-
骨质疏松症和阿尔茨海默病均为退行性疾病,越来越多的研究表明它们的发病机制具有关联[1]。骨质疏松症往往伴随着认知缺陷,Aβ沉积是阿尔茨海默病的典型症状,而阿尔兹海默病小鼠(APPswe/PS1dE9)的骨组织中也会出现Aβ沉积,并出现骨密度降低,骨强度减弱等骨质疏松症状[2]。另有研究表明,氧化应激会导致 Aβ沉积,这种氧化损伤状态可被抗氧化剂改善[3]。因此, Aβ沉积偶联氧化损伤可视为阿尔茨海默病及骨质疏松症的共同发病机制。巴戟天丸收载于明代《古今医统大全》,由君药巴戟天,臣药远志、石菖蒲、茯苓、人参以及地骨皮、茯神组成[4]。前期课题组研究已证实,巴戟天丸组方可以从体内外水平上改善D-半乳糖(D-gal)引发的骨丢失,但巴戟天丸组方治疗Aβ沉积所致的骨丢失及具体作用机制有待进一步阐明[4, 5]。因此,本研究拟以Aβ1-42寡聚体损伤成骨细胞模型,对巴戟天丸组方的抗氧化能力及对骨形成干预作用进行探究,并通过网络药理学方法对潜在的作用机制进行预测。
-
取质量比为5∶10∶10∶10∶10∶10∶3的巴戟天、茯苓、茯神、地骨皮、远志、石菖蒲、人参粉末,混合均匀。按照料液比1∶10加入去离子水,浸泡1 h后,加热煎煮,回流提取2次,每次1 h,所得药液过滤,合并两次的滤液。将收集到的滤液减压浓缩成浓度为2.6 g(生药量)/ml的巴戟天丸水提物母液。-20°C保存备用。
-
新生 24 h Wistar 大鼠(上海西普尔-必凯实验动物有限公司);胎牛血清(以色列BI);MTT(上海碧云天);BCIP/NBT 碱性磷酸酯酶显色试剂盒(上海碧云天)、ALP、CAT、SOD、GSH 、MDA 试剂盒(南京建成);PBS(天津灏洋);DMSO(上海博光);α-MEM 培养基(上海富衡); BMP2、RUNX-2、OPG、GAPDH抗体(美国Abcam)。
-
采用二次消化法从新生 24 h Wistar 大鼠的颅盖骨中分离得到原代成骨细胞,将成骨细胞培养于 α-MEM 培养基中(10% 胎牛血清),置于 37 ℃、5% CO2恒温培养箱中培养,取第 3~5 代成骨细胞进行后续实验。
-
将成骨细胞以 2×104个/孔接种于无菌 96 孔板中,孵育过夜。空白组和模型组更换新的完全培养基,阳性药组加入含N-乙酰半胱氨酸(NAC)的完全培养液(1 mmol/L), 给药组分别加入含不同浓度的巴戟天丸组方完全培养液(0.008、0.04、0.2、1、5 μg/ml),4 h 后模型组和给药组给予Aβ1-42寡聚体进行损伤,使完全培养基中Aβ1-42寡聚体浓度达到 20 mmol/L。培养 48 h 后,采用 MTT 法测定细胞增殖水平。
-
ALP 活性检测:按照“1.4.2”项的方法进行给药,培养 48 h 后,取上清液,根据说明书进行 ALP 活性测定。将成骨细胞以 1×105 个/孔接种于无菌6孔板中,孵育过夜,细胞完全贴壁后,空白组和模型组更换新的完全培养基,阳性药组加入含NAC的完全培养液(1 mmol/L), 给药组分别加入含不同浓度的巴戟天丸组方完全培养基(0.2、1、5 μg/ml),4 h 后模型组和给药组给予Aβ1-42寡聚体进行损伤,使培养基中Aβ1-42寡聚体浓度达到20 mmol/L。培养 48 h 后进行染色,室温下避光孵育 48 h,洗去工作液后置于显微镜下拍照。
-
将成骨细胞以2×104个/孔和5×104 个/孔分别接种于接种于无菌96孔和24孔板中,孵育过夜,细胞完全贴壁后,空白组和模型组更换新的完全培养基,阳性药组加入含NAC的完全培养液(1 mmol/L), 给药组分别加入含不同浓度的巴戟天丸组方完全培养液(0.2、1、5 μg/ml),4 h 后模型组、阳性药组和给药组给予Aβ1-42寡聚体进行损伤,使培养基中Aβ1-42寡聚体浓度达到20 mmol/L。培养 48 h 后根据说明书进行检测。
-
将细胞以 2×105 个/孔接种于 6 孔板中,孵育过夜,细胞完全贴壁后,阳性药组加入含NAC的完全培养液(1 mmol/L), 给药组分别加入含不同浓度的巴戟天丸组方完全培养基(0.2、1、5 μg/ml),4 h 后模型组和给药组加入Aβ1-42寡聚体,使完全培养基中Aβ1-42寡聚体浓度达到20 mmol/L。48 h 后,吸去培养基,PBS 洗涤 3 次。于冰上对细胞进行裂解提取总蛋白,采用 BCA 试剂盒测定蛋白浓度。蛋白变性后,经 SDS-PAGE 凝胶电泳后转移至 PVDF 膜进行转膜,室温封闭1 h后,加入相应的一抗,4 ℃过夜,1×TBST洗涤 3 次,加二抗于室温孵育 50 min,1×TBST洗涤 3 次,采用 ECL 化学发光试剂盒检测。
-
使用 SPSS Staistics 24 统计分析软件进行统计学分析。计量数据均采用(
$\bar{x} $ ±s)表示,选用单因素方差分析(One-Way ANOVA)进行组间变量的比较分析。使用 Graphpad prism 9.0 软件进行统计及绘图。以P<0.05 为差异有显著性意义。 -
通过TCMSP(http://tcmsow.com/tcmsp.php)和ETCM数据库(http://www.tcmip.cn/ETCM/index.php),查找巴戟天、地骨皮、茯苓、人参、石菖蒲和远志6味中药的成分。在TCMSP数据库中,选择药物口服利用度(OB)≥30%,类药性(DL)≥0.18的成分,在ETCM数据库中根据DrugLikeness Grading评分,选择评分为Moderate和Good的成分[6]。
-
通过Uniprot(http://www.uniprot.org/)数据库,将靶点映射成基因,利用Cytoscape 3.6.0软件绘制成分靶点图。
-
通过Disgenet数据库(http://www.disgenet.org/)和Genecards数据库(https://www.genecards.org/)查找阿尔兹海默病和骨质疏松症相关的疾病靶点,绘制PPI蛋白相互作用网络图,整合两大数据库中的靶点基因,去除重复的靶点。利用Venny2.1,将阿尔兹海默病和骨质疏松症相关的疾病靶点图进行交集分析。
-
利用Venny2.1,将阿尔兹海默病、骨质疏松症与巴戟天丸组方相关靶点进行交集分析。将药物疾病交集靶点上传到String数据库进行分析,绘制PPI蛋白相互作用网络图。
-
采用Metascape数据库,对关键靶点进行GO基因富集分析(http://geneontology.org/)和KEGG代谢通路分析(http://www.genome.jp/kegg/),分析巴戟天丸中的主要分子生物过程和信号通路。
-
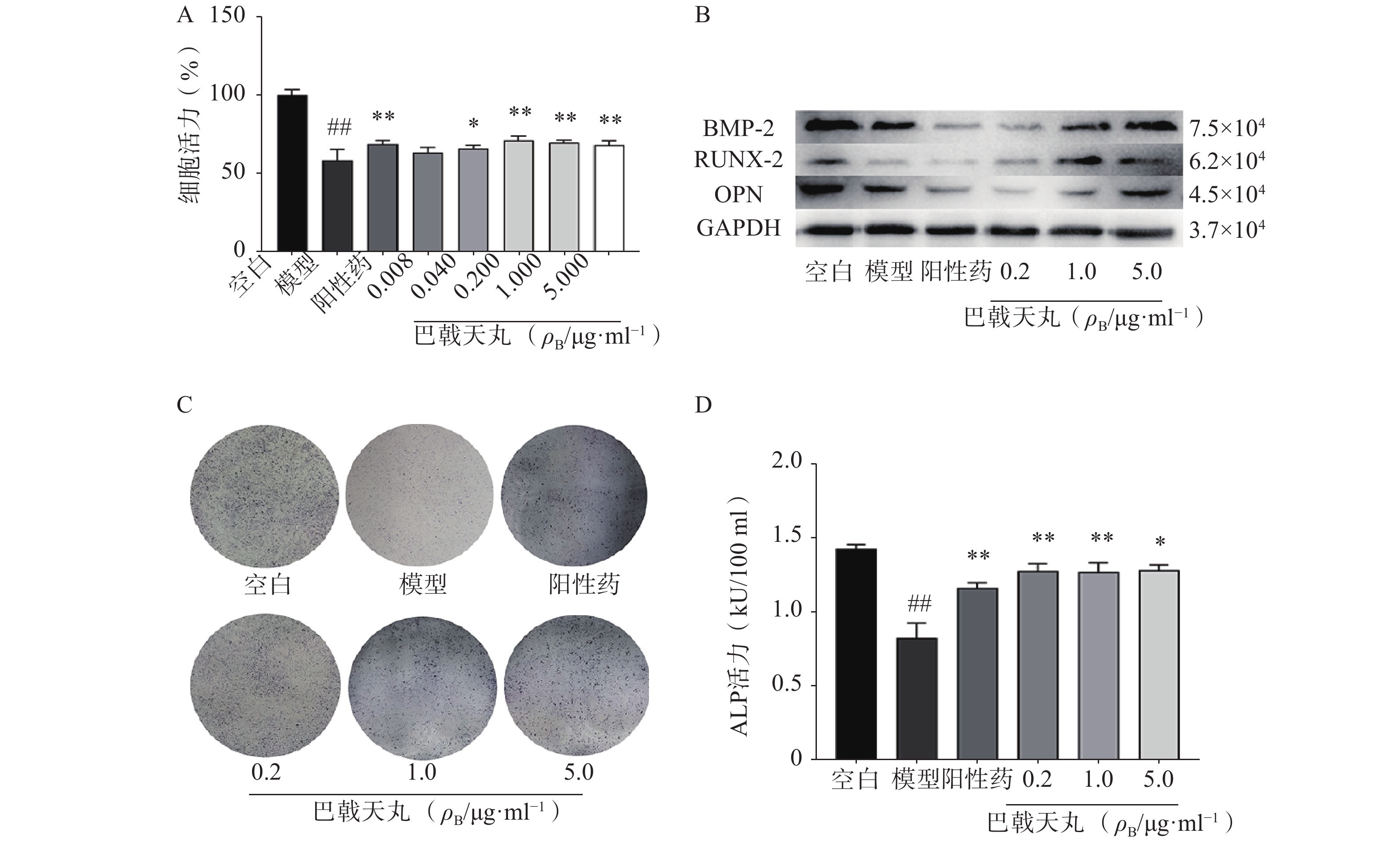
Aβ1-42寡聚体可显著抑制成骨细胞的增殖;巴戟天丸组方在 0.04、0.2、1、5 μg/ml浓度下均能够显著提高成骨细胞的增殖水平(图1A)。Aβ1-42寡聚体会显著抑制成骨细胞中骨形成相关蛋白BMP2、RUNX-2、OPG的表达;给予巴戟天丸组方干预后,BMP2、RUNX-2、OPG的表达显著提高,提示巴戟天丸组方可显著促进Aβ1-42寡聚体损伤成骨细胞的骨形成(图1B)。与空白组相比,Aβ1-42寡聚体可以显著降低成骨细胞的 ALP 活性,而巴戟天丸组方显著逆转了 Aβ 损伤成骨细胞的 ALP 活性,促进成骨细胞的分化(图1C、D)。
-
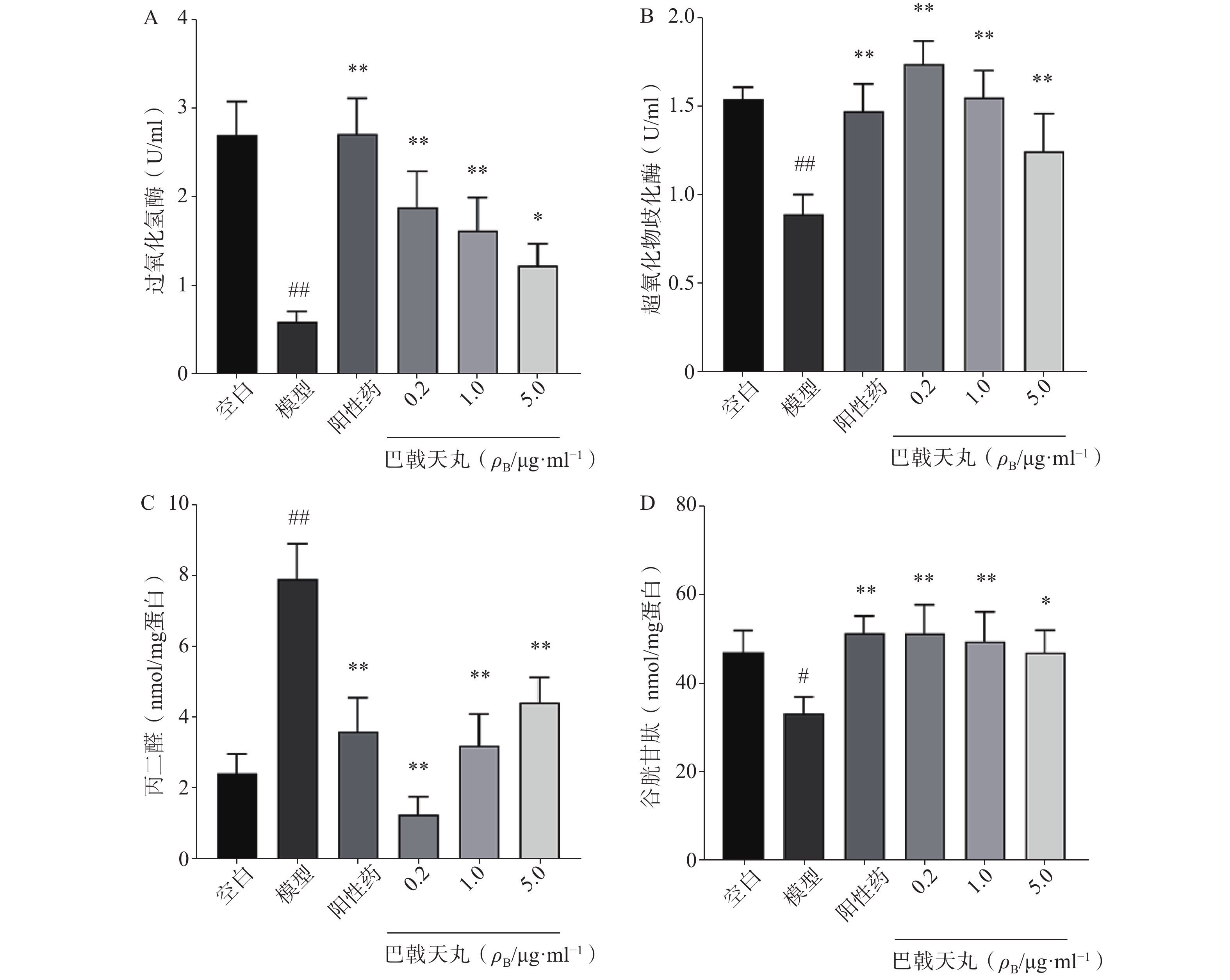
如图2 所示,Aβ1-42寡聚体显著降低了成骨细胞CAT(图2A)、SOD(图2B)、GSH(图2D)的活性,提高MDA的活性(图2C),导致成骨细胞的氧化损伤,而巴戟天丸组方低、中、高剂量均可以显著改善Aβ1-42寡聚体导致的氧化损伤。
-
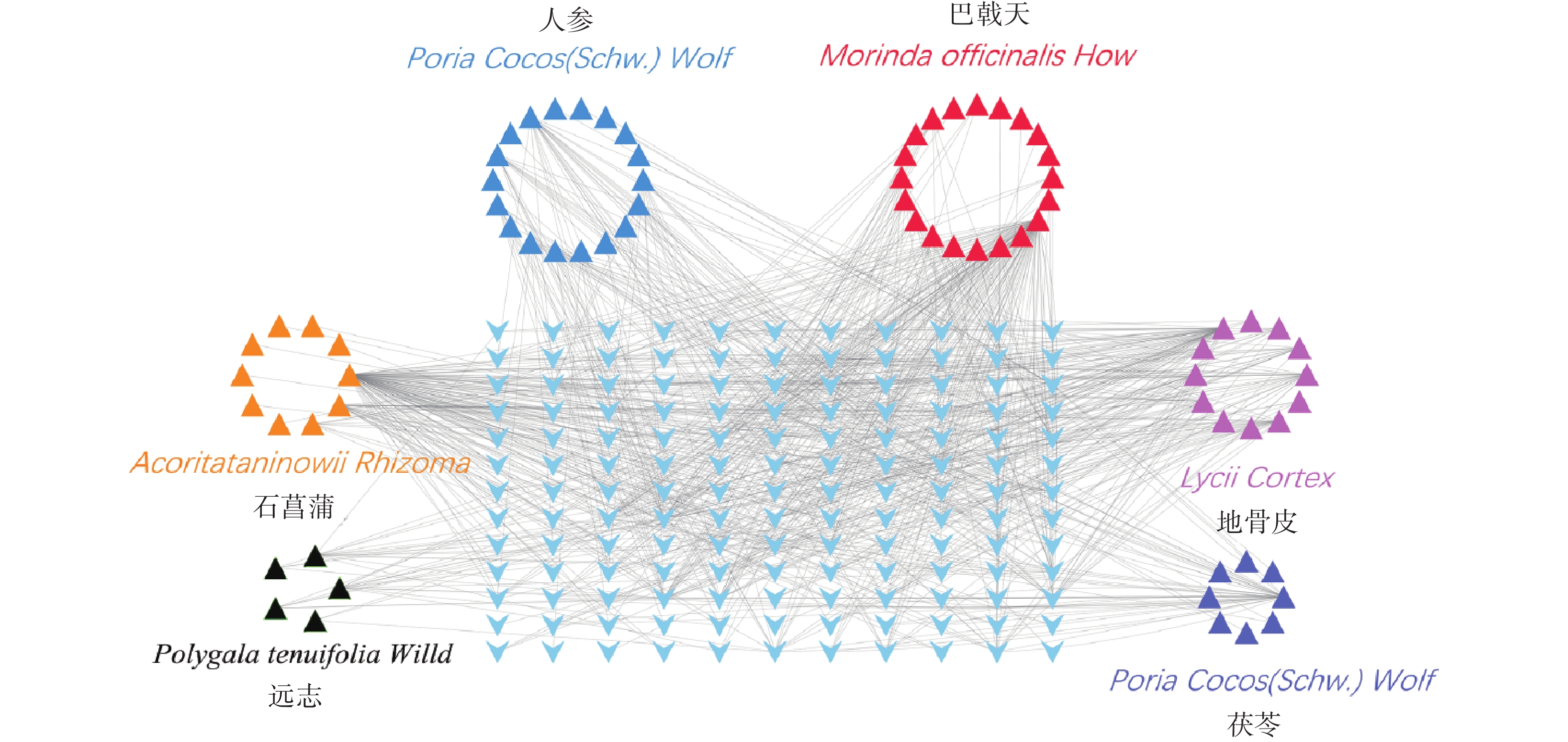
根据条件筛选后,其中,巴戟天成分为20种,地骨皮成分为12种,茯苓成分为10种,人参成分为18种,石菖蒲成分为10种,远志成分为5种,茯神成分0种,共计成分75种。
-
通过TCMSP和ETCM数据库查找满足筛选条件的化学成分对应的靶点,得到151个预测靶点。删除重复靶点后,通过Uniprot数据库映射成基因ID,剔除重复靶点后,得到143个基因symbol(图3)。
-
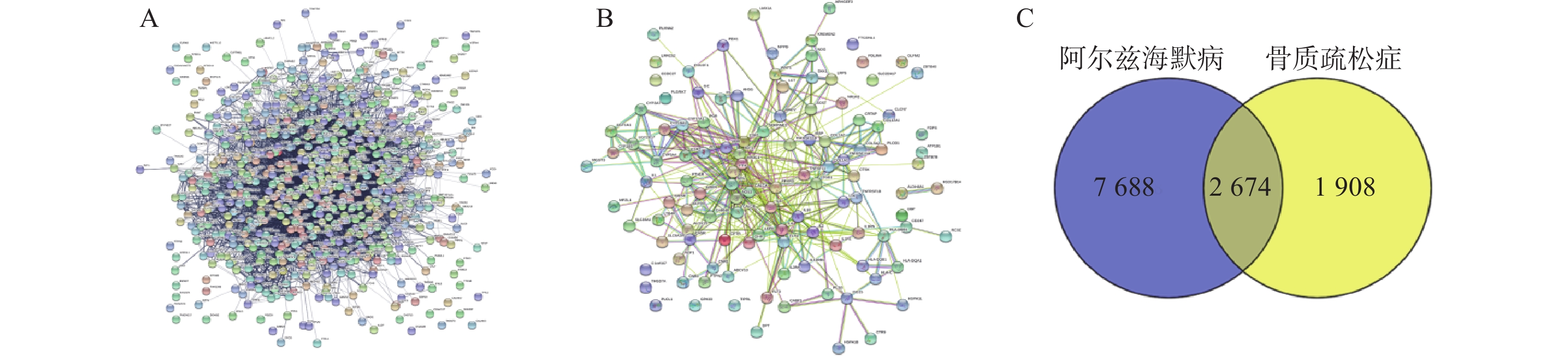
通过Disgenet数据库和Genecards数据库查找得到阿尔兹海默病相关疾病靶点10 362个,骨质疏松症相关疾病靶点4 582个,并将所得靶点进行蛋白相互作用网络分析(图4A、B)。将阿尔兹海默病和骨质疏松症相关的疾病靶点图进行交集分析,得到共有靶点2 674个(图4C)。
-
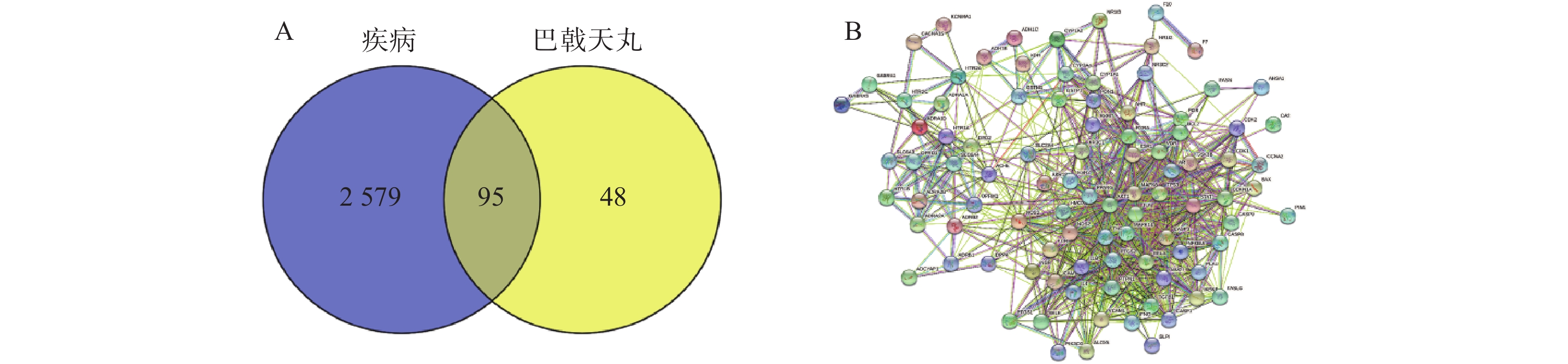
将2 674个疾病交集靶点基因和143个药物成分靶点基因再次进行交集分析,得到共有靶点95个(图5A),将95个药物疾病交集靶点上传到String数据库进行分析,绘制PPI蛋白相互作用网络图。将单独节点去除后,图中共有94个节点,871条边,意味着它们间有特殊的关联,即蛋白质共同贡献一个共同的功能,每个靶点的平均节点度为18.3,说明交集靶点间关系密切而且存在很强的相互作用关系(图5B)。
-
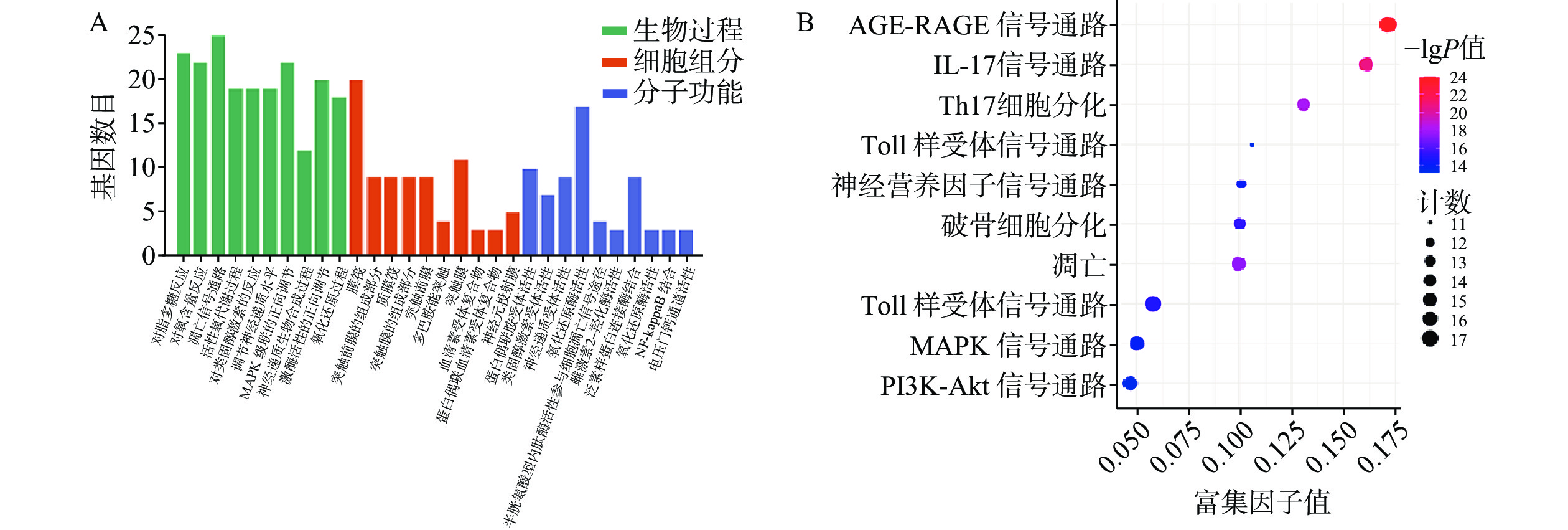
GO富集分析包括生物过程(BP)、分子功能(MF)及细胞组分(CC),将每个结果前 10 的条目可视化为条形图(图6A)。巴戟天丸活性物质参与的BP主要包括对脂多糖的反应、对氧含量的反应、细胞凋亡信号途径、活性氧代谢过程。MF主要包括G蛋白偶合胺受体的活性、神经递质受体的活性、氧化还原酶活性、参与凋亡信号通路的半胱氨酸型内肽酶活性、泛素类蛋白连接酶结合。CC主要包括膜筏、突触膜的整体组成部分、浆膜筏等。 KEGG 通路富集分析中主要涉及与阿尔兹海默症、骨质疏松症相关的通路有AGE-RAGE信号通路、PI3K-Akt信号通路、MAPK信号通路以及神经活性配体与受体的相互作用通路等(图6B)。
-
骨骼重塑是一个重要的生理过程,其主要包括两个阶段,成骨细胞主导的骨形成和破骨细胞主导的骨吸收[7]。其中,成骨细胞的增殖能力反映骨形成的强弱,其分泌的 ALP 是分化阶段的关键酶,可促进骨组织矿化[8]。本研究中,各剂量巴戟天丸均可显著改善Aβ损伤成骨细胞的增殖抑制,且0.2 μg/ml效果最好,5 μg/ml的效果优于0.04 μg/ml,故选择0.2 μg/ml、1 μg/ml、5 μg/ml作为巴戟天丸给药剂量进行后续实验,并发现其可显著提高Aβ损伤成骨细胞的ALP活性和骨形成相关蛋白 BMP2、RUNX-2、OPG的表达,促进骨形成。GSH是细胞抗氧化系统的一个重要成员,高水平的GSH对于清除过多的活性氧(ROS)和解毒异物是不可缺少的[9]。MDA是细胞中多不饱和脂肪酸过氧化的最终产物之一,自由基的增加会导致MDA的过度生产,加剧氧化损伤[10]。SOD和CAT是重要的酶类抗氧化剂,二者活性的降低会导致氧化损伤[11,12]。本实验中巴戟天丸组方可逆转Aβ损伤成骨细胞GSH、SOD、CAT活性的降低, MDA活性的升高,结果所表现的剂量依赖与增殖实验相吻合,表明巴戟天丸组方可作为抗氧化剂改善成骨细胞的氧化损伤。后采用网络药理学方法进一步探究巴戟天丸干预Aβ损伤成骨细胞的作用机制,结果显示,巴戟天丸促进Aβ损伤状态下成骨细胞骨形成的作用可能与AGE-RAGE、PI3K-Akt及MAPK等信号通路有关。其中,AGE-RAGE信号通路的激活会扰乱细胞的氧化还原平衡并调节各种细胞死亡途径 [13]。作为AGE-RAGE信号通路的下游, PI3K-Akt信号通路的激活会增强细胞的抗氧化能力,保护细胞免受氧化应激[14]。此外,MAPK通路的激活同样可以减轻氧化应激状态[15],故网络药理学研究结果同样提示巴戟天丸组方可通过缓解氧化损伤发挥干预Aβ沉积损伤成骨细胞的作用。
The roles of Bajitianwan formula on Aβ-injured osteoblasts and the mechanism based on network pharmacology
-
摘要:
目的 探讨巴戟天丸组方对Aβ损伤成骨细胞的骨形成作用及其机制。 方法 以新生24 h Wistar大鼠所分离的成骨细胞为研究对象,用 Aβ1-42 寡聚体对成骨细胞进行损伤,并用巴戟天丸组方水提物进行药物干预。分别采用MTT法、碱性磷酸酶(ALP)活性检测、过氧化氢酶(CAT)活性检测、超氧化物歧化酶(SOD)活性检测、谷胱甘肽(GSH)活性检测以及丙二醛(MDA)活性检测。采用蛋白质印迹法检测骨形成相关蛋白骨形态发生蛋白2(BMP2)、成骨特异性转录因子(RUNX-2)、骨保护蛋白(OPG)的表达水平;明确巴戟天丸组方对Aβ损伤成骨细胞的作用后,采用网络药理学方法对潜在的作用机制进行预测。 结果 巴戟天丸组方可显著促进Aβ损伤成骨细胞的增殖,提高 ALP、SOD、GSH 活性,抑制MDA活性,并促进骨形成相关蛋白BMP2、RUNX-2、OPG的表达。网络药理学分析显示,巴戟天丸组方发挥改善Aβ损伤成骨细胞的作用主要与AGE-RAGE信号通路、PI3K-Akt信号通路、MAPK信号通路以及神经活性配体与受体的相互作用通路等有关。 结论 明确巴戟天丸组方具有改善Aβ损伤成骨细胞的作用,并通过网络药理学方法探究其相关作用通路,为传统方剂巴戟天丸抗骨质疏松的临床应用提供借鉴。 Abstract:Objective To explore the effect of Bajitianwan(BJTW)formula on bone formation of Aβ-injured osteoblasts and its mechanism. Methods Osteoblasts isolated from neonatal 24-hour Wistar rats were used for the study, and osteoblasts were subjected to damage with Aβ1-42 oligomers, and pharmacological intervention was performed with the aqueous extract of BJTW formula. The MTT assay, alkaline phosphatase(ALP)activity assay, catalase(CAT)activity assay, superoxide dismutase(SOD)activity assay, glutathione(GSH)activity assay and malondialdehyde(MDA)activity assay were carried out respectively. The expression levels of bone morphogenetic protein 2(BMP2), osteogenic specific transcription factor(RUNX-2)and osteoprotective protein(OPG)were detected by Western blotting. After confirming the effect of BJTW formula on Aβ-injured osteoblasts, the network pharmacology method was used to predict the potential pathways. Results The BJTW formula significantly promoted the proliferation of Aβ-injured osteoblasts, increased ALP, SOD and GSH activity, inhibited MDA activity, and promoted the expression of bone formation-related proteins BMP2, RUNX-2 and OPG. Network pharmacological analysis showed that the effect of ameliorating of Aβ-injured osteoblasts by BJTW formula was mainly mediated by AGE-RAGE, PI3K-Akt, MAPK and neuroactive ligand-receptor interaction signaling pathways. Conclusion In this study, the effect of BJTW formula on improving the osteoblasts damaged by Aβ was confirmed for the first time, and its related mechanism was explored based on network pharmacology method. The results lay a strong foundation for the clinical application of traditional formula BJTW against osteoporosis. -
Key words:
- osteoblasts /
- Bajitianwan /
- Aβ deposition /
- network pharmacology
-
放射性核素是指不稳定原子核自发地放出射线通过衰变形成稳定的核素,能够通过皮肤伤口、呼吸道及消化道吸收进入体内,增加了肿瘤、畸变、遗传性病变发生率,严重影响人体健康。放射性材料在诸如核工业、能源生产、研究和开发以及医学等各种领域中的使用越来越多,而产生的放射性废物对人类具有严重危害。自2011年福岛第一核电站事故以来,公众对长期暴露于该核电站释放辐射造成的健康一直高度关注[1]。因此,如何进行核辐射的有效防护一直是研究难点和重点。
有效清除放射性核素的常用手段包括螯合剂或吸附剂,其中多糖是近年发展起来的具有优良吸附作用的分子。多糖(polysaccharide)是由糖苷键结合的糖链,是至少超过10个单糖组成的聚合糖,也是高分子碳水化合物。其中天然多糖,如海藻酸盐、壳聚糖、纤维素等,分子链上具有多种官能团,通过物理和化学相互作用与多种分子结合,也可以通过共混、接枝或与具有额外官能团的各种纳米材料混合的形式引入额外的功能基团,从而增强其吸附能力。多糖的吸附性能不仅与吸附剂上特定官能团的类型和数量有关,还受吸附剂比表面积和吸附位点暴露程度的影响。
本综述概括了主要放射性核素类型及其对人体的危害,总结了多糖在放射性核素清除方面的最新研究进展。多糖作为核战争、核事故等突发核事件中放射性核素有效吸附分子,应用前景广阔。
1. 放射性核素的类型及其对人体危害
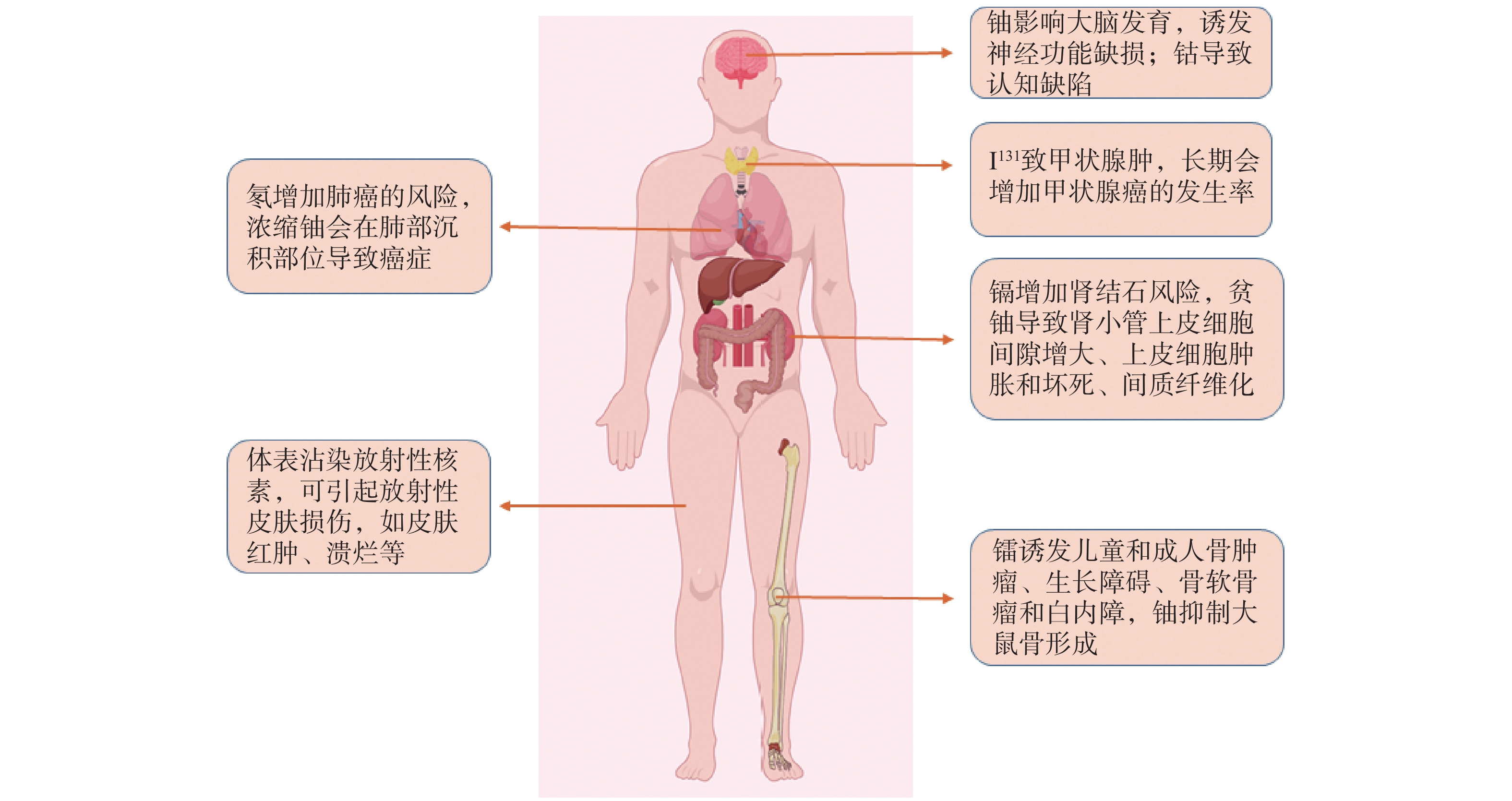
1.1 铀
铀是一种重金属放射性核素,可作为核反应燃料。肾、肝、肺、脑、中枢神经是铀毒性累积的靶向组织[2]。铀污染会诱发行为障碍,影响神经化学和神经生理特性,还会导致肾小管上皮细胞间隙增大、上皮细胞肿胀和坏死、间质纤维化,引起尿素氮和血肌酐升高。在长期接触贫铀的情况下,易发生免疫毒性、胚胎毒性和肝毒性,罹患肿瘤风险增加[3]。
1.2 镉
镉半衰期长达10~30年,不易被生物清除,从而导致其易在生物体内累积[4]。镉暴露可破坏中枢神经系统功能,对另一靶器官肾的主要损伤部位是近端小管,可导致线粒体电子传递链功能障碍和活性氧产生[5]。在单一暴露分析中,单独暴露于镉会显著增加患肾结石的风险,尿镉、钴与肾结石的风险呈正相关,此关联在老年人中尤为显著[6]。对于肥胖、高脂血症、代谢综合征等慢性病患者,镉暴露会进一步增加慢性肾病和肾衰竭风险[7]。
1.3 钴
在切尔诺贝利事故患者中出现的几种心血管损伤可能与钴暴露有关。钴暴露引起的心肌小病灶损伤和血流动力学变化可能会导致心律和血管紧张度紊乱[8]。钴还可能对神经系统造成影响,钴接触工人表现出与注意力和语言记忆困难有关的明显认知缺陷,还会增加精神类疾病和视力下降风险[9, 10]。
1.4 氡
氡是一种比空气重、具有放射性的气体,是镭衰变的结果。氡主要通过吸入进入人体,且氡衍生物是引发肺癌的第二危险因素,仅次于吸烟[11]。2010年室内氡暴露导致的肺癌死亡人数占韩国肺癌死亡总数的12.5%~24.7%[12];2012年,66个国家因氡导致的肺癌死亡总数为226 057例[13]。欧洲和北美地下矿井工人由于长期接触氡气,肿瘤发病率大幅增加,其中以肺癌风险最高[14]。
1.5 镭
镭的所有同位素都具有强烈的放射性,易诱发儿童和成人的骨肿瘤、生长障碍、骨软骨瘤和白内障等。镭与儿童恶性骨肿瘤发病率密切相关,年龄越小,影响越严重[15]。
1.6 碘
碘主要供给甲状腺激素合成。I131半衰期较长,大剂量I131会导致甲状腺肿大,甲状腺结节或萎缩,最终导致甲状腺癌发生率增加,且I131导致的甲状腺机能亢进患者中,60%会出现房颤现象[16]。
2. 用于清除放射性核素的多糖
放射性核素清除最常使用的螯合剂是二乙基三胺五乙酸(DTPA),但由于其选择性差,易与体内 Ca2+、Zn2+等有益金属离子发生配位反应,导致肾毒性、致畸性、胚胎毒性等多种副作用,目前已很少应用[17]。多糖多为天然来源,不仅能通过吸附清除放射性核素,还具有安全性好、生物相容性、清除率高等优点,是极具潜力的放射性核素清除剂。可用于有效清除放射性核素的多糖包括以下几种。
2.1 壳聚糖
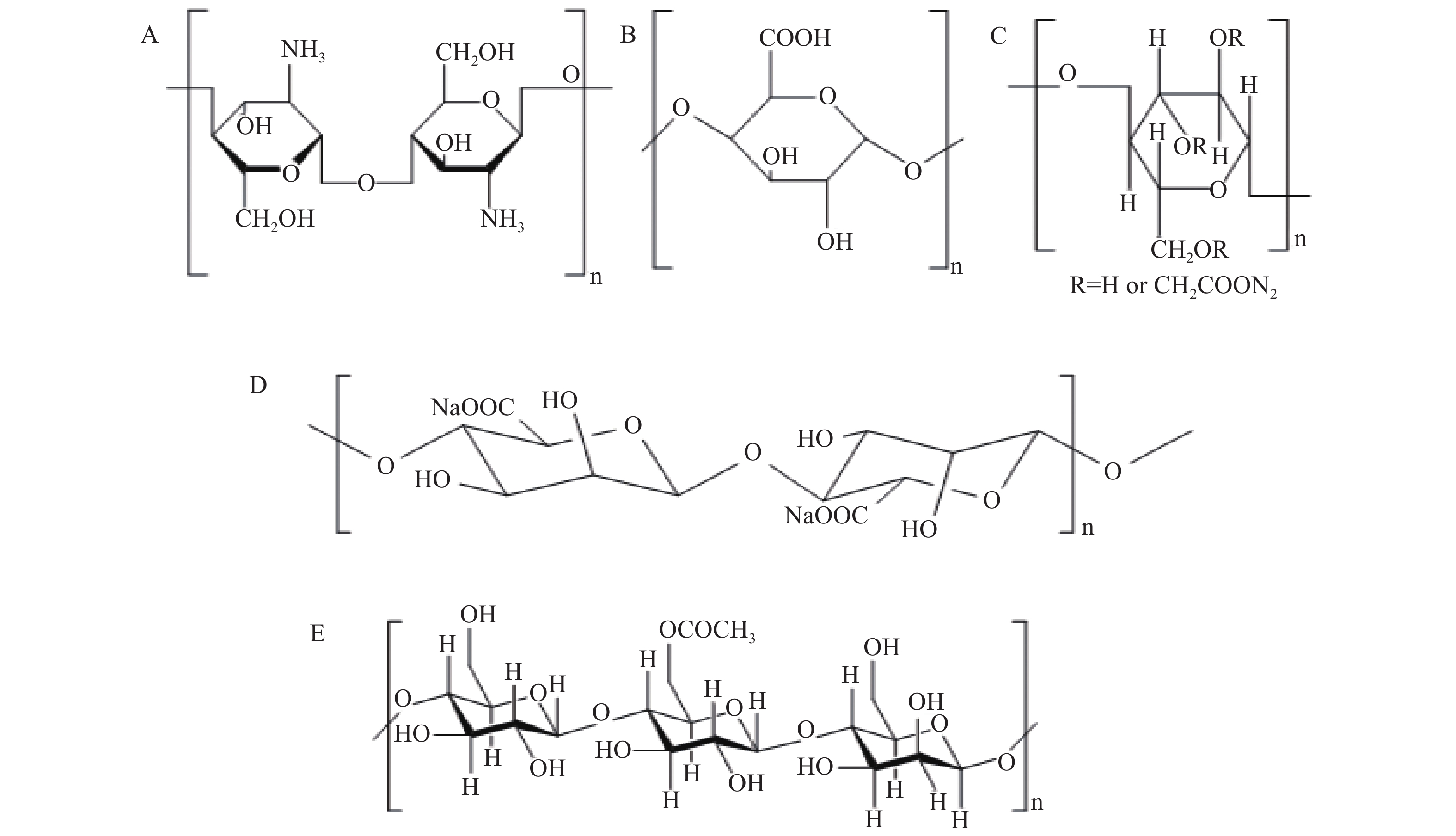
壳聚糖来源于甲壳质的脱乙酰化,由于分子内和分子间氢键的存在,使其具有规则的线性分子链和良好的结晶性能。壳聚糖还具有价廉、资源丰富、吸附能力强、可再生、抗拉强度高、生物降解性和抗菌性能好等优点,被认为是绿色吸附剂[18](图2A)。壳聚糖吸附机制可能包括配位作用、阳离子交换和静电吸附,壳聚糖的羟基团和氨基团还能与铯形成稳定配位键。影响壳聚糖吸附效果的因素主要为pH、温度和吸附时间等。当pH>2时,壳聚糖吸附效率随pH升高而提高;除铜在pH=8时吸附效率最高之外,壳聚糖对铯、铕、铜在pH=8时达到最大吸附效率。在弱酸性条件下吸附效率高可能是因为低浓度H+和H3O+降低了与阳离子壳聚糖的竞争,增加了壳聚糖的吸附位点。
壳聚糖可通过制备成凝胶、静电纺丝、微球等剂型来吸附核素。目前大多数壳聚糖吸附剂呈颗粒状,不利于官能团的暴露,减少了吸附位点,降低了吸附效率。通过制备成微球、无定形粉末、纤维等剂型,可大大增加表面积、暴露更多吸附位点以提高吸附性能。采用湿法纺丝制备的均匀纤维状壳聚糖吸附剂,比颗粒状壳聚糖具有吸附容量高、吸附速率快、比表面积大、机械强度优良等优点,纤维状壳聚糖对Co2+的吸附效率为4.3 mg/g,明显高于颗粒状壳聚糖(2.3 mg/g)[19]。
壳聚糖的实际应用受制于其酸性降解和热稳定性差、机械强度低、比表面积低等缺点,通过化学修饰可克服上述缺点,拓宽其实用价值。羧甲基壳聚糖是壳聚糖经羧甲基化后得到的一种线形水溶性高分子,因分子内含有大量的乙二胺四乙酸式结构而对金属离子具有很好的螯合吸附性能。与壳聚糖水凝胶相比,羧甲基壳聚糖超分子水凝胶在保湿能力、水溶性、生物降解性、生物相容性、抗氧化活性、抗菌性能等方面表现出更明显的优势。同时由于其具有更多的螯合基团,结合其本身吸附能力,因此对放射性核素清除效率更高[20]。壳聚糖与DTPA联用形成的纳米粒对吸入不溶性贫铀粒子具有明显吸附作用;结合肺灌洗,壳聚糖还能帮助DTPA进入细胞内,提高肺组织中贫铀的清除率[21]。
2.2 海藻酸盐
海藻酸盐由1,4-β-D-甘露糖醛酸和α-L-古洛糖醛酸组成(图2D),它可与二价、三价阳离子配位,是一种无毒、可生物降解的低成本天然多糖[22]。
海藻酸盐吸附核素离子有两种方式:物理吸附和化学吸附。物理吸附主要基于静电吸附;化学吸附则分为两种:一种是阳离子之间的离子交换,另一种是核素与海藻酸盐的羟基和羧基产生配位反应,形成配位物[23]。海藻酸盐的吸附效率显著受pH变化的影响。由于其含有大量的羧基和羟基官能团,在低pH溶液中,吸附位点逐渐被质子化,这会导致官能团的螯合作用减弱,并且离子之间产生的静电斥力进一步降低了吸附能力[24-25]。海藻酸盐吸附还依赖温度变化,温度通过影响海藻酸盐表面化学结构来控制吸附。由于吸附过程是吸热过程,适当提高温度可提高吸附容量;但高温也会导致孔径变大,从而使脱吸附率比吸附率高,降低吸附效率[26]。
海藻酸盐可与其他螯合剂联用发挥清除作用。聚丙烯酰胺、海藻酸钠和二乙烯三胺五乙酸混合后制备水凝胶,可有效清除小鼠皮肤创面的放射性锶,并能有效阻止放射性锶通过皮肤创面的吸收。此外,水凝胶剂型还可有效促进放射性核素污染伤口中的肉芽组织形成,促进创面愈合[27]。
不同多糖联用,可通过不同机制协同发挥核素清除作用,提高清除效率。如将海藻酸盐与浒苔多糖复合,为海藻酸钠凝胶引入了更多的活性吸附位点,提高了对重金属的吸附效率[28]。
2.3 羧甲基纤维素
纤维素经羧甲基化后可得到羧甲基纤维素(CMC)(图2C),其结构中含有丰富的羟基和羧基,被称为超吸附材料。同时CMC具有亲水性、生物黏附性、pH敏感性等优点,常用于药物递送和其他生物医学研究[29]。
载普鲁士蓝羧甲基纤维素大孔纳米纤维膜能有效吸附铯,这主要是由于羧甲基纤维素钠纳米纤维膜具有表面积大和高度多孔的结构,增加了吸附位点,提高了吸附容量[30]。通过植酸改性的CMC对137Cs具有特异吸附性,且能大大提高137Cs吸附速率[31]。在Cu(Ⅱ)离子交联法制备的羧甲基纤维素水凝胶中引入聚乙二醇6 000以增加水凝胶羟基数量。该水凝胶在吸水溶胀后,其内部孔隙和表面积显著增加,从而暴露了更多吸附位点,提高了整体吸附效果[32]。羧甲基纤维素钠温敏水凝胶可实现低温吸附、高温脱吸附,其吸附UO22+的主要机制是含氧基团和U(Ⅵ)之间能形成复合物[33]。
2.4 魔芋葡甘聚糖
葡甘聚糖主要来源于自然界广泛存在的魔芋,因此又称为魔芋葡甘聚糖(KGM)。KGM具有良好的持水能力、稳定性、成膜性、增稠性、乳化性等。KGM主链的化学结构是由D-葡萄糖和D-甘露糖缩合形成的共聚物,具有丰富的羧基和羟基[34]。魔芋葡甘聚糖通过羧甲基化可得到羧甲基魔芋葡甘聚糖,能获得更大的吸附容量。其吸附过程主要是通过离子交换和吸附剂表面羟基的配位完成,是一个吸热和自发的过程。KGM带有负电荷,能通过静电作用和氢键提高吸附力;引入结冷胶后,能形成双网络凝胶微球,该凝胶微球具有极高的机械强度,在强酸条件下仍稳定性良好,对铀的最大吸附容量可达到98.10 mg/g[35]。一氯乙酸改性魔芋葡甘聚糖可吸附重金属离子,且羧甲基取代度越高,越有利于吸附。羧甲基魔芋葡甘聚糖可重复使用,且不会明显改变其吸附容量[36]。
2.5 果胶
果胶是一种天然多糖,也可作为可溶性膳食纤维。果胶聚合物链主要通过1,4-α-半乳糖醛酸单元构成。果胶吸附核素主要通过其结构中的羟基,酯化度较低的果胶具有较强的吸附能力。儿童服用果胶后放射性铯含量降低了33%,也可显著降低对铀的吸收[37]。
将果胶和普鲁士蓝制备成珠状杂化吸附剂,可有效吸附铯,其吸附容量可达(36.5±0.8)mg/g,该杂化吸附剂具有明显协同作用,吸附效率显著高于预期。可能普鲁士蓝粉末的存在改变了果胶结构,增加了吸附位点的暴露,使更多铯离子能被捕获和吸附[38]。
多糖清除核素的机制是主要通过其结构中的羟基和羧基等基团与核素发生配位、阳离子交换和静电吸附等作用。除上述多糖之外,甲壳素、木薯淀粉等通过静电作用或螯合作用也可发生吸附,但因其溶解性差、弱机械性能而限制了应用[39, 40]。
3. 未来与展望
核技术在军事、医学等领域的广泛应用,给人们带来便捷的同时,也增加了核辐射暴露的风险。尤其是近期福岛核电站废水的排放更是给我国生态环境、人民健康带来了巨大的安全隐患和挑战。目前,放射性核素清除面临着品种少、清除效率低、副作用大等问题,对螯合剂、吸附剂、促排剂等的深入广泛研究将为清除放射性核素提供更高效、安全的方案。多糖具有安全、生物相容性好、吸附效率高等优势,将其与凝胶、纳米粒、纳米纤维、多孔微球等剂型有机结合,将进一步提高放射性核素吸附效率,为核战争、核事故等紧急条件下高效、安全清除核素提供新策略。
-
[1] FEHSEL K, CHRISTL J. Comorbidity of osteoporosis and Alzheimer’s disease: is 'AKT '-ing on cellular glucose uptake the missing link?[J]. Ageing Res Rev, 2022, 76:101592. doi: 10.1016/j.arr.2022.101592 [2] TATULIAN S A. Challenges and hopes for Alzheimer’s disease[J]. Drug Discov Today, 2022, 27(4):1027-1043. doi: 10.1016/j.drudis.2022.01.016 [3] GUAN L S, MAO Z, YANG S, et al. Dioscin alleviates Alzheimer’s disease through regulating RAGE/NOX4 mediated oxidative stress and inflammation[J]. Biomed Pharmacother, 2022, 152:113248. doi: 10.1016/j.biopha.2022.113248 [4] XU W M, LIU X Y, HE X H, et al. Bajitianwan attenuates D-galactose-induced memory impairment and bone loss through suppression of oxidative stress in aging rat model[J]. J Ethnopharmacol, 2020, 261:112992. doi: 10.1016/j.jep.2020.112992 [5] 徐卫凡, 徐武牧, 丁卢颖, 等. 巴戟天丸防治D-半乳糖损伤成骨细胞骨丢失的作用及机制研究[J]. 药学实践与服务, 2023, 41(3):155-159. [6] 寇柏鑫, 于前, 闫妍, 等. 基于网络药理学和16S rDNA测序的当归六黄汤合煎与单煎治疗阴虚甲亢作用的差异研究[J]. 中草药, 2023, 54(8):2488-2501. [7] SIDDIQUI J A, PARTRIDGE N C. Physiological bone remodeling: systemic regulation and growth factor involvement[J]. Physiol Bethesda Md, 2016, 31(3):233-245. [8] AN J, YANG H, ZHANG Q, et al. Natural products for treatment of osteoporosis: the effects and mechanisms on promoting osteoblast-mediated bone formation[J]. Life Sci, 2016, 147:46-58. doi: 10.1016/j.lfs.2016.01.024 [9] NIU B Y, LIAO K X, ZHOU Y X, et al. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy[J]. Biomaterials, 2021, 277:121110. doi: 10.1016/j.biomaterials.2021.121110 [10] TSIKAS D. Assessment of lipid peroxidation by measuring malondialdehyde(MDA)and relatives in biological samples: analytical and biological challenges[J]. Anal Biochem, 2017, 524:13-30. doi: 10.1016/j.ab.2016.10.021 [11] SAED-MOUCHESHI A, SOHRABI F, FASIHFAR E, et al. Superoxide dismutase(SOD)as a selection criterion for triticale grain yield under drought stress: a comprehensive study on genomics and expression profiling, bioinformatics, heritability, and phenotypic variability[J]. BMC Plant Biol, 2021, 21(1):148. doi: 10.1186/s12870-021-02919-5 [12] GLORIEUX C, CALDERON P B. Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach[J]. Biol Chem, 2017, 398(10):1095-1108. doi: 10.1515/hsz-2017-0131 [13] WAGHELA B N, VAIDYA F U, RANJAN K, et al. AGE-RAGE synergy influences programmed cell death signaling to promote cancer[J]. Mol Cell Biochem, 2021, 476(2):585-598. doi: 10.1007/s11010-020-03928-y [14] ZHUANG Y, WU H R, WANG X X, et al. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/akt-mediated Nrf2 signaling pathway[J]. Oxid Med Cell Longev, 2019, 2019:7591840. [15] LIU M, WANG R B, XING J H, et al. Atractylenolide inhibits apoptosis and oxidative stress of HTR-8/SVneo cells by activating MAPK/ERK signalling in preeclampsia[J]. Phytomedicine, 2021, 93:153773. doi: 10.1016/j.phymed.2021.153773 期刊类型引用(10)
1. 严莹莹,王曼莉,李锦鸿,李承龙,洪光博,黄丽平. 石菖蒲中β-细辛醚的提取工艺优化及其体外抗氧化活性研究. 中国现代应用药学. 2024(01): 18-26 .  百度学术
百度学术2. 孙洁颖,米宝丽,郭芮嘉,王晓,赵楠,刘威. 基于正交实验和Box-Behnken 响应面法优选白芨多糖提取工艺. 广州化工. 2024(05): 116-120 .  百度学术
百度学术3. 周晓丹,赵文静,吴汐柔,邹婕,李鹏晨,汪梦杰,李曹龙. 响应面法优化蛹虫草多糖提取工艺研究. 广州化工. 2024(13): 17-20 .  百度学术
百度学术4. 石震方,伏小刚,高富成,徐垒,李建设,叶林. 基于响应曲面法的露地甘蓝移栽效率优化研究. 南方农机. 2024(24): 1-6 .  百度学术
百度学术5. 柯翠敏,李丝红,胡建萍,谢志新. 运用响应面分析法优化鬼针草总黄酮的提取工艺. 继续医学教育. 2022(03): 109-112 .  百度学术
百度学术6. 邱宏聪,刘布鸣,张华,马军花,吴文华. 复方金钱草颗粒的二次开发研究进展. 广西科学. 2022(01): 45-51 .  百度学术
百度学术7. 杨佳力,陈俊沲,华丽萍,张双双,徐珏雯,石森林. 正交设计与Box-Behnken响应面法优化逍遥散的制备工艺. 华西药学杂志. 2022(03): 285-291 .  百度学术
百度学术8. 解杨,钟凌云,薛晓,王卓,宋金菊,李家晴,张青,王毅彬,曾妍. 基于多指标-响应曲面法优选炆地黄炮制工艺及成分与色泽相关性分析. 中国中药杂志. 2022(18): 4927-4937 .  百度学术
百度学术9. 徐敬朴,李德强,郑丽亚,程新杰,卞广丽. 响应面法优化积雪草的微波提取工艺. 西北药学杂志. 2021(01): 1-5 .  百度学术
百度学术10. 郝明洁,胡德栋,陈晓娜. 撞击流环隙喷嘴在超临界流体技术制备药物超微颗粒中的应用研究. 能源化工. 2020(04): 45-50 .  百度学术
百度学术其他类型引用(7)
-





 下载:
下载:
 下载:
下载: