-
据估计,2017 年全球有 4.51 亿(18~99 岁)糖尿病(DM)患者,预计到 2045 年,这一数字将增至 6.93 亿[1]。糖尿病肾病(DKD)是DM的一种严重并发症,是世界范围内终末期肾病(ESRD)的主要病因[2, 3]。DKD的发生率与DM的发病率和病死率增加密切相关[4]。在美国,开始接受ESRD治疗的DM患者数量从2000年的4万多人显著增加到2014年的5万多人[5]。在我国,DKD的发病率在过去10年中显著增加,2013年的一项调查结果显示,中国DKD患者数量估计达到2 430万[6]。当前,DM患病率持续上升,如果DKD的临床预防策略没有改善,预计DKD的患病率也会随之增加[7, 8]。然而目前除了控制血糖、血压等手段外,临床上尚无其他有效预防DKD的方案。DKD的发病机制复杂,其分子机制还没有得到全面阐明。近年来,越来越多的研究发现,肠道菌群在DKD的发生发展中发挥了重要作用,本文围绕DKD的肠道菌群参与情况,综述其研究进展。
肠道菌群是一个由微生物菌落组成的复杂生态系统,包括至少1 000个不同物种的数万亿细菌,另外还有其他共生生物,如古细菌、病毒、真菌和原生生物。肠道菌群失调的主要特征是细菌和真菌的多样性和丰度下降[9]。近年来,人们对肠道菌群与宿主相互作用产生极大兴趣,众多证据表明,肠道菌群在人类健康和疾病中发挥重要作用,菌群失调已被证明与动脉粥样硬化、高血压、心力衰竭、慢性肾病(CKD)、肥胖和2型糖尿病(T2DM)等疾病有关[10]。肠道菌群有能力产生一系列代谢产物,包括短链脂肪酸(SCFAs)、N-氧化三甲胺(TMAO)、胆汁酸(BA)、蛋白质结合的尿毒症毒素(PBUT)、支链氨基酸(BCAAs)和一些其他未知代谢产物。肠道微生物产生的代谢产物被认为是微生物与宿主之间交流的媒介,对人体的生物活性和代谢有重要影响[11]。近年来,许多研究调查了DM、肥胖和代谢综合征等代谢性疾病患者肠道微生物群的多样性和功能的变化。有研究发现,这些患者的肠道微生物群落发生了显著变化,并导致肠道微生物群失调和肠漏综合征,肠道屏障功能障碍,肠道通透性增加[12]。多种肠道微生物群代谢产物被释放到血液中,如SCFAs、TMAO、脂多糖(LPS)和尿毒症毒素,再通过多种信号通路进一步导致疾病表型的变化[13, 14]。
-
DKD是DM患者的主要微血管并发症,尽管DKD发病机制相当复杂,但最近的研究表明,肠道菌群也参与了DKD的进展。一项研究通过16s rDNA测序分析了DKD患者和健康志愿者之间粪便样本,发现与健康志愿者相比,DKD患者肠道细菌丰富度和多样性显著下降,而许多常见病原体在DKD和DM患者中均富集,如拟杆菌门、毛梭菌门、双歧杆菌门、乳杆菌门、罗斯氏菌门和粪杆菌门。其中,巨球菌属、厌氧菌属和嗜血菌属等属在DKD中的丰富度远高于DM中的丰富度。几个特征属,如巨球菌属、韦荣球菌属、埃希氏菌属、志贺氏菌属、厌氧菌属和嗜血杆菌属则可能是DKD新的潜在微生物标志物[15]。有研究发现,中国人和欧洲女性DM患者中的哈氏梭菌增加,而罗斯伯里氏菌减少,研究者推测这与DM的发展有关[16, 17]。另一项研究则把DKD患者与DM患者进行比较,发现与DM患者相比,DKD患者肠道疣微菌门和梭杆菌门的水平显著升高,二者都属于G-菌,而LPS可通过加速巨噬细胞/单核细胞和中性粒细胞的活化而诱发炎症,导致DKD进展[18]。CKD患者常出现肠道菌群失调、细菌代谢产物累积、肠道屏障功能破坏和慢性炎症,大多数CKD患者肠道细菌过度生长,但细菌多样性下降,如梭杆菌属等在ESRD 患者体内显著富集,而产生SCFAs的细菌,尤其是产生丁酸的细菌丰度随着 ESRD 的恶性进展而逐渐下降,SCFAs通常被认为在维持人类健康方面发挥着多种重要作用[19, 20]。
以上研究表明,健康的肾脏通过细胞和分子信号与肠道微生物群沟通,以确保肠道微生物群的正常稳态。肠道菌群的失衡将导致这种平衡被破坏,肠道菌群多样性下降、特定菌群种类变化可能在DKD的发展中发挥重要作用。
-
肠道的黏液层和上皮结构是肠道屏障最重要的结构,肠道上皮是有正常上皮细胞和几种具有特定功能的细胞组成,包括潘氏细胞、杯状细胞等[21]。潘氏细胞可分泌溶菌酶和防御素等抗菌肽,防止有害细菌定植,杯状细胞通过分泌黏蛋白来维持黏膜层。上皮细胞通过顶端连接复合体连接,该复合体由紧密连接(TJ)和黏附连接组成。TJ由TJ蛋白Claudin、Occludin和连接黏附蛋白分子 A (JAM-A) 以及细胞内斑块蛋白组成[22]。肠道屏障将肠腔中的微生物与内部环境分隔开来,许多微生物系统通过肠壁与内部环境相互作用。DKD患者肠道微生物群组成和功能的改变会导致肠上皮屏障受损和肠道通透性增加[23]。一方面,由于DKD患者肾脏滤过减少,大量尿素水解导致氨重吸收增加,随后肝脏重新合成尿素,导致跨上皮电阻(TER)显著下降,关键TJ蛋白如Claudin-1、Occludin 和ZO-1丢失[24]。另一方面,DKD肠道菌群失调引起相应代谢产物发生变化。肠道菌群代谢会产生多种类型的SCFAs。Nathan等在一项小鼠骨髓移植研究中发现,丁酸盐通过为肠上皮细胞提供能量来维持结肠细胞健康,这可能有助于肠上皮的完整性[25]。Huang等发现,丁酸盐还可能通过改变肠道Claudin-2水平来抑制细胞因子诱导的屏障功能障碍[26]。此外,一些动物研究表明,乙酸盐可直接激活核苷酸结合寡聚物肠上皮细胞中的炎症结构域3(NLRP3)炎症小体,导致IL-18释放,进而通过激活小鼠上皮细胞上的IL-18受体来促进肠屏障的完整性[27]。丙酸盐还可以抑制小鼠结肠组织中 ZO-1、Occludin 和上皮钙黏蛋白(E-cadherin) 下调改善由葡聚糖硫酸钠(DSS)引起的高肠通透性[28]。体外实验中,乙酸盐、丙酸盐和丁酸盐已被证明可以通过改变肠道上皮TJ蛋白的表达促进细胞内通透性,包括体外的 ZO-1[29]。综上所述,SFCAs 被认为是维持肠道屏障的关键因素。
-
BA由肝细胞中的胆固醇在肝脏中合成。初级BA可以通过肠道微生物群转化并分解为次级 BA。肠道微生物群通过初级BA的解结合、脱氢和二羟基化来调节 BA 代谢过程[30]。BA 是G蛋白偶联胆汁酸受体 (TGR5)和核激素受体法尼醇X受体(FXR)的配体。BA与TGR5结合,通过胰高血糖素样肽-1 (GLP-1)提高胰岛素敏感性,并调节肌肉或棕色脂肪组织的能量消耗[31]。FXR的激活会减少脂肪生成和肝脏糖异生,并通过产生抗菌肽来抑制细菌过度生长和易位[32]。Wang等在链脲霉素诱导小鼠糖尿病实验中发现, FXR和TGR5通过调节肾脏信号通路在DM和肥胖相关肾脏疾病中发挥肾脏保护作用[33]。另一项体外实验证明,龙胆苦苷通过 TGR5 激活抑制 NF-κB 信号通路,从而减轻DKD中的炎症和纤维化[34]。熊去氧胆酸(UDCA)是一种继发性BA,已被发现可减轻DKD大鼠肾内质网应激引起的肾功能障碍、足细胞凋亡和氧化应激。给予牛磺酸脱氧胆酸(TUDCA)可以减轻 DM大鼠的肾小球和肾小管损伤,这部分是通过抑制内质网介导的[35]。
-
SCFAs 是肠道菌群代谢的主要产物之一,主要包括由拟杆菌门产生的乙酸盐、丙酸盐和厚壁菌门产生的丁酸盐。丁酸盐通过介导miR7a-5p/P311/TGF-β1通路缓解DKD进展, 转化生长因子-β1(TGF-β1)是触发纤维化信号级联的初始因子[36]。丁酸钠(NaB)是一种已知的核因子E2相关因子2(NRF2)激活剂,具有预防DKD的作用。研究发现,未接受NaB治疗的DM小鼠表现出明显的肾脏病理变化,如氧化损伤、炎症、细胞凋亡、纤维化。NaB 通过激活NRF2抑制组蛋白去乙酰化酶(HDAC)活性改善DKD[37]。Gasdermin D (GSDMD)是一种新发现的焦亡关键执行蛋白,可被炎症性半胱氨酸天冬氨酸酶(caspase)裂解。高糖可增加碘化丙啶(PI)阳性细胞水平,促进乳酸脱氢酶(LDH)、IL-1β、IL-18的释放,并伴有caspase-1水平升高。NaB通过NF-κB/IκB-α信号通路的caspase 1-GSDMD 经典焦烧死亡途径改善了高葡萄糖诱导的肾小球内皮细胞焦亡[37-39]。由以上研究可见,丁酸盐在高糖刺激的肾损伤中发挥重要作用,并可能作为有效的治疗靶点。此外,SCFAs改变导致肠上皮TJ的破坏,进而引起肠道通透性增加,肠腔内微生物代谢物及其他有害物质穿过肠道屏障进入体内循环,如甲酚、吲哚分子、LPS等。LPS会持续浸润门静脉,导致代谢性内毒素血症和炎症细胞因子水平升高,从而加速DKD的进展[40]。 LPS 是G-菌的表面抗原,通过 TLR2 和 TLR4 相关途径介导宿主炎症[41]。TLR2和TLR4通过诱导肿瘤坏死因子-α(TNF-α)、IL-1和IL-1β等促炎细胞因子的释放, NF-κB介导的炎症级联反应,参与DKD的持续炎症反应过程[42]。
其他SCFAs在DKD中的作用仍存在争议,在一项微生物移植治疗DM大鼠的研究中发现,乙酸盐可能会加剧 DKD的疾病进展, 如肠道微生物群产生过量的乙酸盐,通过激活G蛋白偶联受体43(GPR43)破坏胆固醇稳态,导致肾脏出现肾小管间质损伤[43]。另一项动物研究发现,肠道微生物群失调可能与早期DKD肾内肾素-血管紧张素系统(RAS)激活有关,血浆醋酸盐水平与肾内血管紧张素Ⅱ蛋白表达呈正相关,推测醋酸盐也可能参与早期DKD的肾损伤[44]。
-
TMAO主要来源于肠道菌群氧化三甲胺(TMA)。肠道微生物从摄入的卵磷脂和胆碱等营养物质中代谢并产生TMA,这些营养物质通过门静脉循环进入肝脏,并被黄素单加氧酶3或其他黄素单加氧酶氧化,产生TMAO。TMAO水平升高与CKD患者死亡风险增加相关[45]。TMAO及其前体胆碱水平的增加与信号转导蛋白SMAD3和TGF-β信号的磷酸化增强有关,从而加重高脂饮食(HFD)喂养小鼠的肾胶原沉积和肾小管间质纤维化。饮食诱导的肥胖小鼠模型中,TMAO水平升高还与NADPH氧化酶和炎症细胞因子的升高有关,而补充 TMA 形成抑制剂可改善 HFD 诱导的肾损伤纤维化并降低小鼠肾损伤分子-1(KIM-1)和促炎细胞因子的表达[46]。这些研究表明,高水平的TMAO可能是CKD进展的致病介质。近年来,研究发现TMAO在DKD发展中也发挥重要作用。TMAO可激活DKD患者的NF-κB通路,进一步加重体内微炎症,导致DKD恶化[47]。与无肾脏疾病的 T2DM 患者以及健康个体相比,DKD患者表现出更高浓度的TMAO,这与尿白蛋白肌酐比值(UACR) 呈正相关[48]。在一项动物研究中,喂食TMAO的DKD大鼠表现出更严重的肾功能衰退和肾纤维化,该研究证明TMAO可以通过激活NLRP3炎症小体并最终导致IL-1β和IL-18的释放来加速肾脏炎症[49]。
由此可见,肠道微生物群衍生的代谢物,是肠道菌群影响DKD进展的重要参与者,肠道微生物及其代谢物与肾脏之间存在相互作用,称为肠肾轴。
-
肠道微生物群与肾脏疾病相关,已有众多研究证实了肠肾轴的存在。肠道微生物群通过产生无数代谢物来参与宿主体内平衡,这些代谢物充当代谢反应的关键信号分子和底物。基于肠道微生物群的治疗可能是未来预防和治疗DKD的一种有前景的策略。饮食结构的改变有助于改善肠道菌群失调,肠道菌群移植也有望在安全、规范的条件下发挥恢复DKD微生物群生态的作用,宏基因组学和代谢组学的结合有助于研究肠道菌群失调与代谢紊乱之间的关系[49]。然而,目前大部分数据仅限于动物模型,需要更可靠的临床试验来阐明DKD发病机制的关键途径和特定菌株。
Research progress on the mechanism of gut microbiota participating in diabetes nephropathy
-
摘要: 随着糖尿病患病率升高,糖尿病肾病的预防和治疗已成为世界性难题。糖尿病肾病发生发展的分子机制目前尚不明确,但近年来诸多研究表明,肠道菌群在糖尿病肾病的进展中发挥重要作用。综述了肠道菌群参与糖尿病肾病的机制研究进展。Abstract: With the increasing prevalence of diabetes, the prevention and treatment of diabetes nephropathy have become a worldwide problem. The molecular mechanism of the occurrence and development of diabetes nephropathy is still unclear, but many studies in recent years have shown that gut microbiota plays an important role in the progress on diabetes nephropathy. The research progress on the mechanism of gut microbiota participating in diabetes nephropathy was reviewed in this article.
-
Key words:
- diabetes /
- diabetes nephropathy /
- intestinal flora /
- intestinal permeability
-
在全球新型冠状病毒肺炎(coronavirus disease 2019,COVID-19)大流行期间,Moderna和BioNTech/辉瑞公司基于脂质纳米颗粒(lipid nanoparticles,LNP)载体的mRNA疫苗获得FDA紧急批准[1,2],迅速地延缓了新型冠状病毒的传播。基于脂质纳米颗粒-mRNA的COVID-19疫苗取得的巨大成功,使得mRNA疫苗技术迅速成为研究的热点,并促进了mRNA疫苗在肿瘤等重大疾病治疗中的研发和应用。
2024年6月,上海交通大学附属瑞金医院沈柏用教授团队披露mRNA肿瘤疫苗研究新进展,在全球范围内首次报道针对KRAS G12V单靶点的mRNA肿瘤疫苗在实体肿瘤中的治疗效果,为传统治疗无法耐受或者耐药的晚期肿瘤患者带来新希望[3]。同时,针对肿瘤抑制性免疫微环境,编码细胞因子的mRNA肿瘤疫苗可以促进效应T细胞的成熟、调控肿瘤微环境,从而提高在实体瘤局部疫苗接种的抗肿瘤疗效。其中,以MEDI1191、mRNA-
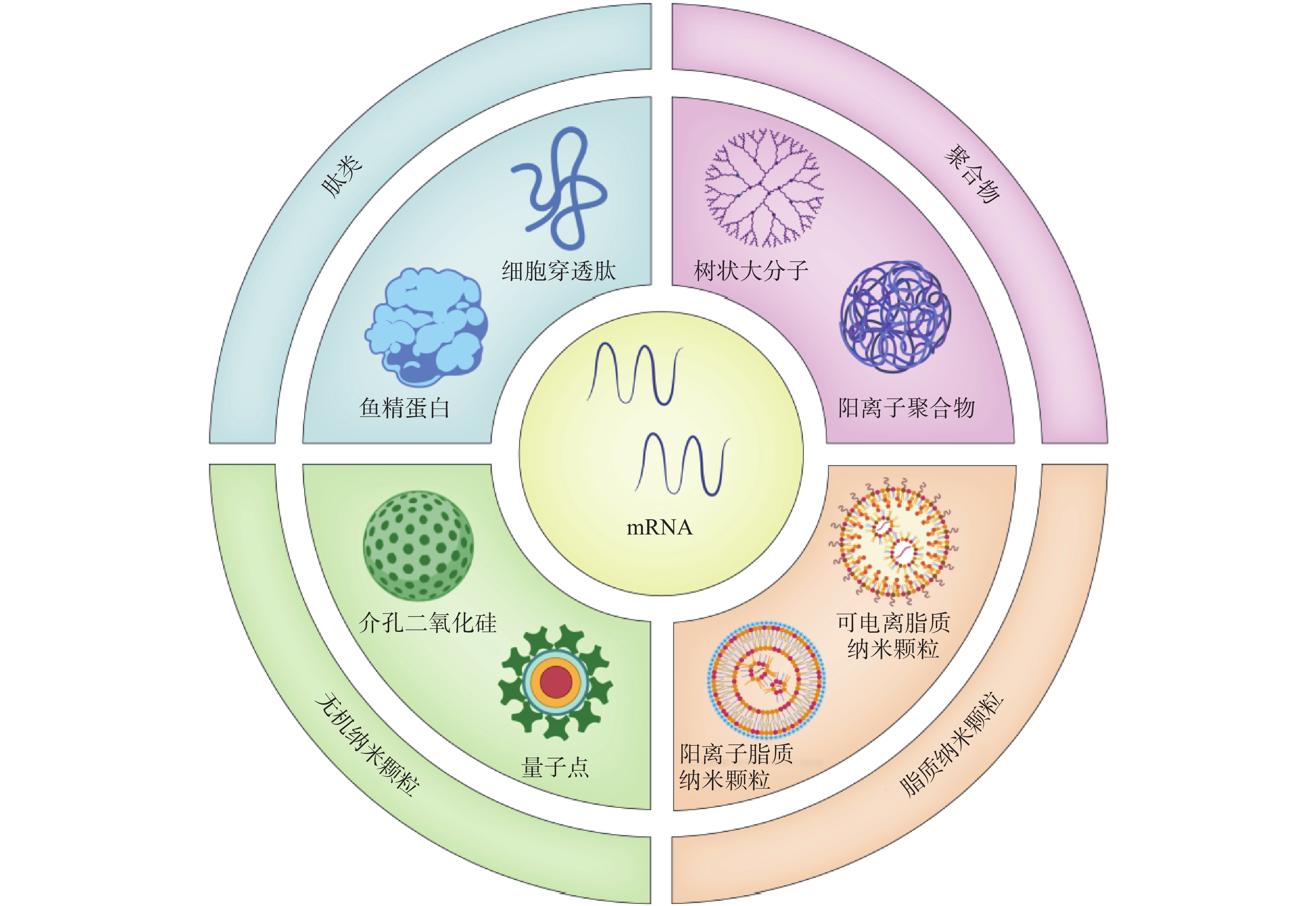
2416 、mRNA-2752 、SAR44100等为代表的 mRNA肿瘤疫苗已进入临床试验阶段。mRNA肿瘤疫苗通过将编码肿瘤抗原的mRNA引入宿主细胞(通常是抗原提呈细胞)的细胞质中表达靶蛋白抗原,然后在抗原提呈细胞表面呈递MHC分子,诱导有效的免疫应答来发挥作用[4,5]。相对于其他类型的治疗性肿瘤疫苗来说,基于mRNA的肿瘤疫苗是一种有前途的策略(图1),其具有如下优势:①与病毒疫苗类似,mRNA肿瘤疫苗能够同时递送多种抗原,引发体液免疫和细胞免疫,增加了肿瘤细胞根除的可能性。②与基于肽的疫苗不同,mRNA肿瘤疫苗不受患者特异性HLA类型的限制。③与基于DNA的肿瘤疫苗相比,mRNA肿瘤疫苗安全且耐受性良好,没有插入宿主基因组的风险[4]。尽管mRNA肿瘤疫苗具有非常广阔的应用前景,但仍然存在以下问题:①mRNA分子在体内非常不稳定,很容易被细胞外的核酸酶降解[6]。②mRNA分子量大且带负电荷,这阻碍了它们通过细胞膜高效递送至靶细胞[7]。因此,推动mRNA药物应用的关键之一是高效的mRNA递送系统的开发。与基于病毒的递送系统相比,非病毒载体在生物安全性和多功能性方面具有重大优势。如基于脂质的纳米颗粒、聚合物纳米颗粒、肽类纳米颗粒和无机纳米粒子,能有效压缩mRNA,使其免受核酸酶的降解[8]。此外,根据靶向器官的生物结构和递送过程中的屏障,非病毒载体可以有目的地进行结构改造,从而更有效地将mRNA递送到身体的特定部位,以提高治疗效果[8]。因此,基于非病毒载体的递送系统的研究已成为mRNA肿瘤疫苗研究领域的热点课题。1. mRNA肿瘤疫苗概况
mRNA肿瘤疫苗的研究发端于 20 世纪 70 年代。1976年,Langer等[9]首次使用了聚合物纳米颗粒和微颗粒作为载体来封装核酸。随后2年,又实现将外源性mRNA通过脂质体递送到宿主细胞,进一步拓展了mRNA技术的应用[10]。1990年,Wolff等[11]将含有氯霉素乙酰转移酶、荧光素酶和β-半乳糖苷酶基因的RNA和DNA表达载体分别注射到小鼠体内骨骼肌中,在所有小鼠体内都检测到了相应蛋白质的表达,为mRNA用作治疗药物的研究奠定了基础。mRNA疫苗的概念可以追溯到1993年,Martinon等[12]在用编码流感病毒蛋白的mRNA免疫的小鼠中观察到细胞毒性T淋巴细胞的诱导,揭示了mRNA作为编码抗原的基因在疫苗研究领域的应用潜能。1995年,Conry 等[13]构建了编码荧光素酶和人癌胚抗原(carcinoem bryonic antigen,CEA)的mRNA剪切体,该mRNA剪切体在体外小鼠成纤维细胞中定向表达CEA, mRNA肿瘤疫苗的概念被首次提出。近年来,COVID-19的流行推动了mRNA疫苗的深入研究和技术的发展,并使mRNA肿瘤疫苗再次受到关注。事实上,COVID-19疫苗的快速发展得益于多年来在临床前和临床试验中以mRNA疫苗作为肿瘤治疗策略的相关研究。因此,mRNA疫苗是未来肿瘤治疗有希望的候选疗法之一。
2. 基于mRNA肿瘤疫苗的非病毒递送系统
由于mRNA存在不稳定性、高免疫原性、递送效率低等问题,开发高效的靶向递送系统是mRNA疫苗亟待解决的问题。mRNA疫苗递送载体主要包括病毒载体和非病毒载体。目前,多数的临床基因治疗试验采用病毒载体。然而,病毒载体存在易于刺激免疫原性反应产生和诱导基因插入突变等问题,其临床安全性一直受到质疑。而包括脂质纳米粒、聚合物、肽类、无机材料在内的非病毒载体,具有强大的基因装载能力、高度的安全性和实用性,且其制备较为简单。因此,非病毒载体在进一步的临床开发和应用中显示出巨大的潜力[14]。
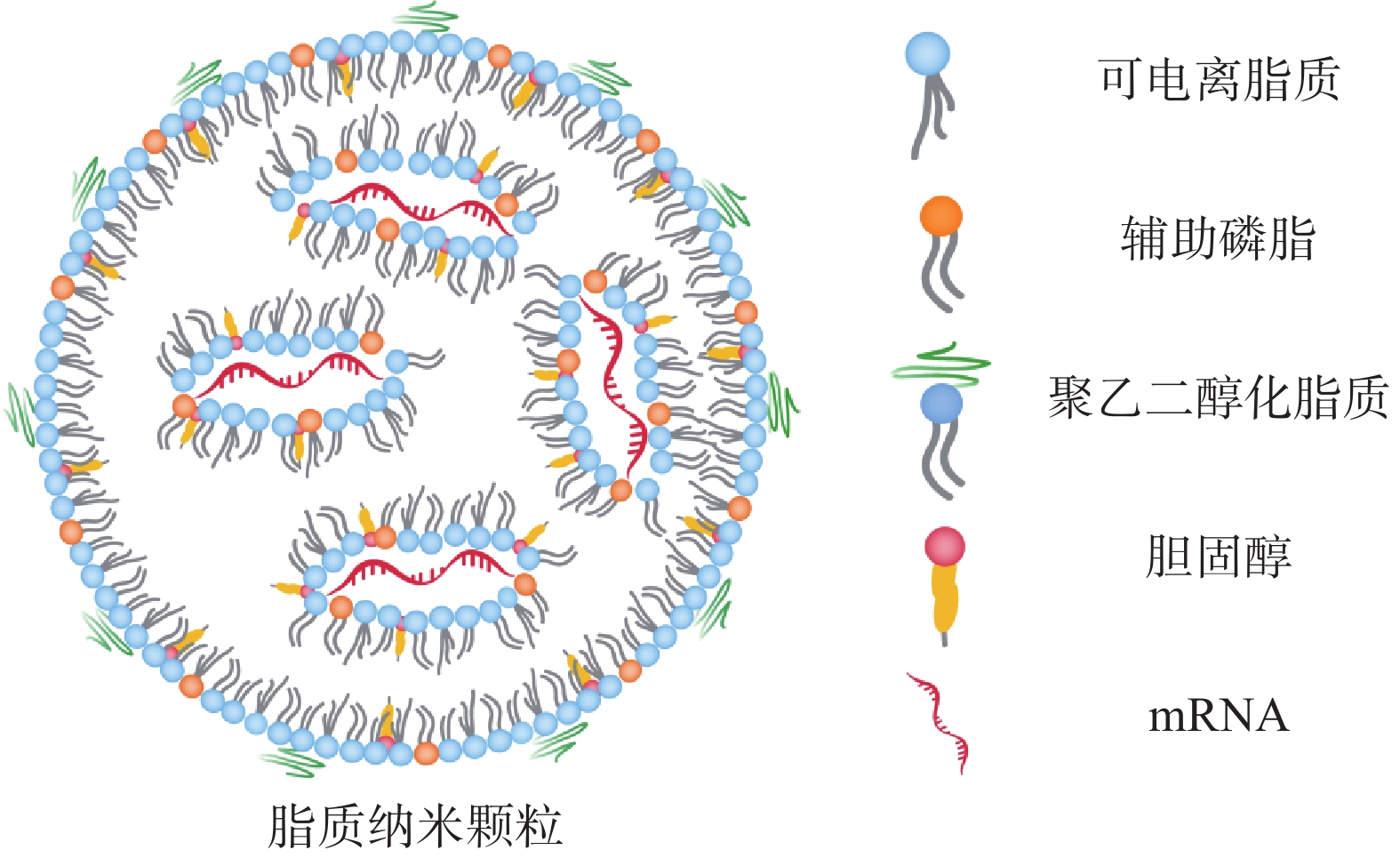
2.1 脂质纳米颗粒(lipid nanoparticles,LNP)
脂质纳米颗粒是一种临床批准的用于核酸递送的多功能平台[15]。截至2023年11月,3种基于LNP的药物(1种siRNA,2种mRNA)已获得美国食品药品监督管理局批准,超过50种候选药物正在临床试验中,用于治疗或预防传染病、肿瘤和遗传性疾病[16]。
LNP脂质壳结构由脂质或类脂材料以及辅助成分(包括胆固醇、辅助磷脂和聚乙二醇化脂质)组成(图2)。这些成分可促进单分散纳米颗粒的形成、提高纳米颗粒的稳定性、实现有效的核酸封装、增加细胞摄取和促进mRNA的内体逃逸。其中,辅助磷脂可以调节 LNP 双分子层的流动性,促进内体逃逸[17];胆固醇通过填充磷脂间的空隙来调节膜的流动性,促进膜融合。聚乙二醇(polyethyleneglycol,PEG)化脂质控制LNP的大小和稳定性,介导LNP的间接靶向能力以及保护LNP免受巨噬细胞介导的清除[18,19];LNP 配方中最重要的成分为阳离子脂质或可电离脂质,在早期的研究中主要使用阳离子脂质,如(2,3-二油酰基-丙基)-三甲基氯化铵[(2, 3-dioleyl-propyl)-trimethyl ammonium chloride, DOTAP]和1,2-双十八烯氧基-3-甲基铵丙烷(1, 2-didecaenooxy-3-methyl ammonium propane, DOTMA)。然而阳离子脂质具有高免疫原性和较强的毒性[20]。
可电离脂质具有pH敏感性,其在酸性条件下带正电荷,在生理pH条件下接近中性[21,22]。这种特性使它们在体内分布时更少地与血清成分相互作用。当可电离脂质纳米粒进入细胞的酸性内体后,它们可以被质子化,从而帮助mRNA逃逸到细胞质中。常用的可电离脂质有1,2-二甲氧基-N,N-二甲基-3-氨基丙烷(DLin-DMA)、N,N-二甲基-2,2-二-(9Z,12Z)-9,12-十八碳二烯-1-基-1,3-二氧戊环-4-乙胺(DLin-KC2-DMA)和4-(N,N-二甲基氨基)丁酸(二亚油基)甲酯(DLin-MC3-DMA)。可电离脂质的结构改变可引起 mRNA递送效率的改变,如Dong等[23]合成了含有不同头部基团的二氨基(DAL)可电离脂质材料,用于包载编码IL-12、IL-27和GM-CSF等细胞因子的mRNA。使用DAL-LNP对携带B16F10黑色素瘤的小鼠进行治疗性疫苗接种,结果显示通过DAL-LNP在瘤内给药IL-12和IL-27 mRNA促进了B16F10黑色素瘤生长的持续抑制,且没有引起明显的毒性。
此外,在LNP的表面进行靶向免疫细胞表面受体的功能性修饰,或与佐剂共同给药,可增强免疫刺激。Shi等[24]将 Pam2Cys(一种可通过 Toll 样受体(TLR)2/6 途径发出信号的简单合成代谢脂氨基酸)引入 LNP,实现了与 mRNA 的共同递送。结果显示,使用由此产生的 mRNA-LNP (Pam2Cys)进行免疫,可通过诱导 IL-12 和 IL-17 等细胞因子改善肿瘤引流淋巴结(tumor-draining lymph nodes,TDLN)的免疫微环境。
2.2 聚合物纳米颗粒(polymer nanoparticles)
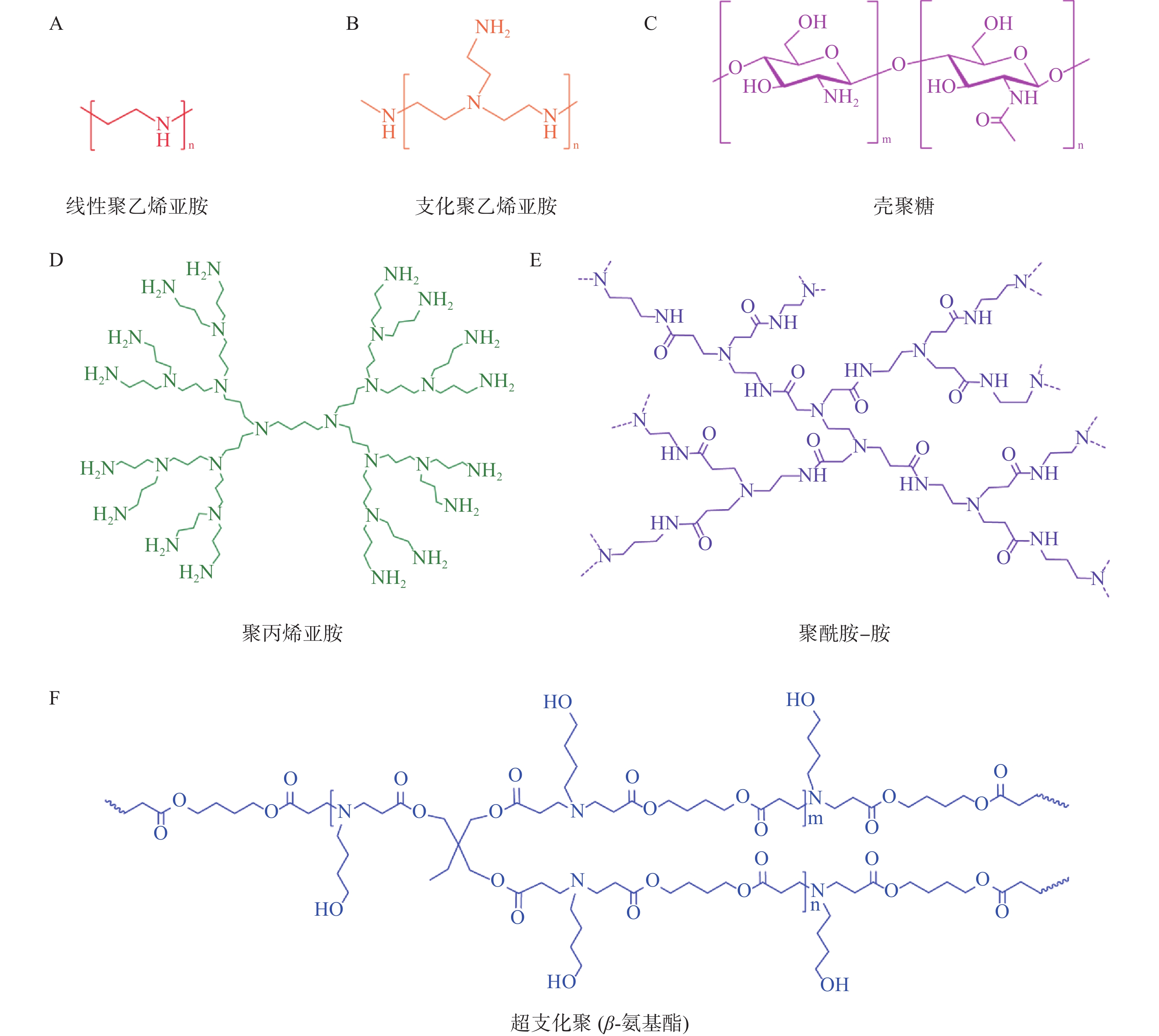
目前常用于mRNA递送的聚合物材料主要有聚乙烯亚胺(polyethyleneimine,PEI)、基于聚酰胺-胺(poly-amindoamine,PAMAM)和聚丙烯亚胺(polypropylenimine,PPI)的树枝状大分子、聚氨基酯(poly β-aminoester,PBAE)、壳聚糖等[25](图3)。与LNP相比,基于聚合物的递送系统具有较高的多分散性、分子量和电荷密度,这导致基于聚合物的递送系统纯度较低、清除率较低且毒性较大。因此,它们在mRNA递送方面的临床应用不如可电离脂质纳米粒广泛。为了提高聚合物材料的转染效率和稳定性,并降低其毒性,人们对其结构进行了改造,包括添加脂质尾部、超支化基团和可生物降解分子[26]。
PEI含有大量的氨基,在生理pH值下可被质子化带正电荷。而mRNA是一种带负电的分子。因此,PEI通过静电相互作用与mRNA紧密地结合形成纳米复合物[27]。此复合物可以有效地压缩mRNA,并在一定程度上保护mRNA免受外界酶的降解[28]。在体内,PEI充当“质子海绵”促进mRNA释放到细胞质。然而,由于高电荷密度和高分子量,PEI具有较大的全身毒性和低生物降解性。用脂肪链修饰的低分子量PEI已被用于mRNA递送以降低毒性,如Peng等[29]合成了一种氟烷烃接枝的聚乙烯亚胺(F-PEI)。结果显示由F-PEI和编码肿瘤抗原的mRNA自组装形成的疫苗,无额外的佐剂,即可诱导树突状细胞成熟并触发有效的抗原呈递,从而引发抗肿瘤免疫反应。
树枝状大分子(dendrimers)是一类具有树枝状结构,由低聚物通过支化单元重复、线性连接而成的大分子,其通常由内核、聚合物主链和树枝单元的侧链组成[30,31]。作为一种阳离子聚合物,树枝状大分子的细胞毒性也需要通过表面改性来降低。England等[32]使用赖氨酸作为位点选择性锚,通过酰胺化反应引入了聚磺酰精氨酸和咪唑基团对PAMAM(P)和赖氨酸(L)树枝状大分子进行了化学改性。实验结果显示,与市售的转染剂jetPEI® 相比,改性的PAMAM树枝状大分子显示出更高的mRNA转染效率。
2.3 肽类纳米粒(peptide-based nanoparticles)
除了基于聚合物和脂质纳米粒的载体外,还可以使用基于肽类的载体递送mRNA。肽类由可生物降解的氨基酸组成,因此其具有高度的生物相容性。鱼精蛋白和细胞穿膜肽(cell-penetrating peptides,CPPs)是两种基于肽的载体,可用于mRNA递送。鱼精蛋白是一种天然的富含精氨酸的阳离子蛋白,可以把带负电的mRNA分子络合成纳米级别的核酸颗粒[33]。此外,鱼精蛋白-mRNA组成的纳米级颗粒还可以进一步制备成脂质纳米颗粒,这种脂质-鱼精蛋白-mRNA(lipid/protamine/mRNA, LPR)的制剂形式,兼有脂质纳米粒和鱼精蛋白的优势[34,35]。Shen等[36]制备了一种由鱼精蛋白/mRNA核和脂质壳组成的mRNA肿瘤疫苗(MVP)。结果显示,MVP 中的mRNA核和脂质壳可充分刺激树突状细胞中I型干扰素和炎症细胞因子的表达,在小鼠结直肠肿瘤和黑色素瘤模型中引起了有效的抗肿瘤免疫。
CPPs是一类由不多于30个氨基酸组成的小分子多肽。CPPs在生理pH值下带正电荷,可以与带负电荷的mRNA形成纳米结构[37]。Men等[38]将肿瘤细胞裂解液引入到DMP纳米颗粒[由DOTAP和(乙二醇)-b-聚(ε-己内酯)(mPEG-PCL)自组装形成]中,并将CPPs修饰在DMP表面,形成CLSV系统。然后将编码IL-22结合蛋白(interleukin-22 binding protein,IL-22BP)的mRNA与CLSV混合形成CLSV/IL-22BP复合物。结果表明,所构建的CLSV达到了激活免疫反应和增强mRNA递送的双重目的,在体外和体内均表现出较强的抑制肿瘤细胞生长的能力。
2.4 无机纳米材料(inorganic nanoparticles,INPs)
无机纳米颗粒是一种多功能纳米平台。虽然INPs的生物相容性不如脂质纳米粒和聚合物,但是INPs可以通过表面功能化修饰,获得具有适当的溶解度和分散性的纳米粒子。并且某些INPs具有磁性和光学性质,可实现对肿瘤的成像和消融[39]。典型的INPs包括金纳米粒子、二氧化硅纳米粒子、氧化铁纳米粒子、量子点等。
常用的INPs是介孔二氧化硅纳米粒子(mesoporous silica, MSN),其具有孔道均匀、高比表面积、大孔容和可生物降解性等优点。Phua等[40]开发了一种介孔二氧化硅纳米颗粒-mRNA (MSN-mRNA)皮下递送系统。结果显示,将编码卵清蛋白和粒细胞巨噬细胞集落刺激因子的裸mRNA和C16@MSNs组成的MSN-mRNA疫苗配方应用于小鼠E.G7-OVA预防性肿瘤模型时,产生了显著的肿瘤抑制作用。其次,INPs还可以与聚合物和脂质纳米粒联合,用于mRNA的递送。Shin等[41]开发了一种基于聚乙烯亚胺修饰的多孔二氧化硅纳米颗粒(PPSN)的递送平台,其携带细胞因子mRNA用于体内局部免疫治疗。结果表明,PPSN在定位mRNA翻译方面明显比美国食品药品监督管理局批准的LNP更有效。该研究显示了PPSN介导的mRNA递送作为肿瘤免疫疗法中基于mRNA治疗的特异性、有效性和安全性平台的潜力。
3. 非病毒递送系统在mRNA肿瘤疫苗中的应用
基于肿瘤的特点编码特异性抗原,使其顺利地被免疫细胞识别以激活免疫应答,是mRNA肿瘤疫苗的核心作用机制。现阶段主要研究的mRNA肿瘤疫苗编码的抗原主要有肿瘤相关抗原(tumor-associated antigen,TAA)、肿瘤特异性抗原(tumor specific antigen,TSA)和免疫调节因子3种类型[42]。通过对非病毒载体和对机体免疫系统的研究不断深入,目前已有多项mRNA肿瘤疫苗进入临床试验。中国进入临床试验阶段的mRNA肿瘤疫苗如表1所示。
表 1 中国进入临床试验阶段的mRNA肿瘤疫苗疫苗登记号 适应证 申办单位 研发阶段 ChiCTR2300071001 EGFR 突变阳性的晚期非小细胞肺癌 苏州艾博生物科技有限公司 探索性研究/预试验 CTR20232018 晚期实体瘤 北京立康生命科技有限公司 Ⅰ期临床 CTR20240438 新诊断的原发性脑胶质母细胞瘤(WHO 4级) 北京启辰生生物科技有限公司 Ⅰ期临床 ChiCTR2300077339 晚期胰腺癌 中国人民解放军总医院 Ⅰ期临床 ChiCTR2300071740 HPV16/18阳性的不可切除的复发性或转移性实体瘤 南阳医学高等专科学校第一附属医院 Ⅰ期临床 ChiCTR2200066118 晚期黑色素瘤 南阳医学高等专科学校第一附属医院 Ⅰ期临床 ChiCTR2200056172 晚期实体瘤 蚌埠医学院第一附属医院 Ⅰ期临床 ChiCTR2000029301 胃癌,食管癌 深圳市新合生物医疗科技有限公司 Ⅰ期临床 ChiCTR1900023000 晚期恶性实体瘤 斯微(上海)生物科技有限公司 Ⅰ期临床 3.1 编码TSA的mRNA肿瘤疫苗
肿瘤特异性抗原是体细胞中的非同义突变产生非自体蛋白[43],仅在肿瘤细胞中表达,而在正常细胞中不表达,因此支持对患者个体肿瘤抗原产生特异性免疫反应[44]。多个编码TSA的mRNA肿瘤疫苗已完成了Ⅰ/Ⅱ期临床试验[45]。BNT 122是BioNTech 和Genentech公司联合研发的一款编码胰腺导管腺癌(pancreatic ductal adenocarcinoma,PDAC)患者的20种新抗原的mRNA疫苗,使用LNP进行静脉注射给药。Ⅰ期临床试验结果显示,在手术切除后的PADC患者中,将BNT 122与化疗和免疫检查点疗法联用时,该mRNA疫苗对延缓PDAC患者的复发具有潜力。目前基于BNT122针对黑色素瘤和结直肠癌的治疗处于Ⅱ期临床试验阶段,针对实体瘤的临床试验即将进行Ⅱ期临床试验[46]。
3.2 编码TAA的mRNA肿瘤疫苗
肿瘤相关抗原是是一种在正常组织中表达但在肿瘤组织中过表达的抗原。具有肿瘤特异性弱、中枢免疫耐受性强、免疫原性弱的特点[47]。目前,利用多种TAA组合开发mRNA疫苗已成为一种趋势。BI
1361849 是Ludwig癌症研究所研发的一款编码NY-ESO-1、MAGE-C2、MAGE-C1、survivin、5T4和MUC1这6种TAA的mRNA疫苗,其使用鱼精蛋白作为载体。Ⅰb期临床研究评估了BI-1361849 联合局部放疗对Ⅳ期非小细胞肺癌(non-small-cell carcinoma,NSCLC)患者的有效性和安全性,结果显示,BI-1361849 具有良好的耐受性和免疫原性。2017年12月,在美国启动了BI-1361849 与 抗程序性死亡配体1 durvalumab和抗细胞毒性T淋巴细胞相关蛋白4(CTLA-4)抗体tremelimumab联用治疗NSCLC的Ⅰ/Ⅱ期临床试验。3.3 编码免疫调节因子的mRNA肿瘤疫苗
免疫调节因子是一类能够刺激或抑制特定免疫细胞功能的分子,包括细胞因子、共刺激因子等[48]。靶向肿瘤微环境实现肿瘤免疫治疗是目前抗肿瘤技术研究的热点。免疫调节因子可以重新激活免疫系统的抗肿瘤免疫反应并重塑积极的免疫微环境[49]。此外,编码免疫调节因子的mRNA疫苗还可作为编码TAA的mRNA疫苗的佐剂。mRNA-2752是Moderna公司研发的一款编码人 OX40L、IL-23 和 IL-36γ 的mRNA疫苗,其使用LNP作为载药系统,通过瘤内注射给药。在Ⅰ期的一项剂量递增研究(NCT03739931)中,mRNA-2752与免疫检查点阻断治疗剂durvalumab共同给药显示出抗肿瘤作用。目前mRNA-2752针对于三阴性乳腺癌、泌尿上皮癌、淋巴瘤和免疫检查点难治性黑色素瘤和非小细胞肺癌的Ⅰ期临床人体耐受性试验正在进行中。
4. 总结与展望
随着分子生物学的发展,mRNA疫苗给肿瘤免疫治疗带来了前所未有的希望。通过对mRNA化学修饰的改进以及开发更高效的非病毒载体递送系统可以提高mRNA肿瘤疫苗的安全性、稳定性和递送效率。基于基因治疗的良好前景,未来mRNA肿瘤疫苗的非病毒递送系统的主要发展方向可能包含以下几个方面:①将不同类型的非病毒载体联用共同递送mRNA疫苗,以结合不同类型载体的优点,实现提高纳米粒子稳定性、生物相容性和降低毒性等目的。②对非病毒载体进行功能化修饰以实现mRNA肿瘤疫苗的靶向性、光响应性、荧光可视性、pH响应性释放等功能。③阐明非病毒载体结构与其功能的关系,以及进入人体后与肿瘤微环境的相互作用机制。目前,正在开展许多基于脂质纳米颗粒、脂质-鱼精蛋白、阳离子脂质复合物和脂质多聚复合物等非病毒递送系统的mRNA肿瘤疫苗的临床试验。相信伴随多组学技术的发展和跨学科的融合,未来肿瘤特异性抗原的筛选将变得越来越精确。随着对非病毒递送系统研究的不断深入,mRNA肿瘤疫苗将在未来的肿瘤治疗中发挥巨大的作用。
-
[1] CHO N, SHAW J, KARURANGA S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045[J]. DIABETES RES CLIN PR, 2018, 138:271-281. doi: 10.1016/j.diabres.2018.02.023 [2] MARTÍNEZ-CASTELAO A, NAVARRO-GONZÁLEZ J, GÓRRIZ J, et al. The concept and the epidemiology of diabetic nephropathy have changed in recent years[J]. J Clin Med, 2015, 4(6):1207-1216. doi: 10.3390/jcm4061207 [3] SARAN R, ROBINSON B, ABBOTT K C, et al. US renal data system 2016 annual data report: epidemiology of kidney disease in the United States[J]. Am J Kidney Dis, 2017, 69(3):A4. doi: 10.1053/j.ajkd.2017.01.036 [4] VALENCIA WM, FLOREZ H.How to prevent the microvascular complications of type 2 diabetes beyond glucose control[J]. BMJ, 2017, 356: j1018. [5] CENTERS FOR DISEASE CONTROL AND PREVENTION (CDC). Incidence of end-stage renal disease attributed to diabetes among persons with diagnosed diabetes: - United States and Puerto Rico, 1996-2007[J]. MMWR Morb Mortal Wkly Rep, 2010, 59(42):1361-1366. [6] ZHANG L X, LONG J Y, JIANG W S, et al. Trends in chronic kidney disease in China[J]. N Engl J Med, 2016, 375(9):905-906. doi: 10.1056/NEJMc1602469 [7] OSAMA G, NASHWA F, NARAYANAN N, et al. Diabetic kidney disease: world wide difference of prevalence and risk factors[J]. J Nephropharmacology, 2016, 5(1):49-56. [8] STENVINKEL P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease[J]. Journal of internal medicine 2010; 268: 456-467. [9] IATCU C O, STEEN A, COVASA M. Gut microbiota and complications of type-2 diabetes[J]. Nutrients, 2021, 14(1):166. doi: 10.3390/nu14010166 [10] WILSON TANG W H, KITAI T, HAZEN S L. Gut microbiota in cardiovascular health and disease[J]. Circ Res, 2017, 120(7):1183-1196. doi: 10.1161/CIRCRESAHA.117.309715 [11] SCHROEDER B O, BÄCKHED F. Signals from the gut microbiota to distant organs in physiology and disease[J]. Nat Med, 2016, 22(10):1079-1089. doi: 10.1038/nm.4185 [12] YANG G, WEI J, LIU P, et al. Role of the gut microbiota in type 2 diabetes and related diseases[J]. METABOLISM, 2021, 117:154712. doi: 10.1016/j.metabol.2021.154712 [13] SHARMA M, LI Y Y, STOLL M L, et al. The epigenetic connection between the gut microbiome in obesity and diabetes[J]. Front Genet, 2020, 10:1329. doi: 10.3389/fgene.2019.01329 [14] JAWORSKA K, KOPACZ W, KOPER M, et al. Enalapril diminishes the diabetes-induced changes in intestinal morphology, intestinal RAS and blood SCFA concentration in rats[J]. Int J Mol Sci, 2022, 23(11):6060. doi: 10.3390/ijms23116060 [15] DU X, LIU J, XUE Y, et al. Alteration of gut microbial profile in patients with diabetic nephropathy[J]. Endocrine, 2021, 73(1):71-84. doi: 10.1007/s12020-021-02721-1 [16] QIN J J, LI Y R, CAI Z M, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes[J]. Nature, 2012, 490(7418):55-60. doi: 10.1038/nature11450 [17] KARLSSON F H, TREMAROLI V, NOOKAEW I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control[J]. Nature, 2013, 498(7452):99-103. doi: 10.1038/nature12198 [18] ZHANG L, LU Q Y, WU H, et al. The intestinal microbiota composition in early and late stages of diabetic kidney disease[J]. Microbiol Spectr, 2023, 11(4):e0038223. doi: 10.1128/spectrum.00382-23 [19] KALANTAR-ZADEH K, JAFAR T H, NITSCH D, et al. Chronic kidney disease[J]. Lancet, 2021, 398(10302):786-802. doi: 10.1016/S0140-6736(21)00519-5 [20] WANG X, YANG S, LI S, et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents[J]. Gut, 2020, 69(12):2131-2142. doi: 10.1136/gutjnl-2019-319766 [21] WANG S L, SHAO B Z, ZHAO S B, et al. Impact of paneth cell autophagy on inflammatory bowel disease[J]. Front Immunol, 2018, 9:693. doi: 10.3389/fimmu.2018.00693 [22] BUCKLEY A, TURNER J R. Cell biology of tight junction barrier regulation and mucosal disease[J]. Cold Spring Harb Perspect Biol, 2018, 10(1):a029314. doi: 10.1101/cshperspect.a029314 [23] MAHMOODPOOR F, RAHBAR SAADAT Y, BARZEGARI A, et al. The impact of gut microbiota on kidney function and pathogenesis[J]. Biomed Pharmacother, 2017, 93:412-419. doi: 10.1016/j.biopha.2017.06.066 [24] VAZIRI N D, YUAN J, NORRIS K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease[J]. Am J Nephrol, 2013, 37(1):1-6. doi: 10.1159/000345969 [25] MATHEWSON N D, JENQ R, MATHEW A V, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease[J]. Nat Immunol, 2016, 17(5):505-513. doi: 10.1038/ni.3400 [26] HUANG X Y, OSHIMA T, TOMITA T, et al. Butyrate alleviates cytokine-induced barrier dysfunction by modifying claudin-2 levels[J]. Biology, 2021, 10(3):205. doi: 10.3390/biology10030205 [27] NOWARSKI R, JACKSON R, GAGLIANI N, et al. Epithelial IL-18 equilibrium controls barrier function in colitis[J]. Cell, 2015, 163(6):1444-1456. doi: 10.1016/j.cell.2015.10.072 [28] TONG L C, WANG Y, WANG Z B, et al. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress[J]. Front Pharmacol, 2016, 7:253. [29] FENG Y H, WANG Y, WANG P, et al. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy[J]. Cell Physiol Biochem, 2018, 49(1):190-205. doi: 10.1159/000492853 [30] SAYIN S, WAHLSTRÖM A, FELIN J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist[J]. Cell Metab, 2013, 17(2):225-235. doi: 10.1016/j.cmet.2013.01.003 [31] SCHAAP F G, TRAUNER M, JANSEN P L M. Bile acid receptors as targets for drug development[J]. Nat Rev Gastroenterol Hepatol, 2014, 11(1):55-67. doi: 10.1038/nrgastro.2013.151 [32] HUANG W D, MA K, ZHANG J, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration[J]. Science, 2006, 312(5771):233-236. doi: 10.1126/science.1121435 [33] WANG X X, WANG D, LUO Y H, et al. FXR/TGR5 dual agonist prevents progression of nephropathy in diabetes and obesity[J]. J Am Soc Nephrol, 2018, 29(1):118-137. doi: 10.1681/ASN.2017020222 [34] XIAO H M, SUN X H, LIU R B, et al. Gentiopicroside activates the bile acid receptor Gpbar1 (TGR5) to repress NF-kappaB pathway and ameliorate diabetic nephropathy[J]. Pharmacol Res, 2020, 151:104559. doi: 10.1016/j.phrs.2019.104559 [35] MARQUARDT A, AL-DABET M M, GHOSH S, et al. Farnesoid X receptor agonism protects against diabetic tubulopathy: potential add-on therapy for diabetic nephropathy[J]. J Am Soc Nephrol, 2017, 28(11):3182-3189. doi: 10.1681/ASN.2016101123 [36] DU Y, YANG Y T, TANG G, et al. Butyrate alleviates diabetic kidney disease by mediating the miR-7a-5p/P311/TGF-β1 pathway[J]. FASEB J, 2020, 34(8):10462-10475. doi: 10.1096/fj.202000431R [37] DONG W P, JIA Y, LIU X X, et al. Sodium butyrate activates NRF2 to ameliorate diabetic nephropathy possibly via inhibition of HDAC[J]. J Endocrinol, 2017, 232(1):71-83. doi: 10.1530/JOE-16-0322 [38] XU Y H, GAO C L, GUO H L, et al. Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice[J]. J Endocrinol, 2018, 238(3): 231-244. [39] RAMEZANI A, MASSY Z A, MEIJERS B, et al. Role of the gut microbiome in uremia: a potential therapeutic target[J]. Am J Kidney Dis, 2016, 67(3):483-498. [40] ZHANG F, QI L L, FENG Q Y, et al. HIPK2 phosphorylates HDAC3 for NF-κB acetylation to ameliorate colitis-associated colorectal carcinoma and sepsis[J]. Proc Natl Acad Sci USA, 2021, 118(28):e2021798118. doi: 10.1073/pnas.2021798118 [41] HU X Y, LI S M, FU Y H, et al. Targeting gut microbiota as a possible therapy for mastitis[J]. Eur J Clin Microbiol Infect Dis, 2019, 38(8):1409-1423. doi: 10.1007/s10096-019-03549-4 [42] HU Z B, LU J, CHEN P P, et al. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis[J]. Theranostics, 2020, 10(6):2803-2816. doi: 10.7150/thno.40571 [43] LU C C, HU Z B, WANG R, et al. Gut microbiota dysbiosis-induced activation of the intrarenal renin-angiotensin system is involved in kidney injuries in rat diabetic nephropathy[J]. Acta Pharmacol Sin, 2020, 41(8):1111-1118. doi: 10.1038/s41401-019-0326-5 [44] GRUPPEN E G, GARCIA E, CONNELLY M A, et al. TMAO is associated with mortality: impact of modestly impaired renal function[J]. Sci Rep, 2017, 7(1):13781. doi: 10.1038/s41598-017-13739-9 [45] SUN G P, YIN Z M, LIU N Q, et al. Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of diet-induced obesity[J]. Biochem Biophys Res Commun, 2017, 493(2):964-970. doi: 10.1016/j.bbrc.2017.09.108 [46] AL-OBAIDE M, SINGH R, DATTA P, et al. Gut microbiota-dependent trimethylamine-N-oxide and serum biomarkers in patients with T2DM and advanced CKD[J]. J Clin Med, 2017, 6(9):86. doi: 10.3390/jcm6090086 [47] YANG M X, ZHANG R, ZHUANG C F, et al. Serum trimethylamine N-oxide and the diversity of the intestinal microbial flora in type 2 diabetes complicated by diabetic kidney disease[J]. Clin Lab, 2022, 68(5): 10.7754/Clin. Lab. 2021.210836. [48] FANG Q, ZHENG B J, LIU N, et al. Trimethylamine N-oxide exacerbates renal inflammation and fibrosis in rats with diabetic kidney disease[J]. Front Physiol, 2021, 12:682482. doi: 10.3389/fphys.2021.682482 [49] MAO Z H, GAO Z X, LIU D W, et al. Gut microbiota and its metabolites–molecular mechanisms and management strategies in diabetic kidney disease[J]. Front Immunol, 2023, 14:1124704. doi: 10.3389/fimmu.2023.1124704 期刊类型引用(1)
1. 王经纬,陈晓颙,袁怡,刘晨曦,胡敏. 基于流变学研究外用半固体制剂质量等同性的应用进展. 药学前沿. 2024(10): 300-309 .  百度学术
百度学术其他类型引用(2)
-

 点击查看大图
点击查看大图
计量
- 文章访问数: 6859
- HTML全文浏览量: 2840
- PDF下载量: 47
- 被引次数: 3




 下载:
下载:

 下载:
下载:
