-
慢性脑缺血(CCI)是指各种原因引发的长期大脑血灌流量不足,在血管性痴呆和阿尔茨海默病等神经系统疾病的发展过程发挥重要作用[1-2]。长期慢性脑缺血会导致慢性神经炎症、海马自噬异常、脑部神经元凋亡[3-4],为此,CCI已经严重威胁人类的健康。肠道被称为人类的“第二大脑”,肠道微生物参与周围神经系统和中枢神经系统的双向调节,并通过脑-肠互动(即脑-肠轴)与机体的神经系统密切相关[5]。研究证明中枢系统疾病会导致肠道菌群失调[6],患者发生脑卒中后,不仅会出现胃肠道并发症,还会发生肠道菌群失调,例如肠道菌群中的阿克曼菌属(Akkermansia)会发生变化[7]。同时,肠道菌群失调也推动了脑卒中等疾病的发展,文献报道厚壁菌门增加与认知功能损伤有一定的关联[8-9]。因此,肠道菌群参与了脑部神经系统疾病的进展。中药复方虎杖清脉饮是上海市著名中医脉管病专家奚九一教授数十年临床诊治经验的总结,临床上对辩证属“热郁毒聚,脉滞络痹”的脑小血管损伤疗效显著。有报道虎杖清脉饮通过调节p38和NF-κB信号通路,抑制高糖诱导的人视网膜毛细血管内皮细胞的损伤[10],提示虎杖清脉饮具有抗炎和抗凋亡作用。然而,虎杖清脉饮对慢性脑缺血的治疗作用缺乏临床前研究,作用机制尚不清楚。因此,本实验研究虎杖清脉饮对慢性脑缺血小鼠认知功能的影响,并基于肠道菌群角度初步探讨其作用机制,为防治慢性脑缺血提供新的思路。
-
24只C57BL/6J雄性小鼠(20±5) g,购自常州卡文斯实验动物有限公司(动物合格证号:202112546)。饲养于海军军医大学药学系动物房,饲养条件为温度(24 ± 2) ℃,相对湿度70 %,每天光照时间为08:00至20:00,自由进食饮水。所有操作均符合海军军医大学实验动物伦理要求。
-
虎杖清脉饮由上海中医药大学附属岳阳中西医结合医院提供。由虎杖、垂盆草、豨莶草、连翘、黄芪、鸡血藤和川芎七味常用中药制备[10]。所有药品粉碎成粗粉,蒸馏水浸泡,煮沸2次,每次40 min,合并滤液,浓缩至0.675 g/ml。银杏叶提取物由上海阿拉丁生物化学技术有限公司提供。
-
Morris 水迷宫(上海奥尔科特生物科技有限公司);组织脱水机、包埋机(武汉俊杰电子有限公司);倒置生物显微镜(日本尼康);病理切片机(上海俫卡仪器有限公司);DYY-6C 型核酸电泳仪、ND2000 型光度计、9700 型PCR 仪、Quantus™型高灵敏荧光计、Miseq PE300测序平台(美吉生物公司)。
-
24只小鼠适应性饲养7 d后,随机分为4组:假手术组、模型组、阳性药组(银杏叶提取物)、虎杖清脉饮组,每组6只。小鼠腹腔注射1 %戊巴比妥钠(10 ml/kg)麻醉,并暴露颈总动脉(CCAs)。在体视显微镜下,将微型弹簧圈(钢丝直径0.08 mm,线圈内径0.18 mm,螺距0.5 mm,总长度2.5 mm)螺旋旋转固定在双侧颈总动脉上致颈总动脉狭窄使脑慢性灌注不足,建立BCAS模型。然后缝合手术部位,并对术后小鼠进行护理,直到它们意识清醒。造模6 w后,各组开始进行灌胃给药12 w。阳性药组给予银杏叶提取物(GBE)30 mg/kg,虎杖清脉饮组(HZQMY)给予虎杖清脉饮13.5 mg/kg,假手术组(Sham)和模型组(BCAS)给予等量生理盐水处理。
-
Morris水迷宫实验分为前5 d的定位航行实验和第6 d的空间探索实验。定位航行实验:主要用于测试小鼠的空间学习能力。实验历时5 d,每天每只小鼠训练3次。训练时随机选择一个象限开始,按顺时针方向训练3次。每次将该象限的池壁中点作为入水点,将小鼠面向池壁轻轻放入水中,尽量保持每次入水方式的一致,减少人为影响。小鼠每次分别从不同象限入水寻找逃逸平台,若小鼠找到登上平台,并停留5 s以上,则计时结束,水迷宫装置自动记录小鼠从入水到寻台成功的时间,即为逃避潜伏期。如果在60 s后仍未登上平台,计时也将停止,将小鼠人为引上平台休息1 min,且当次的逃避潜伏期记录为60 s。
空间探索实验:主要用于测试小鼠的空间记忆能力。在定位航行实验结束的第2d,撤除平台,选择与第四象限最远的第二象限池壁中点为小鼠入水点,将小鼠面向池壁轻轻放入池中,使小鼠自由游泳60 s并记录这60 s内小鼠在原平台的停留时间、穿过原平台所在位置的次数作为判断小鼠空间记忆能力差异的指标。
-
治疗结束后,每组各取6只小鼠,用1 %戊巴比妥钠(10 ml/kg)腹腔注射麻醉,4 %多聚甲醛灌注后,取脑石蜡包埋,切片,厚度5 μm,用于LFB(Luxol Fast Blue stain)染色。将石蜡切片常规脱蜡至水,加入LFB染色液于60 ℃烤箱染色3 h。95 %乙醇洗去多余染色液,蒸馏水冲洗。Luxol分化液分化15 s,70 %乙醇分色。重复分化步骤,直至胼胝体和皮质之间形成鲜明对比。梯度乙醇脱水,二甲苯透明,中性树胶封片,在显微镜下采集图片。
-
末次给药后禁食12 h,脱颈椎处死小鼠,处死方法符合动物福利伦理要求,无菌条件下取盲肠内容物,置于液氮中速冻保存。送至美吉生物技术股份有限公司进行DNA提取,合格样品进一步进行高通量测序。
-
运用美吉生物信息云(https://cloud.majorbio.com/)进行数据的处理,利用美吉生信云UParse软件(v7.0.1090)进行OTU(operational taxonomic units)分析、Alpha多样性分析、稀释曲线分析;利用美吉生信云tax_summary_a文件夹中的数据表和R语言(version 3.3.1)工具统计和作图进行物种组成分析;利用Qiime计算Beta多样性距离矩阵;利用美吉生信云进行线性判别分析(LEfSe),计算多级物种之间的差异。
-
采用统计软件SPSS 19.0、GraphPad 9.0进行分析。符合正态分布的计量数据以(
$\bar{x}$ ±s)表示,多组间比较采用单因素方差分析(One-Way ANOVA)。以P<0.05表示有显著性差异,以P<0.01表示有极显著性差异。 -
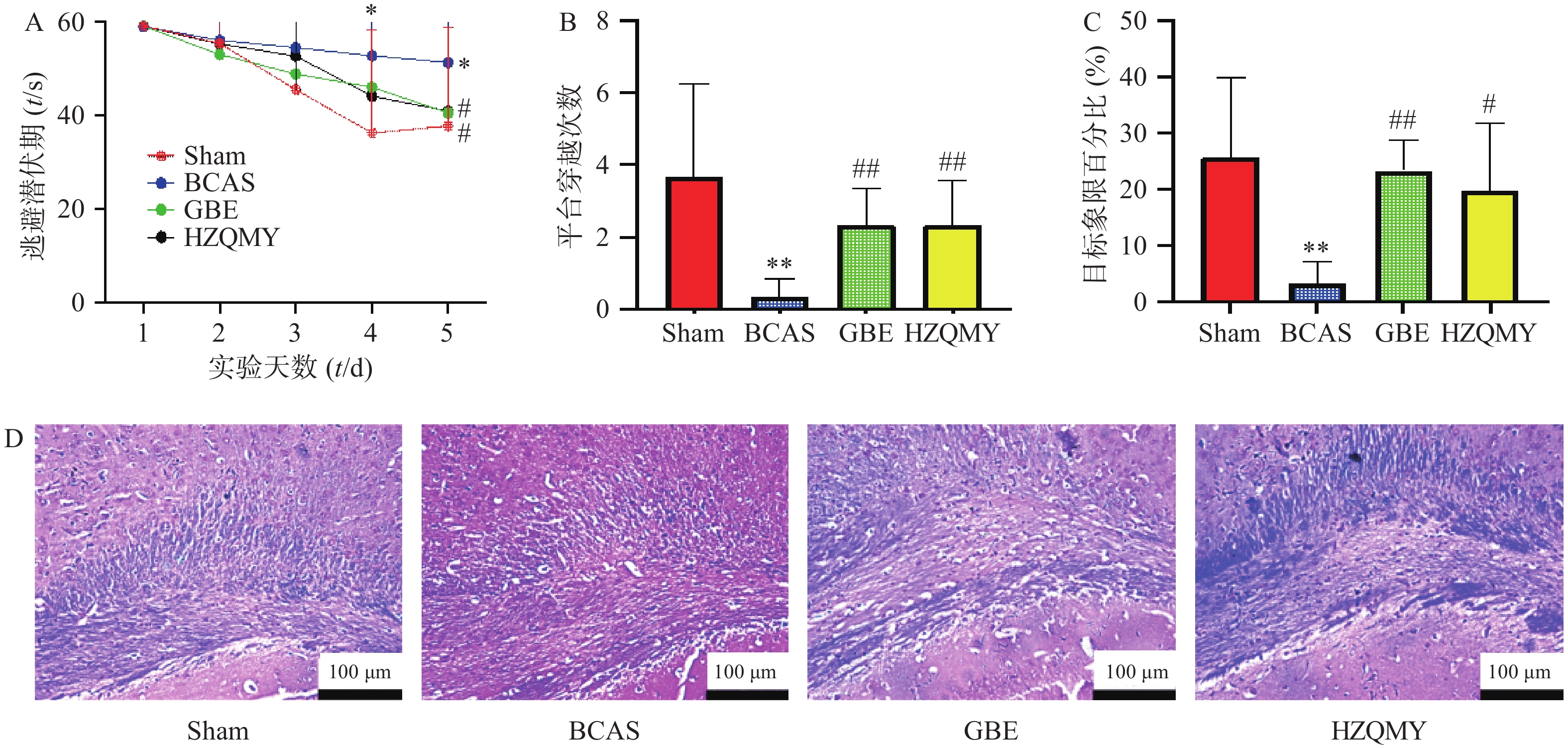
如图1A所示,假手术组小鼠从水迷宫实验第1 d开始到第5 d,逃避潜伏期在不断减少。而模型组小鼠随天数的增加,找到平台的时长并没有明显缩短,并且与假手术组相比,第4天和第5天逃避潜伏期有显著性差异(P<0.05),各给药组小鼠逃避潜伏期在逐渐减少,第5天阳性药组、虎杖清脉饮组与模型组之间的逃避潜伏期差异有统计学意义(P<0.05)。如图1B所示,模型组小鼠的跨越平台次数显著少于假手术组(P<0.01)。而虎杖清脉饮组的跨越平台次数显著大于模型组(P<0.01)。如图1C所示,模型组小鼠的目标象限百分比显著少于假手术组(P<0.05),阳性药组和虎杖清脉饮组的目标象限百分比均显著大于模型组(P<0.05),提示虎杖清脉饮可以改善慢性脑缺血小鼠的学习记忆能力。如图1D所示,假手术组中,小鼠胼胝体的LFB染色,髓鞘排列整齐,无水肿、碎裂及空泡的形成。模型组,LFB着色浅,髓鞘崩解,部分髓鞘空泡化。经银杏叶提取物和虎杖清脉饮治疗,髓鞘病变减轻,染色加深,提示虎杖清脉饮可以改善慢性脑缺血小鼠的白质损伤。
-
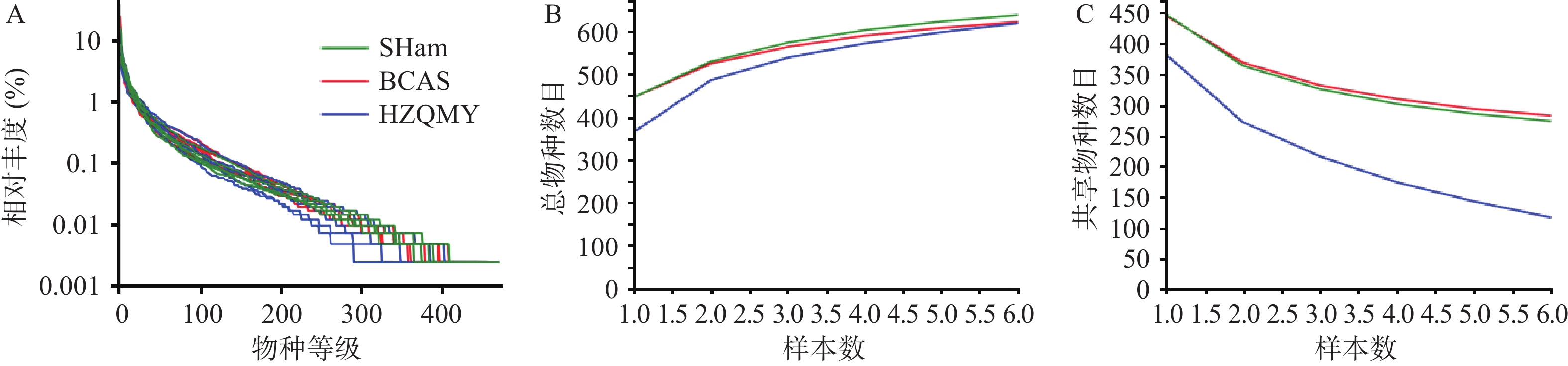
各组小鼠肠道菌群优化后序列数目为964830,碱基数目为398083175,平均长度为412 bp,序列长度分布在400~440 bp内。图2A为3组小鼠肠道微生物等级丰度曲线,从丰度曲线可以看到各组在缓慢平稳下降,说明各组小鼠肠道菌群物种丰富度高并且分布均匀。图2B、2C所示,总物种曲线可分析样本包含的物种总和,共享物种曲线可分析样本共享物种数目,本研究各组总数目和共享项目增加或减少趋势均逐步减缓并趋于平坦,表明各组样本量满足评估物种丰富度和核心物种数的要求。
-
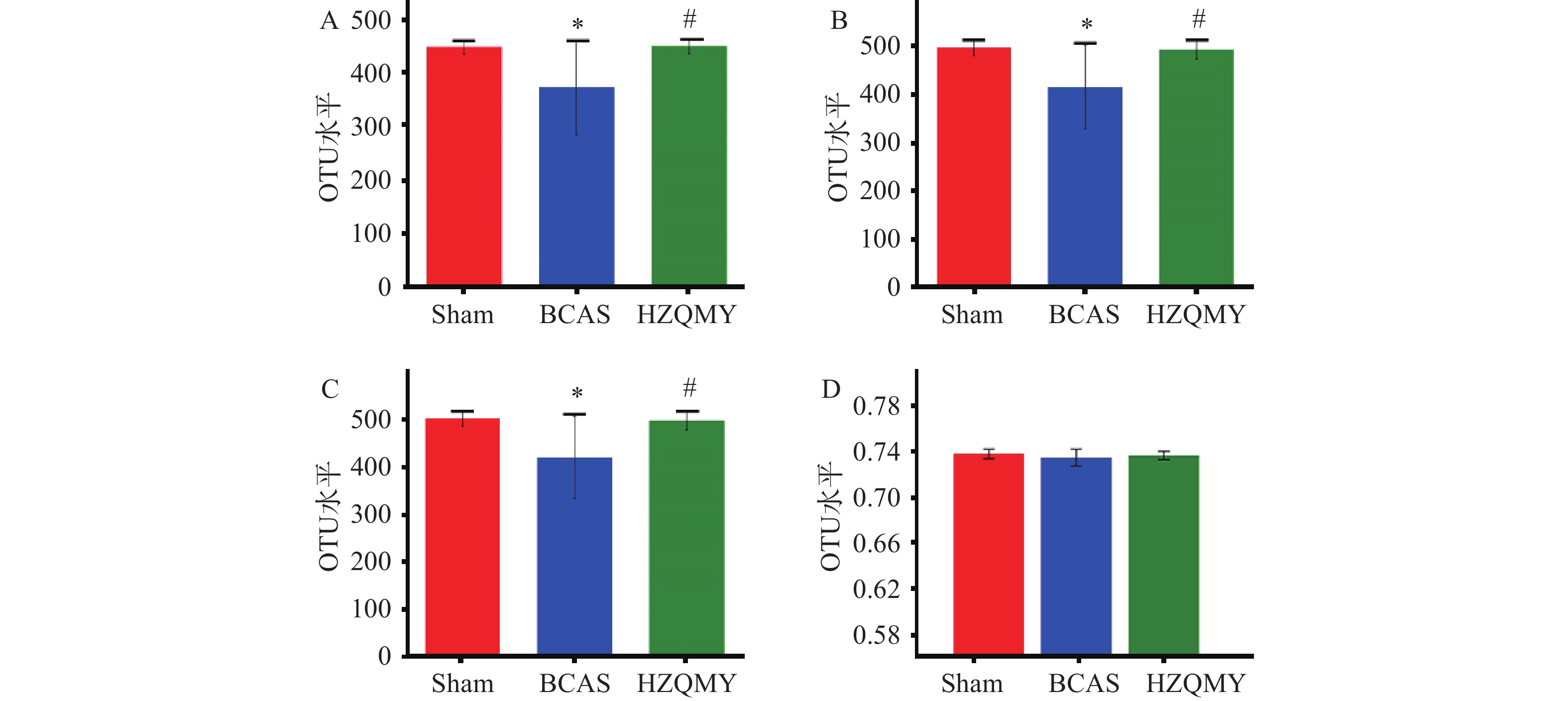
如图3A、3B、3C所示,丰富度实际观测值指数、物种丰富度ACE指数和物种丰富度Chao1指数可解释生物群落丰富度。相较于假手术组,模型组小鼠肠道菌群丰富度显著下降(P<0.05);相较于模型组,虎杖清脉饮组肠道菌群丰富度显著升高(P<0.05),说明虎杖清脉饮可以提升模型组小鼠肠道菌群的丰富度。如图3D所示,3组小鼠肠道菌群的Shannon均匀度测量指数无明显差异。综上,模型组慢性脑缺血小鼠菌群的物种丰富度明显下降,虎杖清脉饮可提升慢性脑缺血小鼠菌群的物种丰富度。
-
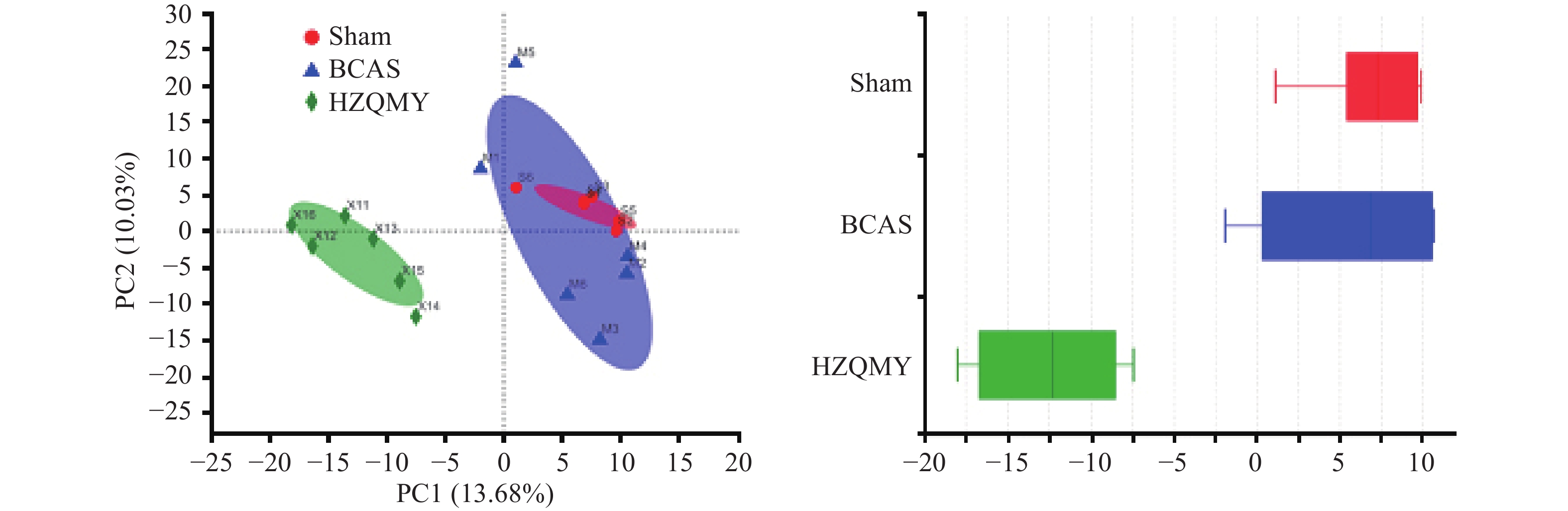
通过主成分分析(PCA)各组小鼠样本菌群群落组成差异,运用反差分解,在图中通过距离反映不同组之间的组成差异,如样本物种组成越相似,反映在主成分分析图中的距离越近。假手术组和模型组聚集在一起,说明模型组对肠道菌群的影响较小。而虎杖清脉饮组与模型组距离较远,说明慢性脑缺血小鼠肠道菌群改变较小,虎杖清脉饮具有调控模型组小鼠肠道菌群组成的作用(图4)。
-
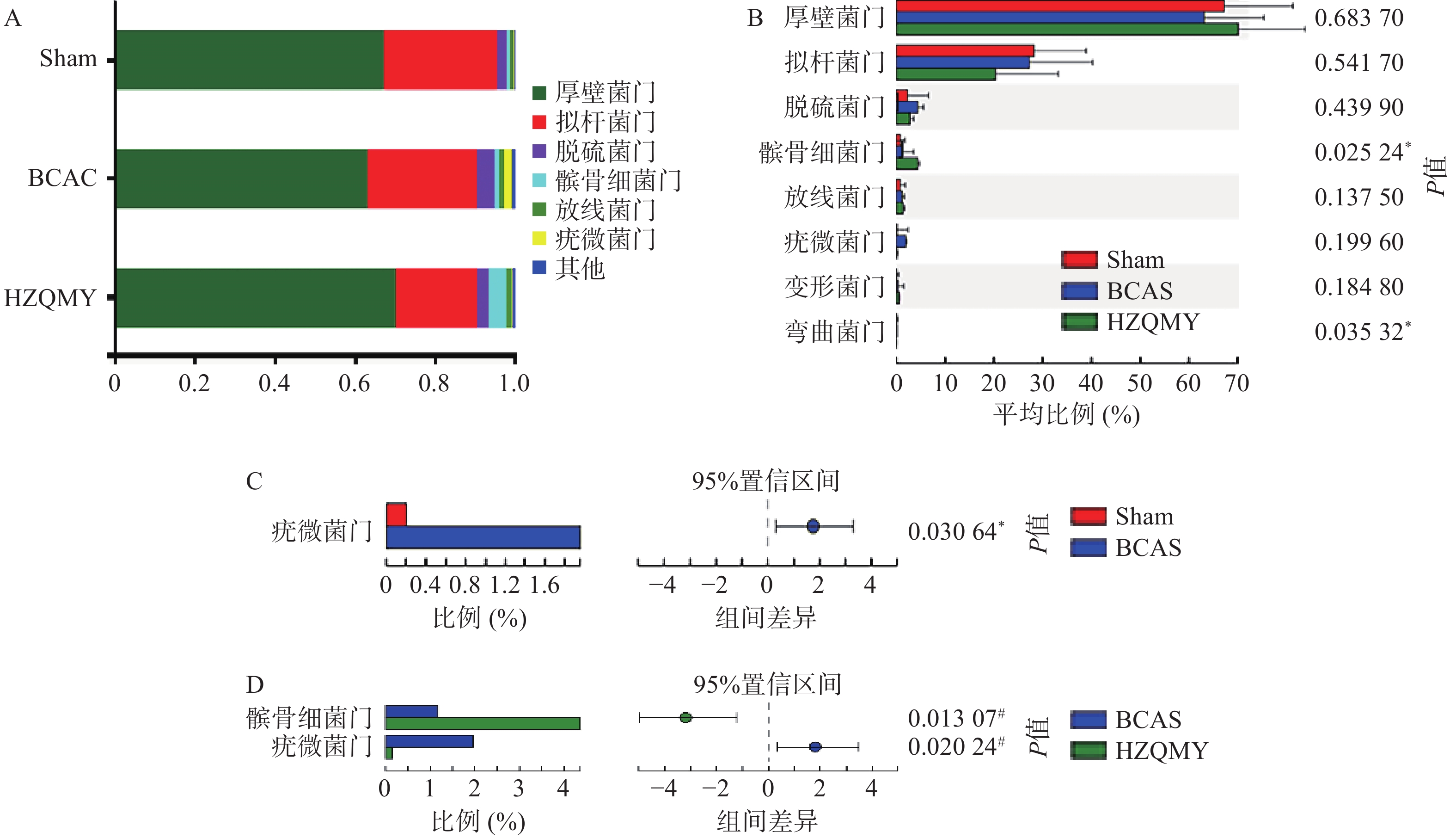
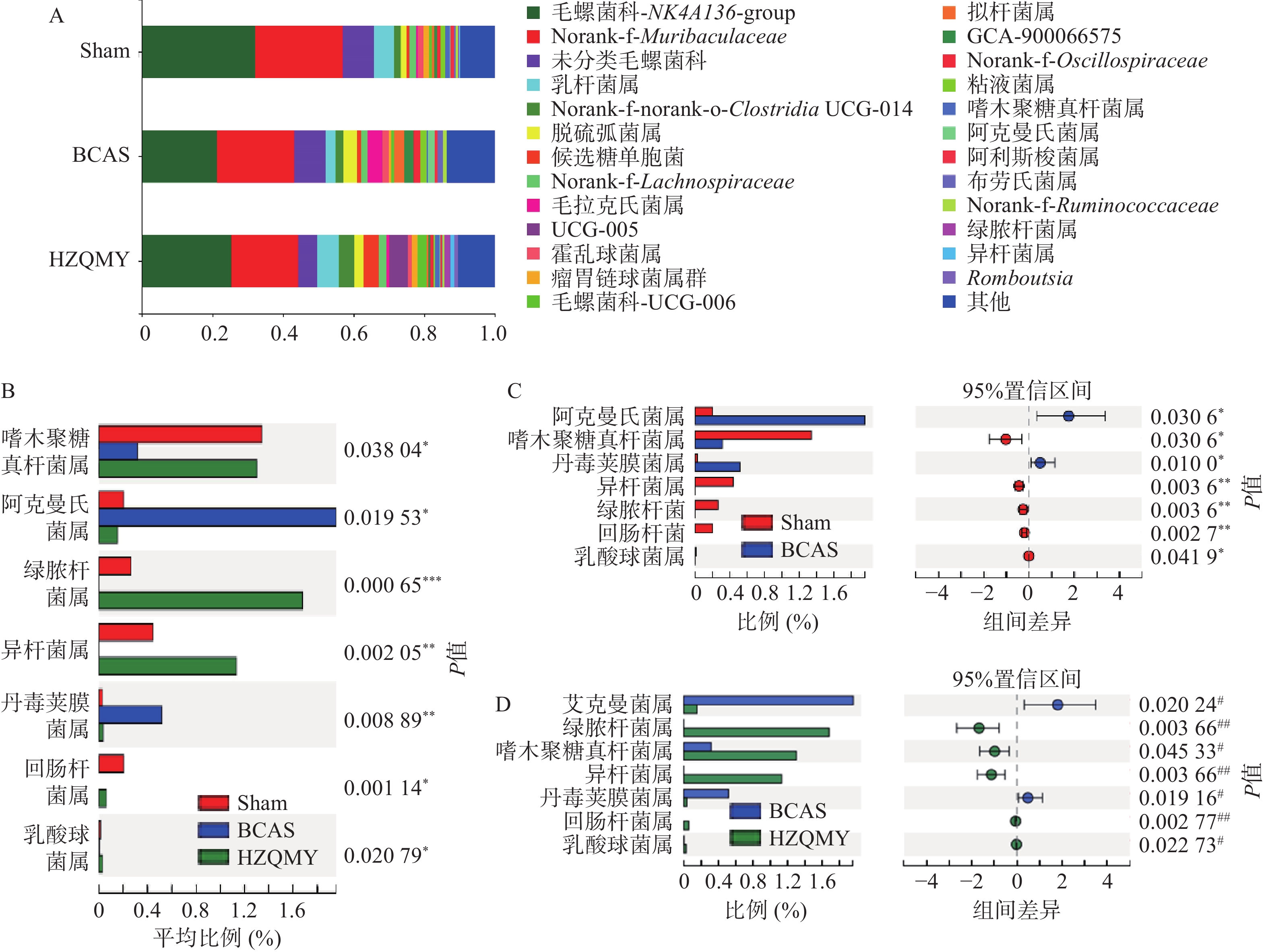
门水平上,3组小鼠肠道中占主导地位的微生物主要有厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidota)、脱硫菌门(Desulfobacterota)、髌骨细菌门(Patescibacteria)、放线菌门(Actinobacteriota),其中后壁菌门和拟杆菌门为优势门类(图5A)。由图5B所示,各组小鼠肠道菌群中髌骨细菌门
和弯曲菌门丰度有显著性差异(P<0.05)。如图5C所示,相较于假手术组,模型组疣微菌门丰度显著升高(P<0.05)。如图5D所示,相较于模型组,虎杖清脉饮组小鼠的疣微菌门丰度显著降低(P<0.05),髌骨细菌门丰度显著增加(P<0.05)。门水平差异性分析显示,慢性脑缺血小鼠疣微菌门丰富度增加;虎杖清脉饮降低慢性脑缺血小鼠疣微菌门和增加髌骨细菌门的群落丰度。 -
属水平上,3组小鼠肠道中优势菌为毛螺菌科_NK4A136_group、norank_f_Muribaculaceae和乳杆菌属(Lactobacillus)等(图6A)。如图6B所示,3组小鼠的肠道菌群中,阿克曼菌属(Akkermansia)、绿脓杆菌(Turicibacter)、嗜木聚糖真杆菌属(Eubacterium_xylanoPhilum_group)、异杆菌属(Allobaculum)、丹毒荚膜菌属(Erysipelatoclostridium)、回肠杆菌(Ileibacterium)、乳酸球菌属(Lactococcus)群落丰度具有显著性差异(P<0.05,P<0.01)。如图6C所示,与假手术组相比,模型组阿克曼菌属、丹毒荚膜菌属丰度显著上升(P<0.05);嗜木聚糖真杆菌属、异杆菌属、绿脓杆菌、回肠杆菌和乳酸球菌属丰度显著下降(P<0.05,P<0.01)。如图6D所示,与模型组相比,虎杖清脉饮组的阿克曼菌属、丹毒荚膜菌属丰度显著下降(P<0.05);嗜木聚糖真杆菌属、异杆菌属、绿脓杆菌、回肠杆菌和乳酸球菌属丰度显著上升(P<0.05,P<0.01)。
-
如表1所示,LEfSe可以检测各组之间存在显著丰度差异特征。相较于假手术组,模型组疣微菌门(P_Verrucomicrobiota)、疣微菌纲(c_Verrucomicrobiae)、疣微菌目(o_Verrucomicrobiales)、阿克曼菌科(f_Akkermansiaceae)等相对丰度显著上调;嗜木聚糖真杆菌属(g_Eubacterium_xylanophilum_group)、罗氏菌属(g_Roseburia)、回肠杆菌属(g_Ileibacterium)等相对丰度显著下调,表明慢性脑缺血小鼠上述肠道菌群丰度发生显著性变化。
表 1 各组小鼠肠道菌群LEfSe多级物种差异判别分析
菌群 模型组vs
假手术组虎杖清脉饮组vs
模型组疣微菌门(P_Verrucomicrobiota) ↑ ↓ 疣微菌纲(c_Verrucomicrobiae) ↑ ↓ 疣微菌目(o_Verrucomicrobiales) ↑ ↓ 克里斯滕菌目(o_Christensenellales) ↑ 阿克曼菌科(f_Akkermansiaceae) ↑ ↓ 克里斯滕菌科(f_Christensenellaceae) ↑ 粪芽孢菌属(g_Coprobacillus) ↑ ↓ 阿克曼菌属(g_Akkermansia) ↑ ↓ 嗜胆菌属(g_Bilophila) ↑ 克里斯滕菌属
(g_Christensenellaceae_R-7_group)↑ 丹毒荚膜菌属(g_Erysipelatoclostridium) ↑ ↓ 嗜木聚糖真杆菌属(g_Eubacterium_xylanophilum_group) ↓ 罗氏菌属(g_Roseburia) ↓ 未分类克里斯滕森菌科(g_unclassified_f_Christensenellaceae) ↓ 异杆菌属(g_Allobaculum) ↓ ↑ 理研菌属(g_Rikenella) ↓ 绿脓杆菌属(g_Turicibacter) ↓ ↑ g_Tyzzerella ↓ 回肠杆菌属(g_Ileibacterium) ↓ 蓝细菌纲(c_Cyanobacteriia) ↓ f_Tannerellaceae ↓ f_norank_o_Chloroplas ↓ g_norank_f_norank_o_Chloroplast ↓ 副杆菌属(g_Parabacteroides) ↓ o_PeptostrePtococcales-Tissierellales ↑ 髌骨细菌门(P_Patescibacteria) ↑ c_Saccharimonadia ↑ o_Saccharimonadales ↑ f_Saccharimonadaceae ↑ 丹毒丝菌科(f_Erysipelotrichaceae) ↑ 消化链球菌科(f_Peptostreptococcaceae) ↑ g_Candidatus_Saccharimonas ↑ g_UCG-005 ↑ g_Romboutsia ↑ 嗜木聚糖真杆菌属(g_Eubacterium_xylanophilum_group) ↑ g_norank_f_Atopobiaceae ↑ 安德克氏菌属(g_Adlercreutzia) ↑ 分节丝状菌属(g_Candidatus_Arthromitus) ↑ g_Lachnospiraceae_UCG-006 ↑ g_NK4A214_group ↑ 注:↑表示相对丰度增加,↓表示相对丰度降低。 相较于模型组,虎杖清脉饮组疣微菌纲(c_Verrucomicrobiae)、蓝细菌纲(c_Cyanobacteriia)、疣微菌门(P_Verrucomicrobiota)、阿克曼菌科(f_Akkermansiaceae)、阿克曼菌属(g_Akkermansia)、粪芽孢菌属(g_Coprobacillus)、丹毒荚膜菌属(g_Erysipelatoclostridium)、副杆菌属(g_Parabacteroides)等相对丰度显著下调;丹毒丝菌科(f_Erysipelotrichaceae)、消化链球菌科(f_PeptostrePtococcaceae)、绿脓杆菌属(g_Turicibacter)、异杆菌属(g_Allobaculum)、嗜木聚糖真杆菌属(g_Eubacterium_xylanophilum_group)等相对丰度显著上调。说明虎杖清脉饮通过调节嗜木聚糖真杆菌属、异杆菌属、绿脓杆菌、疣微菌、阿克曼菌和丹毒荚膜菌的丰度,达到治疗慢性脑缺血的治疗作用。
-
慢性脑缺血会损害脑血管,导致患者身体残疾、认知障碍或抑郁样行为。慢性脑缺血(CCI)被认为是血管性痴呆的一个病理生理标志[1-2],它是血管认知障碍(VCI)最严重的阶段和形式,与推理、学习和记忆方面的问题有关[11-12]。
近年来,大量的研究展示了肠道微生物对人体的生长发育有重要作用,当肠道微生物失调时,可能造成神经功能缺陷,进而出现神经退行性疾病[13-14]。肠道微生物参与周围神经系统和中枢神经系统的双向调节,并通过脑-肠轴调节参与机体的免疫、代谢、神经、内分泌等系统,进而影响多种神经系统疾病的发生[15-16]。研究显示,神经类疾病如阿尔兹海默症、抑郁症、帕金森疾病等都存在宿主肠道菌群紊乱的现象,并且发现肠道菌群能通过神经内分泌和自主神经实现脑-肠信号转导,从而参与调节宿主的神经-内分泌-免疫网络,影响宿主的情绪、认知或行为改变[17]。
虎杖清脉饮由虎杖、垂盆草、豨莶草、连翘、黄芪、鸡血藤和川芎组成,虎杖为君药,《药性论》曰:“虎杖,压一切热毒”。虎杖性味苦,寒,在方中起清热解毒、凉血祛瘀的功效。虎杖对神经具有保护作用,如虎杖苷可通过调控lncRNA-MALAT1发挥脑微血管内皮细胞保护作用;虎杖苷可以激活SIRT1信号通路减轻大鼠创伤性脑损伤[18]。连翘[19]、垂盆草及豨莶草[20-21]共为臣药。连翘味苦,微寒,属于清热解毒药,《医学衷中参西录》:连翘具升浮宣散之力,可流通气血。垂盆草味甘、淡,性凉,豨莶草性味辛、苦,寒,二者具有清热解毒,祛风通络之功。三药联用助虎杖清热解毒,又能增强通络的作用。鸡血藤[22]、黄芪共为方中佐药。鸡血藤温而不烈,活血祛瘀兼有补血功效;黄芪甘温,具有益气扶正之功,治久病气虚。三者可治血瘀气虚兼症,并能制君药和臣药苦寒,保护脾胃。川芎[23]辛温,既能祛风,又能行气活血,引诸药上行巅顶,为佐而兼使之用。其中的川芎嗪、阿魏酸、藁本内酯等[24-25]化学成分有明显脑保护作用。诸药合用,共奏清热解毒、活血通络之功。本研究通过减少颈总动脉的血流量,成功建立慢性脑缺血小鼠的模型。
通过水迷宫实验和LFB染色表明虎杖清脉饮对小鼠慢性脑缺血具有保护作用,从肠道菌群的角度观察发现慢性脑缺血将会导致小鼠菌群的物种的丰富度有所下降,并且其中疣微菌门、阿克曼菌属和丹毒荚膜菌属相对丰度上升,而嗜木聚糖真杆菌属和异杆菌属的相对丰度下降;虎杖清脉饮具有增加小鼠肠道菌群物种丰富度的作用,以及回调因为慢性脑缺血而导致菌属变化的作用。阿克曼菌隶属于疣微菌门,是肠道微生物中唯一一个来自疣微菌门的细菌。阿克曼菌[26] 是一种以肠黏膜层中的黏蛋白为食,降解黏蛋白和黏液层。当艾克曼菌异常增加时,会导致肠道屏障的受损,进而诱发肠道炎症和LPS大量进入血液影响其他脏器。有相关文献报道[27] ,在中风患者的肠道艾克曼菌中异常增长。同时,丹毒荚膜菌[28]的丰度也随着帕金森患者胃肠功能障碍增加而增加,这与我们的实验结果相符。嗜木聚糖真杆菌和异杆菌[29-30] 属于短链脂肪酸产生菌,短链脂肪酸[31-32] 可以降低肠道的通透性以及加强肠道屏障的防御力,调节免疫细胞趋化性、活性氧(ROS)释放以及细胞因子释放而具有抗炎功能,短链脂肪酸也可以促进脑内海马神经的发生和延长。虎杖清脉饮减少阿克曼菌属的相对丰度;增加嗜木聚糖真杆菌属和异杆菌属的相对丰度,进而维持肠道屏障功能的完整性,降低机体内的炎症反应,保护血脑屏障[5]。综上所述,虎杖清脉饮对慢性脑缺血小鼠的治疗作用与其对慢性脑缺血小鼠肠道菌群失调的改善作用具有相关性。
Effects of Huzhang Qingmai decoction on cognitive function and intestinal flora in mice with chronic cerebral ischemia
-
摘要:
目的 从肠道菌群角度探讨虎杖清脉饮对慢性脑缺血小鼠认知功能改善的作用机制。 方法 放置微弹簧圈于双侧颈总动脉致狭窄制备慢性脑缺血小鼠模型。灌胃给药12 w后,采用Morris水迷宫和LFB染色测定小鼠的认知功能和白质的损伤程度;提取各组小鼠盲肠内容物,进行16S rRNA基因V3+V4去扩增子信息采集与分析。 结果 水迷宫实验结果显示,虎杖清脉饮组小鼠逃避潜伏期显著缩短,跨越平台次数和目标象限百分比增加,学习记忆功能明显改善。LFB染色显示,模型组白质损伤严重;虎杖清脉饮组白质损伤程度较轻。16S rRNA测序结果显示,与模型组相比,虎杖清脉饮组小鼠肠道菌群中疣微菌门、阿克曼菌属和丹毒荚膜菌属的丰度显著降低(P<0.05),而嗜木聚糖真杆菌属和异杆菌属的丰度显著增加(P<0.05)。 结论 虎杖清脉饮具有改善慢性脑缺血小鼠认知功能的作用,其保护作用可能与调节慢性脑缺血小鼠肠道菌群有关。 Abstract:Objective To investigate the mechanism of action of Huzhang Qingmai decoction (HZQMY) on the improvement of cognitive function in mice with chronic cerebral ischemia from the perspective of intestinal flora. Methods A mouse model of chronic cerebral ischemia was established by placing microcoils around the bilateral common carotid arteries to induce bilateral carotid artery stenosis (BCAS). After 12 weeks of intragastric administration, the cognitive function of the mice was measured by the Morris water maze; the myelin damage was analyzed by LFB staining; The contents of the cecum of the mice in each group were extracted and analyzed by 16S rRNA sequencing. Results The results of the water maze experiment showed that the mice in the HZQMY group had a significantly shorter escape latency, increased the number of crossings platform and the percentage of target quadrants. LFB staining showed that the white matter damage in the model group was severe; the white matter damage in the HZQMY group was milder. The results of 16S rRNA sequencing showed that compared with the model group, the abundance of Verrucomicrobiota, Akkermansia, and ErysiPelatoclostridium capsulatum in the intestinal flora in HZQMY group was significantly reduced (P<0.05), while the abundances of Eubacterium_xylanoPhilum and Allobaculum were significantly increased (P<0.05). Conclusion The protective effect of HZQMY, which has the effect of improving cognitive function in mice with chronic cerebral ischemia, may be related to the regulation of intestinal flora in mice with chronic cerebral ischemia. -
Key words:
- HZQMY /
- chronic cerebral ischemia /
- cognitive function /
- 16S rRNA /
- intestinal flora
-
目前全球尚无预防和治疗新冠肺炎(COVID-19)确认的特效药物。有学者试图遴选已有的抗病毒药物,以期尽快开发“老药新用”的治疗方案。国家卫生健康委员会陆续发布了7版试行《新型冠状病毒肺炎诊疗方案》,其中,广谱抗病毒药物利巴韦林被作为可试用的临床治疗药物。
利巴韦林(ribavirin)常用剂型有注射剂、片剂、口服液、气雾剂等,国外临床上多以雾化剂用于成人和儿童的呼吸道合胞病毒性肺炎的治疗[1]。体外研究表明[2],利巴韦林可增强干扰素的体外活性,在治疗SARS-CoV感染者中,诊断后立即使用利巴韦林时治疗效果较好[3]。利巴韦林对DNA和RNA病毒均具有抑制作用,通常以雾化剂用于成人和儿童的呼吸道合胞病毒性肺炎的治疗。美国食品药品管理局(FDA)于1986年首次批准利巴韦林吸入溶液气雾剂(Virazole®,USP)[4],于1992年上市,而说明书中明确规定用于严重下呼吸道感染的住院患儿,且全程气雾治疗3~7 d,可见其应用的严格限制。根据国内多家临床中心的试验数据表明,与利巴韦林颗粒剂相比,国内的利巴韦林气雾剂(信韦林)达到同等的治疗效果时,虽然用药剂量大幅下降,但不良反应率无明显差异[5-8],表明利巴韦林吸入溶液气雾剂(Virazole®,USP)和利巴韦林气雾剂(信韦林)可能存在不良反应较大的缺陷,临床应用受到一定的限制。
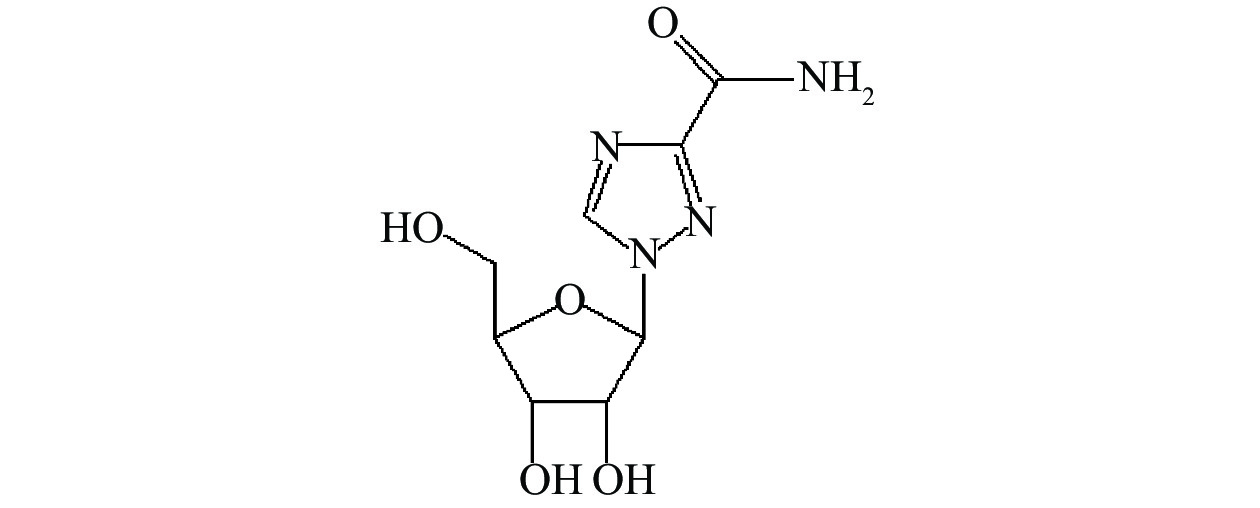
脂质体定量吸入粉雾剂(DPI)是肺部给药系统的研究热点之一。脂质体由于类似细胞结构而具有被动靶向性,作为药物载体常用于肺部给药,有利于抗病毒药物直接递送并作用于肺部细胞,抑制病毒的繁殖,特定粒径的脂质体粉雾剂可使药物不同程度地的分布于人体上、下呼吸道,从而发挥更好的临床治疗效果[9-10]。利巴韦林水溶性较好,亲脂性较差,如图1的结构式所示,不易被细胞渗透吸收,采用脂质体包裹,并制成粉雾剂给药至呼吸道或肺泡组织,可实现肺部沉积增强的作用效果,同时降低不良反应。本文根据临床急需及利巴韦林的药物理化性质,结合吸入制剂肺部给药方面的治疗优势,设计研制了一种利巴韦林脂质体吸入粉雾剂。以此开展设计研究,尝试开发抗病毒药物新型给药系统,以期为扩大利巴韦林临床使用范围,也为制剂学基础研究提供参考。
1. 仪器与材料
1.1 仪器
高效液相色谱仪(Agilent 1200);ME104E电子天平(METTLER TOLEDO);EYELA N-1000旋转蒸发仪(日本东京理化器械株式会社);Niro NS2006L高压匀质机(意大利GEA Niro Soavi公司);Nano NS90粒度检测仪(英国马尔文仪器有限公司);Hitachi S-3400N扫描电子显微镜(日本日立公司)。
1.2 材料
利巴韦林原料药(纯度>98%,西安康诺化工有限公司);利巴韦林对照品(629-200202,中国食品药品检定研究院);大豆卵磷脂(SPC)、脱氧胆酸钠(SDC)(北京化学试剂公司);N,N-二甲基甲酰胺、无水乙醇、磷酸氢二钠、磷酸二氢钠(分析纯,北京化学试剂公司);蒸馏水。
2. 方法与结果
2.1 利巴韦林脂质体冻干粉含量测定方法
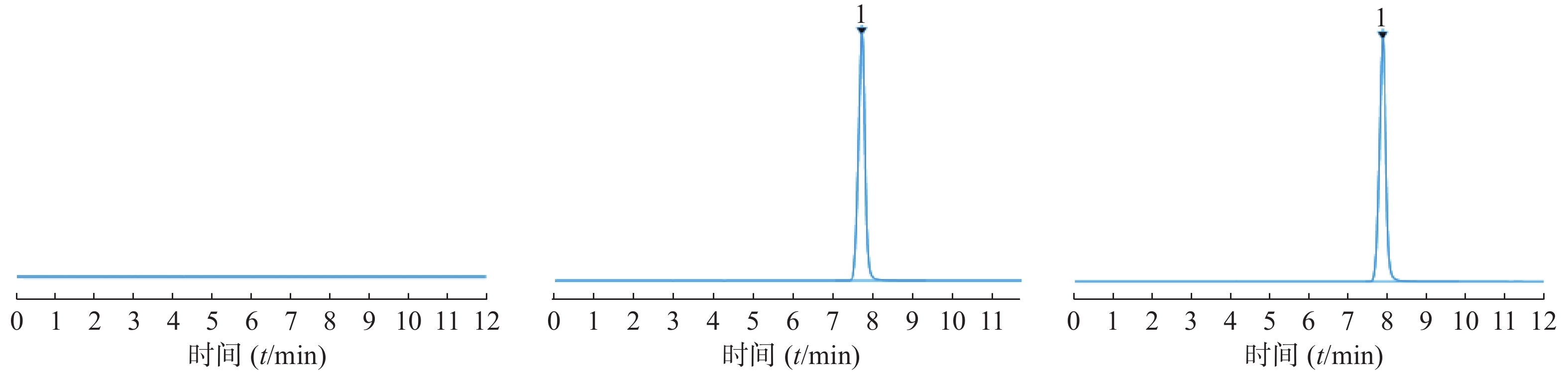
参照美国药典第40版中“利巴韦林”含量测定方法[11],采用高效液相色谱法测定。色谱条件:色谱柱为Carbomix H-NP5(7.8 mm×100 mm,5 μm);流动相硫酸溶液调整pH至2.5±0.1;流速:0.6 ml/min;柱温:60 ℃;检测波长UV 207 nm;进样量10 μl,取利巴韦林对照品适量,流动相溶解稀释至浓度为0.025 mg/ml,过滤,进样检测。取利巴韦林脂质体冻干粉适量,加入无水乙醇少量破乳,呈透明溶液后流动相稀释,制成浓度0.025 mg/ml供试品溶液;同样方式处理阴性样品溶液。在该色谱条件下得到图2的色谱图,图中利巴韦林峰型较佳,出峰时间适宜,阴性对照无干扰。
2.2 利巴韦林脂质体粉雾剂的制备
取大豆卵磷脂(5 mmol/L),脱氧胆酸钠(5 mmol/L),为制备具有高稳定性的双分子膜,在处方中加入适量维生素E置于250 ml的茄形瓶中,加入无水乙醇溶解,超声,至呈透明黄色溶液;将此溶液置旋转蒸发仪上旋转蒸发除去溶剂成膜;另取利巴韦林原料药溶解于磷酸盐缓冲液中(20 mg/ml),溶解完全后匀速加入至茄形瓶内,并于40 ℃水浴温度水化薄膜,直到完全水化呈近均一透明的乳白色溶液;高压均质机下将上述溶液均质,即得到粒径100~200 nm的利巴韦林脂质体溶液。加入冻干保护剂乳糖, 溶解摇匀后置西林瓶中,启动冷冻干燥程序,即得疏松利巴韦林脂质体冻干粉。
2.3 外观形态
取载药脂质体经处理后于透射电镜下观察,呈类圆形球状物,直径约200 nm,见图3。
2.4 流动性、松密度
将粉末通过下孔径为5 mm 的玻璃漏斗,从10 cm的高度缓慢、均匀地落入平板上,形成圆锥体。测量圆锥体的高度(h)和基底的半径(r),按公式计算休止角(θ,tanθ=h/r)。粉末休止角为37~40°,流动性较好。取量筒,自由落体方式装入脂质体冻干粉,记录质量与体积,计算松密度约0.25 g/ml。
2.5 包封率、冻干粉含量测定
取利巴韦林脂质体冻干粉适量,纯化水溶解得脂质体复溶物,取复溶物适量加入无水乙醇少量破乳,呈透明溶液后用流动相稀释,制成0.025 mg/ml供试品溶液,按“2.1”项下色谱条件检测冻干粉含量。
经过前期实验对比,本研究采用Sephadex G-50柱层析法测定包封率。分别精密量取1 ml脂质体和冻干粉复溶物上柱,用纯化水洗脱,流速0.5 d/s,从洗脱液由澄清变乳白色开始收集至洗脱液再次变澄清(约15 ml),置50 ml容量瓶中,以乙醇定容至刻度,振摇破乳;10 μl进样分析,记录色谱图,计算包封药物含量(W包);另各取1 ml脂质体溶液及冻干粉复溶物置50 ml容量瓶中,乙醇稀释至刻度,振摇破乳,10μl进样分析,记录色谱图,计算总药量(W总)。以包封率=(W包/W总)×100%,计算结果见表1。
表 1 利巴韦林脂质体冻干粉含量与包封率项目 样品 No.1 No.2 No.3 平均值 含量(mg/g) 脂质体冻干粉末 9.4 8.6 9.3 9.1 包封率 /% 冻干前脂质体溶液 64.23 63.83 66.34 64.80 脂质体冻干粉复溶液 63.43 61.65 64.10 63.06 结果表明,按照初步拟定方法制得的脂质体包封率约63%,冻干前后数据无明显差别,复溶效果较好,载药量较高,但包封率较低,处方工艺需要进一步优化。
2.6 复溶液粒径、聚合物分散指数与电位测定
取本品复溶液适量,纯化水再次稀释100倍,于马尔文粒径仪检测,如表2所示,本品冻干前后溶液粒径约160 nm,聚合物分散指数(PDI)、电位均较好,表明稳定性较好。
表 2 利巴韦林脂质体冻干复溶液粒径、PDI与电位样品 No.1 No.2 No.3 平均值 粒径/PDI 电位 粒径/PDI 电位 粒径/PDI 电位 粒径 电位 冻干前溶液 148.3 nm/0.57 −45.4 mv 163.7 nm/0.63 −41.8 mv 172.6 nm/0.39 −40.5 mv 161.5 nm −42.6 mv 冻干复溶液 147.8 nm/0.58 −42.5 mv 161.8 nm/0.65 −38.6 mv 181.7 nm/0.41 −39.1 mv 163.8 nm −40.1 mv 2.7 冻干粉溶解性
脂质体具有优良的两亲性,冻干保护剂亦有很好的亲水性。取本品冻干粉5 g置10 ml容量瓶中,加水10 ml,轻轻振摇即刻溶解完成,形成均一的乳白色胶束溶液,说明本品溶解性较好。
3. 讨论
病毒通过侵入人体呼吸道黏膜上皮细胞而感染,并在细胞内进行复制与表达,再释放至细胞外感染宿主其他细胞,最终导致机体产生过度免疫反应。与一些在下呼吸道的细胞内进行复制繁殖的高致病性病毒类似,COVID-19导致下呼吸道症状较为明显。故若将抗病毒药物递送至下呼吸道,直达病灶,并进入被感染的机体上皮细胞内,可能更好地发挥抑制或清除病毒的作用。通常肺吸入给药后可直接将药物运送至肺组织,在局部起效,与全身给药治疗肺部疾病相比较,可明显减少药物用量,降低药物在其他部位分布与吸收造成的不良反应,加之粉雾剂具备给药剂量准确、无需抛射剂、方便、易用及肺部靶标部位药物沉积量高等优势,是肺部疾病治疗药物较为理想的给药途径。
将利巴韦林通过脂质体包裹技术实现粉雾剂肺部细胞靶向治疗,相比普通口服与常规气雾剂可减少用药剂量和降低不良反应,理论上具有一定的可行性。因而,对轻、中度COVID-19感染患者也许是一种潜在的抗病毒药物治疗方式。
经对利巴韦林脂质体粉雾剂的初步制剂技术探究,结果证明制备的制剂满足粉雾剂的基本要求。由于粉雾剂具有无需抛射剂、药物相对稳定的优势[12],而且给药装置易于携带,操作简单、给药剂量相对准确等优点,故适合开发研制成利巴韦林脂质体吸入粉雾剂。此外,制备的利巴韦林脂质体冻干粉还可开发为溶液气雾剂,故在药剂开发方面也有较好前景。然而,本实验尚为初步研究,仍需要对制剂处方工艺进一步优化,以进一步提高包封率与改善整体粒径分布等性能。如要进入临床试用,还需对给药装置筛选、体内外药物沉积、药效学等进行深入考察,以满足临床使用的基本要求。
-
表 1 各组小鼠肠道菌群LEfSe多级物种差异判别分析
菌群 模型组vs
假手术组虎杖清脉饮组vs
模型组疣微菌门(P_Verrucomicrobiota) ↑ ↓ 疣微菌纲(c_Verrucomicrobiae) ↑ ↓ 疣微菌目(o_Verrucomicrobiales) ↑ ↓ 克里斯滕菌目(o_Christensenellales) ↑ 阿克曼菌科(f_Akkermansiaceae) ↑ ↓ 克里斯滕菌科(f_Christensenellaceae) ↑ 粪芽孢菌属(g_Coprobacillus) ↑ ↓ 阿克曼菌属(g_Akkermansia) ↑ ↓ 嗜胆菌属(g_Bilophila) ↑ 克里斯滕菌属
(g_Christensenellaceae_R-7_group)↑ 丹毒荚膜菌属(g_Erysipelatoclostridium) ↑ ↓ 嗜木聚糖真杆菌属(g_Eubacterium_xylanophilum_group) ↓ 罗氏菌属(g_Roseburia) ↓ 未分类克里斯滕森菌科(g_unclassified_f_Christensenellaceae) ↓ 异杆菌属(g_Allobaculum) ↓ ↑ 理研菌属(g_Rikenella) ↓ 绿脓杆菌属(g_Turicibacter) ↓ ↑ g_Tyzzerella ↓ 回肠杆菌属(g_Ileibacterium) ↓ 蓝细菌纲(c_Cyanobacteriia) ↓ f_Tannerellaceae ↓ f_norank_o_Chloroplas ↓ g_norank_f_norank_o_Chloroplast ↓ 副杆菌属(g_Parabacteroides) ↓ o_PeptostrePtococcales-Tissierellales ↑ 髌骨细菌门(P_Patescibacteria) ↑ c_Saccharimonadia ↑ o_Saccharimonadales ↑ f_Saccharimonadaceae ↑ 丹毒丝菌科(f_Erysipelotrichaceae) ↑ 消化链球菌科(f_Peptostreptococcaceae) ↑ g_Candidatus_Saccharimonas ↑ g_UCG-005 ↑ g_Romboutsia ↑ 嗜木聚糖真杆菌属(g_Eubacterium_xylanophilum_group) ↑ g_norank_f_Atopobiaceae ↑ 安德克氏菌属(g_Adlercreutzia) ↑ 分节丝状菌属(g_Candidatus_Arthromitus) ↑ g_Lachnospiraceae_UCG-006 ↑ g_NK4A214_group ↑ 注:↑表示相对丰度增加,↓表示相对丰度降低。 -
[1] IADECOLA. The pathobiology of vascular dementia[J]. Neuron, 2013, 80(4): 844-866. [2] VAN DER FLIER W M, SKOOG I, SCHNEIDER J A, et al. Vascular cognitive impairment[J]. Nat Rev Dis Primers,2018,4:18003. doi: 10.1038/nrdp.2018.3 [3] MELAZZINI L, CODARI M, VITALI P, et al. Brain vascular changes in adults with congenital heart disease: a systematic review[J]. Neuroimage Clin,2019,23:101873. doi: 10.1016/j.nicl.2019.101873 [4] LO COCO D, LOPEZ G, CORRAO S. Cognitive impairment and stroke in elderly patients[J]. Vasc Health Risk Manag,2016,12:105-116. [5] BODUKAM V, HAYS R D, MARANIAN P, et al. Association of gastrointestinal involvement and depressive symptoms in patients with systemic sclerosis[J]. Rheumatology (Oxford),2011,50(2):330-334. doi: 10.1093/rheumatology/keq296 [6] 唐莉莉. 基于脑肠轴理论探讨温肾养肝方调节肠道菌群治疗帕金森病的临床观察和实验研究[D]. 南京: 南京中医药大学, 2020. [7] 安云英, 吴敏娜, 李璞泽, 等. 口服硫酸链霉素对帕金森小鼠症状的改善及其对肠道菌群的影响[J]. 微生物学报, 2019, 59(9):1636-1650. doi: 10.13343/j.cnki.wsxb.20180390 [8] SANGUINETTI E, COLLADO M C, MARRACHELLI V G, et al. Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet[J]. Sci Rep,2018,8(1):4907. doi: 10.1038/s41598-018-23261-1 [9] ROSARIO D, BIDKHORI G, LEE S, et al. Systematic analysis of gut microbiome reveals the role of bacterial folate and homocysteine metabolism in Parkinson’s disease[J]. Cell Rep,2021,34(9):108807. doi: 10.1016/j.celrep.2021.108807 [10] YU Y Y, LIU Q P, LI M T, et al. Hu-Zhang-Qing-Mai-Yin inhibits proliferation of human retinal capillary endothelial cells exposed to high glucose[J]. Front Pharmacol,2021,12:732655. doi: 10.3389/fphar.2021.732655 [11] KHAN A, KALARIA R N, CORBETT A, et al. Update on vascular dementia[J]. J Geriatr Psychiatry Neurol,2016,29(5):281-301. doi: 10.1177/0891988716654987 [12] WOLTERS F J, IKRAM M A. Epidemiology of vascular dementia[J]. Arterioscler Thromb Vasc Biol,2019,39(8):1542-1549. doi: 10.1161/ATVBAHA.119.311908 [13] SENDER R, FUCHS S, MILO R. Revised estimates for the number of human and bacteria cells in the body[J]. PLoS Biol,2016,14(8):e1002533. doi: 10.1371/journal.pbio.1002533 [14] LEE J, D’AIGLE J, ATADJA L, et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice[J]. Circ Res,2020,127(4):453-465. doi: 10.1161/CIRCRESAHA.119.316448 [15] ROUND J L, MAZMANIAN S K. The gut microbiota shapes intestinal immune responses during health and disease[J]. Nat Rev Immunol,2009,9(5):313-323. doi: 10.1038/nri2515 [16] DELZENNE N M, RODRIGUEZ J, OLIVARES M, et al. Microbiome response to diet: focus on obesity and related diseases[J]. Rev Endocr Metab Disord,2020,21(3):369-380. doi: 10.1007/s11154-020-09572-7 [17] YU X B, ZHOU G Y, SHAO B, et al. Gut microbiota dysbiosis induced by intracerebral hemorrhage aggravates neuroinflammation in mice[J]. Front Microbiol,2021,12:647304. doi: 10.3389/fmicb.2021.647304 [18] 胡炜, 金海涛, 王沛, 等. 虎杖苷激活SIRT1信号通路减轻大鼠创伤性脑损伤[J]. 西部医学, 2020, 32(11):1580-1583,1588. doi: 10.3969/j.issn.1672-3511.2020.11.005 [19] 杜会枝, 马红. 连翘对神经系统的药理作用研究进展[J]. 中国药学杂志, 2021, 56(7):526-530. [20] 于静, 王婧, 苏素文, 等. 豨莶草提取物抗多柔比星致大鼠急性心肌损伤的作用[J]. 医药导报, 2013, 32(7):843-847. [21] 孟倩超, 金若敏, 王聃, 等. 豨莶草抗血栓组分对血小板聚集的影响[J]. 上海中医药杂志, 2008, 42(5):89-91. doi: 10.3969/j.issn.1007-1334.2008.05.040 [22] 余弯弯, 双鹏程, 张凌. 鸡血藤化学成分及药理作用研究概况[J]. 江西中医药大学学报, 2014, 26(4):89-92. [23] 刘传亮, 陈国华, 李蕾. 复方丹参川芎中药配方颗粒干预老年颈动脉粥样硬化的研究[J]. 世界最新医学信息文摘, 2019, 19 82): 15-16, 19. [24] LI W X, TANG Y P, WANG H, et al. Research on Chinese medicine pairs (VII): Angelicae sinensis radix-Chuanxiong rhizoma[J]. Zhongguo Zhong Yao Za Zhi,2013,38(24):4220-4226. [25] CHU S H, YANG D, WANG Y P, et al. Effect of resveratrol on the repair of kidney and brain injuries and its regulation on klotho gene in d-galactose-induced aging mice[J]. Bioorg Med Chem Lett,2021,40:127913. doi: 10.1016/j.bmcl.2021.127913 [26] LI N, WANG X C, SUN C C, et al. Change of intestinal microbiota in cerebral ischemic stroke patients[J]. BMC Microbiol,2019,19(1):191. doi: 10.1186/s12866-019-1552-1 [27] HASANI A, EBRAHIMZADEH S, HEMMATI F, et al. The role of Akkermansia muciniphila in obesity, diabetes and atherosclerosis[J]. J Med Microbiol, 2021, 70(10): [28] WANG Y B, XIE Q H, SUN S, et al. Probiotics-fermented Massa Medicata Fermentata ameliorates weaning stress in piglets related to improving intestinal homeostasis[J]. Appl Microbiol Biotechnol,2018,102(24):10713-10727. doi: 10.1007/s00253-018-9438-y [29] DIAO H, JIAO A R, YU B, et al. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets[J]. Genes Nutr,2019,14:4. doi: 10.1186/s12263-019-0626-x [30] SUN M M, WU W, LIU Z J, et al. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases[J]. J Gastroenterol,2017,52(1):1-8. doi: 10.1007/s00535-016-1242-9 [31] KUNDU P, LEE H U, GARCIA-PEREZ I, et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice[J]. Sci Transl Med,2019,11(518):eaau4760. doi: 10.1126/scitranslmed.aau4760 [32] KUNDU P, LEE H U, GARCIA-PEREZ I, et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice[J]. Sci Transl Med, 2019, 11(518): eaau4760. -





 下载:
下载:

 下载:
下载: