格列酮显著提高了棕色脂肪和肾脏METRNL的表达,但是对脾脏、肝脏、肌肉、白色脂肪、脑组织METRNL的表达没有影响,说明罗格列酮可能主要通过棕色脂肪和肾脏提高血液METRNL浓度。此外,进一步实验发现,罗格列酮促进了棕色脂肪中代谢和棕色脂肪标记蛋白的表达,这与以往的研究结果一致[10],既往研究表明,METRNL可促进白色脂肪棕色化,提示罗格列酮促进棕色脂肪代谢的作用可能有METRNL蛋白参与。
本研究发现了胰岛素增敏剂罗格利酮治疗可能通过提高棕色脂肪和肾组织的METRNL表达来升高血清METRNL水平,提示METRNL可能参与了罗格列酮对糖尿病的治疗过程。
-
表 1 两组患者临床资料的单因素分析
项目 非早期预后不良组(n=61) 早期预后不良组(n=31) 统计量 P值 年龄(岁, $ \bar{x} $ ±s)77.16±16.47 74.42±14.52 0.785 0.434 性别[女,n(%)] 16(26.23) 7(22.58) 0.146 0.702 体重(m/kg, $ \bar{x} $ ±s)63.99±10.09 60.63±11.63 1.430 0.156 发病天数(t/d, $ \bar{x} $ ±s)7.72±5.46 12.48±6.56 −3.693 <0.001 血氧饱和度(%, $ \bar{x} $ ±s)94.01±4.97 90.91±10.76 1.894 0.061 核酸检测CT值( $ \bar{x} $ ±s)28.31±5.64 26.90±5.24 1.158 0.250 淋巴细胞计数(×109/L, $ \bar{x} $ ±s)0.98±0.59 0.62±0.44 2.992 0.040 eGFR[ml/(min·1.73 m2), $ \bar{x} $ ±s]68.29±30.56 57.20±38.44 1.505 0.136 ALT[U/L,M(P25,P75)] 22.90(9.00,121.00) 23.00(6.00,237.20) −0.450 0.653a AST[U/L,M(P25,P75)] 31.00(13.50,88.00) 37.40(21.00,306.20) 2.747 0.006a TG(mmol/L, $ \bar{x} $ ±s)1.36±0.87 1.50±0.76 −0.782 0.436 TC(mmol/L, $ \bar{x} $ ±s)3.95±0.94 3.59±1.19 1.555 0.123 LDL-C(mmol/L, $ \bar{x} $ ±s)2.19±0.69 2.01±0.97 0.998 0.321 HDL-C(mmol/L, $ \bar{x} $ ±s)1.14±0.32 1.00±0.44 1.664 0.100 CK(U/L, $ \bar{x} $ ±s)145.23±253.51 148.21±107.09 −0.062 0.950 CRP(mg/L, $ \bar{x} $ ±s)52.41±46.71 118.72±82.74 −4.918 <0.001 PCT[ng/ml,M(P25,P75)] 0.07(0.01,27.60) 1.40(0.01,42.95) 4.366 <0.001a D−二聚体[μg/ml,M(P25,P75)] 0.79(0.19,11.39) 2.17(0.36,41.83) 4.254 <0.001a 用药前已使用其他抗新冠病毒药物治疗[n(%)] 1(1.64) 5(16.13) 7.079 0.008 用药前已行呼吸机辅助通气[n(%)] 10(16.39) 19(61.29) 19.194 <0.001 合并疾病 高血压[n(%)] 39(63.93) 20(64.52) 0.003 0.956 糖尿病[n(%)] 16(26.23) 14(45.16) 3.352 0.067 ASCVD[n(%)] 29(47.54) 15(48.39) 0.006 0.939 慢性肺病[n(%)] 19(31.15) 5(16.13) 2.404 0.121 治疗方案 Paxlovid疗程(t/d, $ \bar{x} $ ±s)4.74±0.68 5.26±2.05 −1.805 0.074 联合免疫抑制剂[n(%)] 42(68.85) 29(93.55) 7.116 0.008 联合抗凝药[n(%)] 36(59.02) 29(93.55) 11.821 0.001 联合抗菌药物[n(%)] 46(75.41) 30(96.77) 6.530 0.011 联合俯卧位治疗[n(%)] 8(13.11) 9(29.03) 3.457 0.088 联合呼吸机辅助通气[n(%)] 11(18.03) 25(80.65) 33.831 <0.001 注:a表示Mann-Whitney U检验。 表 2 Paxlovid早期预后不良多因素logistic分析
项目 回归系数B 标准误S.E 卡方值Waldχ2 自由度df 比值比OR 95%CI置信区间 P值 发病天数(t/d) 0.126 0.061 4.237 1 1.135 1.006~1.279 0.040 淋巴细胞计数 2.019 0.892 5.126 1 7.527 1.311~43.208 0.024 AST 0.023 0.009 6.578 1 1.023 1.005~1.041 0.010 CRP 0.016 0.007 5.744 1 1.016 1.003~1.029 0.017 联合呼吸机辅助通气 3.528 1.054 11.194 1 34.051 4.311~268.936 0.001 常量 −8.371 2.080 16.195 1 <0.001 表 3 各危险因素对Paxlovid早期预后不良的预测价值
项目 最佳临界值 敏感度 特异性 约登指数 AUC(95%CI) P值 发病天数(t/d) 14.500 0.387 0.951 0.338 0.722(0.614~0.831) 0.001 淋巴细胞计数 1.685 0.355 0.934 0.289 0.582(0.449~0.715) 0.202 AST 31.950 0.839 0.557 0.396 0.676(0.566~0.786) 0.006 CRP 104.500 0.548 0.869 0.417 0.772(0.672~0.871) <0.001 联合呼吸机辅助通气 0.500 0.806 0.820 0.626 0.813(0.715~0.911) <0.001 联合预测因子 447.920 0.903 0.852 0.756 0.939(0.885~0.993) <0.001 -
[1] WHO. WHO Coronavirus (COVID-19) Dashboard[EB/OL]. (2023-03)[2023-03-13]. https://covid19.who.int/. [2] WANG Y, ZHAO D Y, LIU X B, et al. Early administration of Paxlovid reduces the viral elimination time in patients infected with SARS-CoV-2 Omicron variants[J]. J Med Virol, 2023, 95(1):e28443. doi: 10.1002/jmv.28443 [3] 国家药品监督管理局. 国家药监局应急附条件批准辉瑞公司新冠病毒治疗药物奈玛特韦片/利托那韦片组合包装进口注册[EB/OL]. (2023-02)[2023-03-13]. https://www.nmpa.gov.cn/directory/web/nmpa/yaowen/ypjgyw/20220212085753142.html. [4] ZHENG Q, MA P F, WANG M W, et al. Efficacy and safety of paxlovid for COVID-19: a meta-analysis[J]. J Infect, 2023, 86(1):66-117. [5] NAJJAR-DEBBINY R, GRONICH N, WEBER G, et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients[J]. Clin Infect Dis, 2023, 76(3):e342-e349. doi: 10.1093/cid/ciac443 [6] 国家卫生健康委员会. 新型冠状病毒感染诊疗方案(试行第十版)[EB/OL]. (2023-01)[2023-03-13]. http://www.nhc.gov.cn/xcs/zhengcwj/202301/32de5b2ff9bf4eaa88e75bdf7223a65a.shtml. [7] DE VRIES M, MOHAMED A S, PRESCOTT R A, et al. A comparative analysis of SARS-CoV-2 antivirals characterizes 3CLpro inhibitor PF-00835231 as a potential new treatment for COVID-19[J]. J Virol, 2021, 95(7):e01819-e01820. [8] MÓTYÁN J A, MAHDI M, HOFFKA G, et al. Potential resistance of SARS-CoV-2 main protease (mpro) against protease inhibitors: lessons learned from HIV-1 protease[J]. Int J Mol Sci, 2022, 23(7):3507. doi: 10.3390/ijms23073507 [9] HAMMOND J, LEISTER-TEBBE H, GARDNER A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19[J]. N Engl J Med, 2022, 386(15):1397-1408. doi: 10.1056/NEJMoa2118542 [10] WANG L, BERGER N A, DAVIS P B, et al. COVID-19 rebound after paxlovid and molnupiravir during January-June 2022[J]. medRxiv, 2022: 2022.06. 21.22276724. [11] WENG C Z, XIE R C, HAN G J, et al. Safety and efficacy of paxlovid against Omicron variants of coronavirus disease 2019 in elderly patients[J]. Infect Dis Ther, 2023, 12(2):649-662. doi: 10.1007/s40121-023-00760-x [12] 潘敏, 张田婧, 常双, 等. 老年人新型冠状病毒感染小分子抗病毒药物治疗建议[J/OL]. 中国药理学通报, 2023(3): 425-430(2023-03)[2023-03-14]. http://kns.cnki.net/kcms/detail/34.1086.r.20230308.1807.010.html. [13] PUHACH O, ADEA K, HULO N, et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2[J]. Nat Med, 2022, 28(7):1491-1500. doi: 10.1038/s41591-022-01816-0 [14] WÖLFEL R, CORMAN V M, GUGGEMOS W, et al. Virological assessment of hospitalized patients with COVID-2019[J]. Nature, 2020, 581(7809):465-469. doi: 10.1038/s41586-020-2196-x [15] Ruirui, Wang. Epidemiological and clinical features of 125 Hospitalized Patients with COVID-19 in Fuyang, Anhui, China[J]. Int J Infect Dis, 2020, 95:421-428. doi: 10.1016/j.ijid.2020.03.070 -





 下载:
下载:
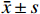

 下载:
下载:




