-
在全球范围内,缺血性脑卒中(IS)仍然是导致死亡和严重残疾的最主要原因之一[1]。在IS的治疗过程中,除因出血风险而存在禁忌外,几乎所有IS的患者都应给予抗血小板药物进行二次卒中预防[2], 阿司匹林联合氯吡格雷的双重抗血小板治疗在IS发病早期发挥着重要作用[3]。然而临床应用中,脑血管病患者在服用氯吡格雷药物治疗期间存在个体差异,一些患者在服药后出现了病情反复发作或者病情加重的现象,称为氯吡格雷抵抗(CR)[4]。研究表明,导致氯吡格雷抵抗的因素是多种多样的,尚无统一的结论。包括性别[5-6]、年龄[6]、BMI[6-7]、糖尿病[5,8]、高脂血症[8]、高同型半胱氨酸[5,8]、药物间相互作用[8](钙离子拮抗剂、质子泵抑制剂)、遗传基因多态性[9-10](参与氧化代谢的相关基因CYP2C19、与其活化有关基因ABCB1)等均会导致该药物出现抵抗现象。但亦有部分研究报道得出相反结论[11]。且研究主要集中于心血管领域,尤其是经皮冠状动脉介入(PCI)术后患者,而针对IS患者发生CR影响因素的报道相对较少。氯吡格雷属于噻吩吡啶类化学衍生物,需要在多种酶的参与下才能够生成有活性的化合物,该活性代谢产物与血小板上的二磷酸腺苷(ADP)受体P2Y12共价特异性地结合,减少ADP所刺激的血小板凝聚,阻断ADP诱导的糖蛋白与纤维蛋白原PIIb/IIIa 受体的结合,发挥抗栓的效应[12-13]。故本研究利用血栓弹力图(TEG)测定ADP数值,对脑卒中患者的基线资料及CYP2C19基因多态性进行统计分析,评估产生CR现象的影响因素,为IS临床个体化用药、预测卒中复发风险提供精准医疗方案。
-
本研究利用HIS系统选取2021年4月至2022年7月在中部战区总医院神经内科住院治疗的IS患者。(1)纳入标准:①符合《中国脑急性缺血性脑卒中诊治指南》[14]或IS的相关诊断,且经CT或MRI影像学等证实;②年龄均>18岁;③未进行静脉溶栓或取栓治疗;④均采用氯吡格雷联合阿司匹林进行抗血小板治疗。(2)排除标准:①存在出血性疾病或颅内感染者;②合并恶性肿瘤或严重心、肝、肺、肾脏器官功能衰竭者;③有认知障碍者;④依从性差者;⑤对本研究药物过敏者。该研究已获得中部战区总医院医学伦理委员会审查批准,审查号:[2021]025-01。
-
所有入组患者入院后均给予改善循环(银杏达莫)、稳定血斑块(阿托伐他汀)、护胃(泮托拉唑)等常规治疗。在常规治疗基础上给予阿司匹林(拜耳医药保健有限公司,国药准字:J20171021,规格:100 mg/片)100 mg每日一次和硫酸氢氯吡格雷[赛诺菲(杭州)制药有限公司,国药准字:H20056410,规格:75 mg/片]75 mg 每日一次,均为3个月一个疗程。同时根据基础疾病给予降血压、降血糖及调血脂治疗。
-
收集所有入组患者的基线资料,包括姓名、性别、年龄、既往有无高血压病、糖尿病、冠心病、高脂血症、吸烟史(戒烟小于5年)、饮酒史(戒酒小于5年)、既往有无缺血性脑卒中病史及患者住院期间合并用药等。记录患者在住院后次日清晨空腹采集的静脉血各项检验指标,包括血常规、肝肾功能、凝血功能等。
-
患者在服用阿司匹林联合氯吡格雷药物7~14 d,空腹采集静脉血,分别注射入柠檬酸钠抗凝管1和肝素抗凝管2中。利用TEG-5000型TEG凝血分析仪(美国Haemoscope公司)检测柠檬酸-高岭土样品,得出ADP抑制率。将血小板聚集抑制率>30%定义为氯吡格雷非抵抗,血小板聚集抑制率≤30%定义为氯吡格雷抵抗[15]。
-
患者入院后次日清晨空腹抽取静脉血4 ml于EDTA抗凝管中,利用全自动杂交仪系统(BR-526-24,上海百傲科技公司)对CYP2C19*1、CYP2C19*2及CYP2C19*3位点进行检测。该检测过程主要包括全血DNA的提取、PCR扩增、芯片杂交显色,利用基因芯片识读仪自动读取结果。根据CYP2C19基因多态性进行药物代谢分组[16],即:快代谢组:EM,CYP2C19*1*1,中代谢组:IM,CYP2C19*1*2、CYP2C19*1*3,慢代谢组:PM,CYP2C19*2*2、CYP2C19*2*3、CYP2C19*3*3。
-
采用SPSS 26.0统计软件进行数据分析,计量资料服从正态分布,采用(
$\bar x $ ±s)表示,组间比较采用独立样本t检验。若计量资料属于非正态分布,则以中位数和四分位数[M(P25,P75)]表示,组间比较采用Mann-Whitney U检验。计数资料以例数和百分比[例(%)]表示,组间比较采用χ2检验,将IS患者发生氯吡格雷抵抗的影响因素进一步进行多因素Logisti回归分析,以P<0.05为差异有统计学意义。 -
本研究共纳入缺血性脑卒中患者202例,年龄35~86岁,其中,CR组87例,NCR组115例,CR组患者合并糖尿病的比例明显高于NCR组,差异有统计学意义(P<0.05)。在性别、年龄、吸烟、饮酒、既往有无缺血性脑卒中、高血压病、冠心病、高脂血症及合并用药方面,两组间差异均无统计学意义(P>0.05),见表1。
表 1 抵抗组和非抵抗组患者基线资料比较
基本信息参数 CR组(n=87,%) NCR组(n=112,%) x2/t P 年龄 64.52±1.07 62.39±0.97 −1.47 0.14 性别(男) 65(74.7) 86(74.8) 0.00 0.99 吸烟史 43(49.4) 56(48.7) 0.11 0.92 饮酒史 37(42.5) 41(35.7) 0.99 0.32 IS既往史 16(18.4) 22(19.1) 0.02 0.89 高血压 83(95.4) 106(92.2) 0.41 0.53 糖尿病 35(40.2) 22(19.6) 10.15 0.03 冠心病 7(8.0) 10(8.7) 0.03 0.87 高脂血症 40(46.0) 56(48.7) 0.15 0.70 合并用药 质子泵抑制剂 61(70.1) 84(73.0) 0.21 0.67 丁苯酞注射液 67(77.0) 83(72.2) 0.61 0.44 -
氯吡格雷抵抗组血常规的白细胞计数水平高于非抵抗组,且组间差异有统计学意义(P<0.05)。两组患者红细胞、血红蛋白、血小板计数、凝血酶原时间、D-二聚体、血糖、总胆固醇、甘油三酯、高密度脂蛋白胆固醇、低密度脂蛋白、超敏C反应蛋白、血同型半胱氨酸、直接胆红素、总胆红素、谷草转氨酶、谷丙转氨酶的组间差异均无统计学意义(P>0.05),见表2。
表 2 两组患者实验室检测指标比较
检测项目 抵抗组(n=87) 非抵抗组(n=115) 检验值 P 白细胞 6.70(5.60,8.40) 6.40(5.30,7.30) 2.14b 0.03 红细胞 4.56±0.34 4.44±0.05 −0.40a 0.69 血红蛋白 131(119,139) 134(120,143) −1.15b 0.11 血小板 187(148,227) 206(138,232) −1.62b 0.08 凝血酶原时间 11.21±0.65 11.18±0.85 −0.32a 0.92 D-二聚体 0.30±0.07 0.29±0.09 −0.78a 0.44 血糖 6.03±0.22 6.45±0.21 1.33a 0.18 总胆固醇 4.37(3.72,5.04) 4.32(3.73,5.05) −0.19b 0.85 甘油三酯 1.81±0.13 1.75±0.11 −0.36a 0.72 高密度脂蛋白 1.19(0.99,1.35) 1.14(1.00,1.32) −1.07b 0.29 低密度脂蛋白 2.41(1.94,2.80) 2.27(1.87,2.75) −0.50b 0.62 超敏C反应蛋白 1.91(1.04,5.40) 1.76(0.84,3.50) 1.65b 0.09 血同型半胱氨酸 17.32±1.18 15.81±0.61 −1.22a 0.23 直接胆红素 3.32±1.25 3.79±0.33 1.47a 0.14 总胆红素 13.33±0.47 13.71±0.42 0.60a 0.54 谷草转氨酶 23.00(19.00,28.00) 22.00(18.00,27.00) −0.89b 0.37 谷丙转氨酶 22.00(17.00,31.00) 21.00(15.00,28.79) −1.42b 0.15 注: a为 χ2 值,b为z值。 -
入选的202名患者中,CYP2C19 *1、CYP2C19 *2及CYP2C19*3等位基因的频率分别为62.26%、33.17%及4.21%,所有基因位点均符合Hardy-Weinberg平衡定律,差异无统计学意义(P>0.05),表明该群体具有代表性,详见表3。
表 3 202例IS患者 CYP2C19 基因型及等位基因的频率分布
CYP2C19等位基因 等位基因数/个 基因频率/% CYP2C19 *1 253 62.62 CYP2C19 *2 134 33.17 CYP2C19 *3 17 4.21 -
CYP2C19 IM组患者发生氯吡格雷抵抗的比例显著高于EM组和PM组,差异具有统计学意义(P>0.05),见表4。
表 4 不同CYP2C19代谢类型的CR发生率比较(n,%)
CYP2C19
代谢类型氯吡格雷
抵抗[n(%)]非氯吡格雷
抵抗[n(%)]χ2 p EM 24(27.59) 56(48.70) 9.95 0.007 IM 50(57.47) 43(37.39) PM 13(14.94) 16(13.91) -
3组IS患者中ADP抑制率均不符合正态分布,因此采用非参数检验。EM组ADP抑制率的中位数为39.6%,IM组为26.10%,PM组为31.95%。3组血小板抑制率中位数差异具有统计学意义(P<0.05);两两比较,IM组的血小板抑制率明显低于EM组,差异具有统计学意义(P<0.05);其他组间差异无统计学意义(P>0.05),详见表5。
表 5 不同基因型血小板抑制率的比较
CYP2C19基因分组 基因型数/个 血小板抑制率/% EM 39.60(26.20,53.95) CYP2C19*1*1 80 IM 26.10(15.20,53.60)* CYP2C19*1*2 81 CYP2C19*1*3 12 PM 31.95(20.65,65.40) CYP2C19*2*2 24 CYP2C19*2*3 5 χ2 6.31 P 0.04 注:3组间比较采用 Kruskal-Wallis H检验;两两比较采用 Mann- Whitney U检验;* P<0.05,与EM组比较。 -
以发生CR作为因变量,将上述研究中存在统计学差异(P<0.05)的项目作为自变量进行赋值,包括合并糖尿病(未合并糖尿病者赋值0,合并糖尿病者赋值1)、CYP2C19代谢类型(IM赋值1,其余赋值0),白细胞计数为连续型数值变量,纳入二元Logistic回归模型中进行分析。结果显示,合并糖尿病、高白细胞计数水平、CYP2C19中代谢型为发生氯吡格雷抵抗的独立危险因素,见表6。
表 6 发生氯吡格雷抵抗危险因素的Logistic回归分析结果
因素 B SE Wald P OR 95%CI 合并糖尿病 0.595 0.227 4.496 0.026 0.851 (0.707,1.191) 白细胞计数 0.211 0.076 7.774 0.005 1.235 (1.065,1.432) CYP2C19
中代谢型0.901 0.291 9.565 0.002 0.368 (0.197,0.686) -
氯吡格雷是继阿司匹林后在心脑血管中被广泛使用的抗血小板药物。有报道表明氯吡格雷联合阿司匹林在治疗缺血性脑卒中疗效优于单独使用阿司匹林,且不会增加脑卒中患者发生出血的风险[3,17]。在临床应用中仍有一些患者在服用氯吡格雷后,不能够抑制ADP诱导的血小板凝聚,也称为氯吡格雷抵抗或者氯吡格雷低反应性[4]。目前国际上关于氯吡格雷抵抗的诊断并无确切的标准[18],有文献称化学比浊法属于比较经典的血小板功能检测方法,但是由于在操作过程中步骤比较繁多且尚无统一的标准化流程,现已逐渐被其它检测方法所取代[19]。有国外研究报道将床旁快速血小板分析(Verify Now)检测系统的检测结果定义为“金标准”,但是由于该检测方法成本较高,因此在国内临床中并没有被大规模应用。而现在国内较常使用的抗血小板效果的监测方法有TEG、血小板分析仪、PL-11等,TEG检测血小板功能操作便捷、迅速、受外界影响较小等优点,现已在临床中被广泛使用[20]。
氯吡格雷本身属于无活性的前体药物,需在多种酶的参与下生成有活性的化合物,才能发挥抑制血小板聚集作用。细胞色素P450酶参与了两步氧化代谢过程。国外有研究报道[21]CYP2C19*2、CYP2C19*3等位基因的突变主要以亚洲人为主,发生在非洲和欧洲的突变率较低,携带CYP2C19*2、CYP2C19*3基因的突变可降低细胞色素P450酶的活性,使得活性代谢物的水平减少,从而导致该药物的临床疗效不佳,所以IM和PM组氯吡格雷抗血小板活性会受到CYP2C19基因多态性的影响。本研究结果显示,CYP2C19 IM组患者发生氯吡格雷抵抗的比例显著高于EM组和PM组,血小板抑制率明显低于EM组,差异均具有统计学意义,而PM组血小板抑制率虽然低于EM组,但差异无统计学意义,可能是PM组(14.36%)人群占比显著低于IM组(46.04%)和EM组(39.60%)造成的。
目前已有研究表明糖尿病患者在服用氯吡格雷后血小板抑制率较差[22-23],但其机制尚不明确。在小鼠模型中的研究结果表明,高血糖可以通过下调CYP酶影响氯吡格雷药物活化,从而导致抗血小板浓度降低[24]。Angiolillo等[25]针对冠心病合并2型糖尿病患者诱导的血小板抑制率减弱的研究中,解释其原因可能是长期的高血糖水平使血小板信号通路上调降低了抗血小板药物的药效,从而导致药物抵抗的发生。此外,糖尿病会影响肝脏代谢与胃肠道吸收,这可能会影响药物的吸收和代谢[26]。本研究结果也显示,合并糖尿病是发生氯吡格雷抵抗的独立风险因素。
本研究结果还表明高白细胞计数可能是IS患者发生CR的独立危险因素(OR=1.235,95% CI:1.065,1.432),但目前在IS患者中有关白细胞计数与CR之间关系的研究较少。有研究发现炎症标志物升高增加心血管事件的风险,血小板聚集和炎性因子之间的相互作用在急性冠脉综合征中起着非常重要的作用,而炎症调节越来越被认为是当代的治疗靶点,接受氯吡格雷联合阿司匹林双重抗血小板治疗的急性冠状动脉综合征的患者中,白细胞计数与临床疗效密切相关[27]。也有研究者在冠心病患者中发现白细胞中的3个长链非编码RNA与CR的发生有关[28]。IS随着细胞死亡和脑组织受损,分子危险信号通过激活更多的小胶质细胞和浸润白细胞进行前馈反应,释放更多的具有促炎作用的细胞因子。炎症加剧了脑细胞受损程度,使外周血中释放更多的细胞因子,这些细胞因子参与了脑卒中的进展,影响其预后[29-30]。因此,在临床中,高白细胞计数患者服用氯吡格雷时应警惕CR的发生。
本研究的局限性主要包括以下几点:①样本量相对较少;②TEG检测过程中受血凝速度、红细胞聚集状态等因素影响;③患者服药7~14 d只检测1次ADP抑制率,不能反应整体水平;④未直接检测血浆氯吡格雷及其活性代谢物水平。上述因素可能对研究结果产生偏倚,今后还需要进一步的验证。
Analysis on risk factors of clopidogrel resistance in patients with ischemic stroke
-
摘要:
目的 探讨缺血性脑卒中患者服用氯吡格雷治疗后发生药物抵抗的危险因素,为促进临床个体化药物治疗提供依据。 方法 选取202例诊断为缺血性脑卒中的住院患者,入中部战区总医院后均给予双抗治疗(阿司匹林+氯吡格雷),住院期间通过芯片杂交法检测CYP2C19基因型,将CYP2C19基因多态性根据药物代谢类型分为快代谢组、中代谢组和慢代谢组。患者服药7~14 d根据血栓弹力图(TEG)检测由腺苷二磷酸(ADP)诱导的血小板抑制率,将ADP<30%为氯吡格雷药物抵抗组,ADP≥30%为非抵抗组。采用Logistic回归分析研究发生氯吡格雷抵抗的危险因素。 结果 202例缺血性脑卒中患者中,抵抗组87例,非抵抗组115例。氯吡格雷抵抗组合并糖尿病的患者比例和白细胞计数水平高于非抵抗组,差异均具有统计学意义(P<0.05)。CYP2C19中代谢组患者发生氯吡格雷抵抗的比例显著高于快代谢组,血小板抑制率也明显低于快代谢组,差异均具有统计学意义(P<0.05)。 结论 合并糖尿病、高白细胞计数水平及CYP2C19中代谢型是缺血性脑卒中患者发生氯吡格雷抵抗的独立危险因素。 -
关键词:
- 缺血性脑卒中 /
- 氯吡格雷抵抗 /
- 血栓弹力图 /
- 细胞色素氧化酶P450酶2C19 /
- 危险因素
Abstract:Objective To investigate the risk factors of drug resistance in patients with ischemic stroke by clopidogrel therapy and provide references for promoting clinical individualized drug therapy. Methods A total of 202 inpatients diagnosed with ischemic stroke were admitted and given dual anti-treatment (aspirin+clopidogrel). CYP2C19 genotype was detected by microarray hybridization during hospitalization, and CYP2C19 gene polymorphisms were classified into fast metabolism group, medium metabolism group and slow metabolism group according to the type of drug metabolism. Patients were tested for platelet inhibition induced by adenosine diphosphate (ADP) according to thromboelastographic (TEG) on 7~14 d of drug administration. ADP <30% was classified as clopidogrel drug resistance group and ADP ≥30% as non-resistance group. Logistic regression analysis was used to study the risk factors for the development of clopidogrel resistance. Results Among 202 patients with ischemic stroke, 87 were in the resistant group and 115 in the non-resistant group. The proportion of patients with clopidogrel resistance combined with diabetes and the level of white blood cell count were higher than that in the non-resistant group, and the differences were statistically significant (P<0.05).The proportion of patients with clopidogrel resistance in the CYP2C19 intermediate metabolism group was significantly higher than that in the fast metabolism group, and the rate of platelet inhibition was also significantly lower than that in the fast metabolism group, all with statistically significant differences (P<0.05). Conclusion Combined diabetes mellitus, high white blood cell count levels and CYP2C19 mid-metabolic phenotype are independent risk factors for the development of clopidogrel resistance in patients with ischemic stroke. -
在全球范围内,缺血性脑卒中(IS)仍然是导致死亡和严重残疾的最主要原因之一[1]。在IS的治疗过程中,除因出血风险而存在禁忌外,几乎所有IS的患者都应给予抗血小板药物进行二次卒中预防[2], 阿司匹林联合氯吡格雷的双重抗血小板治疗在IS发病早期发挥着重要作用[3]。然而临床应用中,脑血管病患者在服用氯吡格雷药物治疗期间存在个体差异,一些患者在服药后出现了病情反复发作或者病情加重的现象,称为氯吡格雷抵抗(CR)[4]。研究表明,导致氯吡格雷抵抗的因素是多种多样的,尚无统一的结论。包括性别[5-6]、年龄[6]、BMI[6-7]、糖尿病[5,8]、高脂血症[8]、高同型半胱氨酸[5,8]、药物间相互作用[8](钙离子拮抗剂、质子泵抑制剂)、遗传基因多态性[9-10](参与氧化代谢的相关基因CYP2C19、与其活化有关基因ABCB1)等均会导致该药物出现抵抗现象。但亦有部分研究报道得出相反结论[11]。且研究主要集中于心血管领域,尤其是经皮冠状动脉介入(PCI)术后患者,而针对IS患者发生CR影响因素的报道相对较少。氯吡格雷属于噻吩吡啶类化学衍生物,需要在多种酶的参与下才能够生成有活性的化合物,该活性代谢产物与血小板上的二磷酸腺苷(ADP)受体P2Y12共价特异性地结合,减少ADP所刺激的血小板凝聚,阻断ADP诱导的糖蛋白与纤维蛋白原PIIb/IIIa 受体的结合,发挥抗栓的效应[12-13]。故本研究利用血栓弹力图(TEG)测定ADP数值,对脑卒中患者的基线资料及CYP2C19基因多态性进行统计分析,评估产生CR现象的影响因素,为IS临床个体化用药、预测卒中复发风险提供精准医疗方案。
1. 材料和方法
1.1 研究对象
本研究利用HIS系统选取2021年4月至2022年7月在中部战区总医院神经内科住院治疗的IS患者。(1)纳入标准:①符合《中国脑急性缺血性脑卒中诊治指南》[14]或IS的相关诊断,且经CT或MRI影像学等证实;②年龄均>18岁;③未进行静脉溶栓或取栓治疗;④均采用氯吡格雷联合阿司匹林进行抗血小板治疗。(2)排除标准:①存在出血性疾病或颅内感染者;②合并恶性肿瘤或严重心、肝、肺、肾脏器官功能衰竭者;③有认知障碍者;④依从性差者;⑤对本研究药物过敏者。该研究已获得中部战区总医院医学伦理委员会审查批准,审查号:[2021]025-01。
1.2 治疗方法
所有入组患者入院后均给予改善循环(银杏达莫)、稳定血斑块(阿托伐他汀)、护胃(泮托拉唑)等常规治疗。在常规治疗基础上给予阿司匹林(拜耳医药保健有限公司,国药准字:J20171021,规格:100 mg/片)100 mg每日一次和硫酸氢氯吡格雷[赛诺菲(杭州)制药有限公司,国药准字:H20056410,规格:75 mg/片]75 mg 每日一次,均为3个月一个疗程。同时根据基础疾病给予降血压、降血糖及调血脂治疗。
1.3 资料收集
收集所有入组患者的基线资料,包括姓名、性别、年龄、既往有无高血压病、糖尿病、冠心病、高脂血症、吸烟史(戒烟小于5年)、饮酒史(戒酒小于5年)、既往有无缺血性脑卒中病史及患者住院期间合并用药等。记录患者在住院后次日清晨空腹采集的静脉血各项检验指标,包括血常规、肝肾功能、凝血功能等。
1.4 TEG及相关参数测定
患者在服用阿司匹林联合氯吡格雷药物7~14 d,空腹采集静脉血,分别注射入柠檬酸钠抗凝管1和肝素抗凝管2中。利用TEG-5000型TEG凝血分析仪(美国Haemoscope公司)检测柠檬酸-高岭土样品,得出ADP抑制率。将血小板聚集抑制率>30%定义为氯吡格雷非抵抗,血小板聚集抑制率≤30%定义为氯吡格雷抵抗[15]。
1.5 CYP2C19 基因检测
患者入院后次日清晨空腹抽取静脉血4 ml于EDTA抗凝管中,利用全自动杂交仪系统(BR-526-24,上海百傲科技公司)对CYP2C19*1、CYP2C19*2及CYP2C19*3位点进行检测。该检测过程主要包括全血DNA的提取、PCR扩增、芯片杂交显色,利用基因芯片识读仪自动读取结果。根据CYP2C19基因多态性进行药物代谢分组[16],即:快代谢组:EM,CYP2C19*1*1,中代谢组:IM,CYP2C19*1*2、CYP2C19*1*3,慢代谢组:PM,CYP2C19*2*2、CYP2C19*2*3、CYP2C19*3*3。
1.6 统计学方法
采用SPSS 26.0统计软件进行数据分析,计量资料服从正态分布,采用(
$\bar x $ ±s)表示,组间比较采用独立样本t检验。若计量资料属于非正态分布,则以中位数和四分位数[M(P25,P75)]表示,组间比较采用Mann-Whitney U检验。计数资料以例数和百分比[例(%)]表示,组间比较采用χ2检验,将IS患者发生氯吡格雷抵抗的影响因素进一步进行多因素Logisti回归分析,以P<0.05为差异有统计学意义。2. 结果
2.1 基线资料分析
本研究共纳入缺血性脑卒中患者202例,年龄35~86岁,其中,CR组87例,NCR组115例,CR组患者合并糖尿病的比例明显高于NCR组,差异有统计学意义(P<0.05)。在性别、年龄、吸烟、饮酒、既往有无缺血性脑卒中、高血压病、冠心病、高脂血症及合并用药方面,两组间差异均无统计学意义(P>0.05),见表1。
表 1 抵抗组和非抵抗组患者基线资料比较基本信息参数 CR组(n=87,%) NCR组(n=112,%) x2/t P 年龄 64.52±1.07 62.39±0.97 −1.47 0.14 性别(男) 65(74.7) 86(74.8) 0.00 0.99 吸烟史 43(49.4) 56(48.7) 0.11 0.92 饮酒史 37(42.5) 41(35.7) 0.99 0.32 IS既往史 16(18.4) 22(19.1) 0.02 0.89 高血压 83(95.4) 106(92.2) 0.41 0.53 糖尿病 35(40.2) 22(19.6) 10.15 0.03 冠心病 7(8.0) 10(8.7) 0.03 0.87 高脂血症 40(46.0) 56(48.7) 0.15 0.70 合并用药 质子泵抑制剂 61(70.1) 84(73.0) 0.21 0.67 丁苯酞注射液 67(77.0) 83(72.2) 0.61 0.44 2.2 实验室检验指标分析
氯吡格雷抵抗组血常规的白细胞计数水平高于非抵抗组,且组间差异有统计学意义(P<0.05)。两组患者红细胞、血红蛋白、血小板计数、凝血酶原时间、D-二聚体、血糖、总胆固醇、甘油三酯、高密度脂蛋白胆固醇、低密度脂蛋白、超敏C反应蛋白、血同型半胱氨酸、直接胆红素、总胆红素、谷草转氨酶、谷丙转氨酶的组间差异均无统计学意义(P>0.05),见表2。
表 2 两组患者实验室检测指标比较检测项目 抵抗组(n=87) 非抵抗组(n=115) 检验值 P 白细胞 6.70(5.60,8.40) 6.40(5.30,7.30) 2.14b 0.03 红细胞 4.56±0.34 4.44±0.05 −0.40a 0.69 血红蛋白 131(119,139) 134(120,143) −1.15b 0.11 血小板 187(148,227) 206(138,232) −1.62b 0.08 凝血酶原时间 11.21±0.65 11.18±0.85 −0.32a 0.92 D-二聚体 0.30±0.07 0.29±0.09 −0.78a 0.44 血糖 6.03±0.22 6.45±0.21 1.33a 0.18 总胆固醇 4.37(3.72,5.04) 4.32(3.73,5.05) −0.19b 0.85 甘油三酯 1.81±0.13 1.75±0.11 −0.36a 0.72 高密度脂蛋白 1.19(0.99,1.35) 1.14(1.00,1.32) −1.07b 0.29 低密度脂蛋白 2.41(1.94,2.80) 2.27(1.87,2.75) −0.50b 0.62 超敏C反应蛋白 1.91(1.04,5.40) 1.76(0.84,3.50) 1.65b 0.09 血同型半胱氨酸 17.32±1.18 15.81±0.61 −1.22a 0.23 直接胆红素 3.32±1.25 3.79±0.33 1.47a 0.14 总胆红素 13.33±0.47 13.71±0.42 0.60a 0.54 谷草转氨酶 23.00(19.00,28.00) 22.00(18.00,27.00) −0.89b 0.37 谷丙转氨酶 22.00(17.00,31.00) 21.00(15.00,28.79) −1.42b 0.15 注: a为 χ2 值,b为z值。 2.3 CYP2C19基因多态性分布
入选的202名患者中,CYP2C19 *1、CYP2C19 *2及CYP2C19*3等位基因的频率分别为62.26%、33.17%及4.21%,所有基因位点均符合Hardy-Weinberg平衡定律,差异无统计学意义(P>0.05),表明该群体具有代表性,详见表3。
表 3 202例IS患者 CYP2C19 基因型及等位基因的频率分布CYP2C19等位基因 等位基因数/个 基因频率/% CYP2C19 *1 253 62.62 CYP2C19 *2 134 33.17 CYP2C19 *3 17 4.21 2.4 不同CYP2C19代谢类型患者发生氯吡格雷抵抗的比较
CYP2C19 IM组患者发生氯吡格雷抵抗的比例显著高于EM组和PM组,差异具有统计学意义(P>0.05),见表4。
表 4 不同CYP2C19代谢类型的CR发生率比较(n,%)CYP2C19
代谢类型氯吡格雷
抵抗[n(%)]非氯吡格雷
抵抗[n(%)]χ2 p EM 24(27.59) 56(48.70) 9.95 0.007 IM 50(57.47) 43(37.39) PM 13(14.94) 16(13.91) 2.5 CYP2C19不同基因型血小板抑制率的比较
3组IS患者中ADP抑制率均不符合正态分布,因此采用非参数检验。EM组ADP抑制率的中位数为39.6%,IM组为26.10%,PM组为31.95%。3组血小板抑制率中位数差异具有统计学意义(P<0.05);两两比较,IM组的血小板抑制率明显低于EM组,差异具有统计学意义(P<0.05);其他组间差异无统计学意义(P>0.05),详见表5。
表 5 不同基因型血小板抑制率的比较CYP2C19基因分组 基因型数/个 血小板抑制率/% EM 39.60(26.20,53.95) CYP2C19*1*1 80 IM 26.10(15.20,53.60)* CYP2C19*1*2 81 CYP2C19*1*3 12 PM 31.95(20.65,65.40) CYP2C19*2*2 24 CYP2C19*2*3 5 χ2 6.31 P 0.04 注:3组间比较采用 Kruskal-Wallis H检验;两两比较采用 Mann- Whitney U检验;* P<0.05,与EM组比较。 2.6 发生氯吡格雷抵抗的危险因素分析
以发生CR作为因变量,将上述研究中存在统计学差异(P<0.05)的项目作为自变量进行赋值,包括合并糖尿病(未合并糖尿病者赋值0,合并糖尿病者赋值1)、CYP2C19代谢类型(IM赋值1,其余赋值0),白细胞计数为连续型数值变量,纳入二元Logistic回归模型中进行分析。结果显示,合并糖尿病、高白细胞计数水平、CYP2C19中代谢型为发生氯吡格雷抵抗的独立危险因素,见表6。
表 6 发生氯吡格雷抵抗危险因素的Logistic回归分析结果因素 B SE Wald P OR 95%CI 合并糖尿病 0.595 0.227 4.496 0.026 0.851 (0.707,1.191) 白细胞计数 0.211 0.076 7.774 0.005 1.235 (1.065,1.432) CYP2C19
中代谢型0.901 0.291 9.565 0.002 0.368 (0.197,0.686) 3. 讨论
氯吡格雷是继阿司匹林后在心脑血管中被广泛使用的抗血小板药物。有报道表明氯吡格雷联合阿司匹林在治疗缺血性脑卒中疗效优于单独使用阿司匹林,且不会增加脑卒中患者发生出血的风险[3,17]。在临床应用中仍有一些患者在服用氯吡格雷后,不能够抑制ADP诱导的血小板凝聚,也称为氯吡格雷抵抗或者氯吡格雷低反应性[4]。目前国际上关于氯吡格雷抵抗的诊断并无确切的标准[18],有文献称化学比浊法属于比较经典的血小板功能检测方法,但是由于在操作过程中步骤比较繁多且尚无统一的标准化流程,现已逐渐被其它检测方法所取代[19]。有国外研究报道将床旁快速血小板分析(Verify Now)检测系统的检测结果定义为“金标准”,但是由于该检测方法成本较高,因此在国内临床中并没有被大规模应用。而现在国内较常使用的抗血小板效果的监测方法有TEG、血小板分析仪、PL-11等,TEG检测血小板功能操作便捷、迅速、受外界影响较小等优点,现已在临床中被广泛使用[20]。
氯吡格雷本身属于无活性的前体药物,需在多种酶的参与下生成有活性的化合物,才能发挥抑制血小板聚集作用。细胞色素P450酶参与了两步氧化代谢过程。国外有研究报道[21]CYP2C19*2、CYP2C19*3等位基因的突变主要以亚洲人为主,发生在非洲和欧洲的突变率较低,携带CYP2C19*2、CYP2C19*3基因的突变可降低细胞色素P450酶的活性,使得活性代谢物的水平减少,从而导致该药物的临床疗效不佳,所以IM和PM组氯吡格雷抗血小板活性会受到CYP2C19基因多态性的影响。本研究结果显示,CYP2C19 IM组患者发生氯吡格雷抵抗的比例显著高于EM组和PM组,血小板抑制率明显低于EM组,差异均具有统计学意义,而PM组血小板抑制率虽然低于EM组,但差异无统计学意义,可能是PM组(14.36%)人群占比显著低于IM组(46.04%)和EM组(39.60%)造成的。
目前已有研究表明糖尿病患者在服用氯吡格雷后血小板抑制率较差[22-23],但其机制尚不明确。在小鼠模型中的研究结果表明,高血糖可以通过下调CYP酶影响氯吡格雷药物活化,从而导致抗血小板浓度降低[24]。Angiolillo等[25]针对冠心病合并2型糖尿病患者诱导的血小板抑制率减弱的研究中,解释其原因可能是长期的高血糖水平使血小板信号通路上调降低了抗血小板药物的药效,从而导致药物抵抗的发生。此外,糖尿病会影响肝脏代谢与胃肠道吸收,这可能会影响药物的吸收和代谢[26]。本研究结果也显示,合并糖尿病是发生氯吡格雷抵抗的独立风险因素。
本研究结果还表明高白细胞计数可能是IS患者发生CR的独立危险因素(OR=1.235,95% CI:1.065,1.432),但目前在IS患者中有关白细胞计数与CR之间关系的研究较少。有研究发现炎症标志物升高增加心血管事件的风险,血小板聚集和炎性因子之间的相互作用在急性冠脉综合征中起着非常重要的作用,而炎症调节越来越被认为是当代的治疗靶点,接受氯吡格雷联合阿司匹林双重抗血小板治疗的急性冠状动脉综合征的患者中,白细胞计数与临床疗效密切相关[27]。也有研究者在冠心病患者中发现白细胞中的3个长链非编码RNA与CR的发生有关[28]。IS随着细胞死亡和脑组织受损,分子危险信号通过激活更多的小胶质细胞和浸润白细胞进行前馈反应,释放更多的具有促炎作用的细胞因子。炎症加剧了脑细胞受损程度,使外周血中释放更多的细胞因子,这些细胞因子参与了脑卒中的进展,影响其预后[29-30]。因此,在临床中,高白细胞计数患者服用氯吡格雷时应警惕CR的发生。
本研究的局限性主要包括以下几点:①样本量相对较少;②TEG检测过程中受血凝速度、红细胞聚集状态等因素影响;③患者服药7~14 d只检测1次ADP抑制率,不能反应整体水平;④未直接检测血浆氯吡格雷及其活性代谢物水平。上述因素可能对研究结果产生偏倚,今后还需要进一步的验证。
-
表 1 抵抗组和非抵抗组患者基线资料比较
基本信息参数 CR组(n=87,%) NCR组(n=112,%) x2/t P 年龄 64.52±1.07 62.39±0.97 −1.47 0.14 性别(男) 65(74.7) 86(74.8) 0.00 0.99 吸烟史 43(49.4) 56(48.7) 0.11 0.92 饮酒史 37(42.5) 41(35.7) 0.99 0.32 IS既往史 16(18.4) 22(19.1) 0.02 0.89 高血压 83(95.4) 106(92.2) 0.41 0.53 糖尿病 35(40.2) 22(19.6) 10.15 0.03 冠心病 7(8.0) 10(8.7) 0.03 0.87 高脂血症 40(46.0) 56(48.7) 0.15 0.70 合并用药 质子泵抑制剂 61(70.1) 84(73.0) 0.21 0.67 丁苯酞注射液 67(77.0) 83(72.2) 0.61 0.44 表 2 两组患者实验室检测指标比较
检测项目 抵抗组(n=87) 非抵抗组(n=115) 检验值 P 白细胞 6.70(5.60,8.40) 6.40(5.30,7.30) 2.14b 0.03 红细胞 4.56±0.34 4.44±0.05 −0.40a 0.69 血红蛋白 131(119,139) 134(120,143) −1.15b 0.11 血小板 187(148,227) 206(138,232) −1.62b 0.08 凝血酶原时间 11.21±0.65 11.18±0.85 −0.32a 0.92 D-二聚体 0.30±0.07 0.29±0.09 −0.78a 0.44 血糖 6.03±0.22 6.45±0.21 1.33a 0.18 总胆固醇 4.37(3.72,5.04) 4.32(3.73,5.05) −0.19b 0.85 甘油三酯 1.81±0.13 1.75±0.11 −0.36a 0.72 高密度脂蛋白 1.19(0.99,1.35) 1.14(1.00,1.32) −1.07b 0.29 低密度脂蛋白 2.41(1.94,2.80) 2.27(1.87,2.75) −0.50b 0.62 超敏C反应蛋白 1.91(1.04,5.40) 1.76(0.84,3.50) 1.65b 0.09 血同型半胱氨酸 17.32±1.18 15.81±0.61 −1.22a 0.23 直接胆红素 3.32±1.25 3.79±0.33 1.47a 0.14 总胆红素 13.33±0.47 13.71±0.42 0.60a 0.54 谷草转氨酶 23.00(19.00,28.00) 22.00(18.00,27.00) −0.89b 0.37 谷丙转氨酶 22.00(17.00,31.00) 21.00(15.00,28.79) −1.42b 0.15 注: a为 χ2 值,b为z值。 表 3 202例IS患者 CYP2C19 基因型及等位基因的频率分布
CYP2C19等位基因 等位基因数/个 基因频率/% CYP2C19 *1 253 62.62 CYP2C19 *2 134 33.17 CYP2C19 *3 17 4.21 表 4 不同CYP2C19代谢类型的CR发生率比较(n,%)
CYP2C19
代谢类型氯吡格雷
抵抗[n(%)]非氯吡格雷
抵抗[n(%)]χ2 p EM 24(27.59) 56(48.70) 9.95 0.007 IM 50(57.47) 43(37.39) PM 13(14.94) 16(13.91) 表 5 不同基因型血小板抑制率的比较
CYP2C19基因分组 基因型数/个 血小板抑制率/% EM 39.60(26.20,53.95) CYP2C19*1*1 80 IM 26.10(15.20,53.60)* CYP2C19*1*2 81 CYP2C19*1*3 12 PM 31.95(20.65,65.40) CYP2C19*2*2 24 CYP2C19*2*3 5 χ2 6.31 P 0.04 注:3组间比较采用 Kruskal-Wallis H检验;两两比较采用 Mann- Whitney U检验;* P<0.05,与EM组比较。 表 6 发生氯吡格雷抵抗危险因素的Logistic回归分析结果
因素 B SE Wald P OR 95%CI 合并糖尿病 0.595 0.227 4.496 0.026 0.851 (0.707,1.191) 白细胞计数 0.211 0.076 7.774 0.005 1.235 (1.065,1.432) CYP2C19
中代谢型0.901 0.291 9.565 0.002 0.368 (0.197,0.686) -
[1] MAIDA C D, NORRITO R L, DAIDONE M, et al. Neuroinflammatory mechanisms in ischemic stroke: focus on cardioembolic stroke, background, and therapeutic approaches [J]. Int J Mol Sci, 2020, 21(18): E6454. doi: 10.3390/ijms21186454 [2] 王陇德, 彭斌, 张鸿祺, 等. 《中国脑卒中防治报告2020》概要[J]. 中国脑血管病杂志, 2022, 19(2): 136-144. [3] POWERS W J, RABINSTEIN A A, ACKERSON T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association [J]. Stroke, 2019, 50(12): e344-e418. [4] SUGUNARAJ J P, PALANISWAMY C, SELVARAJ D R, et al. Clopidogrel resistance [J]. Am J Ther, 2010, 17(2): 210-215. doi: 10.1097/MJT.0b013e3181bdc3e4 [5] ZOU X, DENG X L, WANG Y M, et al. Genetic polymor-phisms of high platelet reactivity in Chinese patients with coronary heart disease under clopidogrel therapy[J]. Int J Clin Pharm, 2020, 42((1):): 158-166. doi: 10.1007/s11096-019-00953-w [6] AL-HUSEIN B A, AL-AZZAM S I, ALZOUBI K H, et al. Investigating the effect of demographics, clinical characteristics, and polymorphism of MDR-1, CYP1A2, CYP3A4, and CYP3A5 on clopidogrel resistance[J]. J Cardiovasc Pharmacol, 2018, 72(6): 296-302. doi: 10.1097/FJC.0000000000000627 [7] WU Z K, WANG J J, WANG T, et al. Clopidogrel resistance response in patients with coronary artery disease and metabolic syndrome: the role of hyperglycemia and obesity[J]. J Geriatr Cardiol, 2015, 12(4): 378-382. [8] SU J F, HU X H, LI C Y. Risk factors for clopidogrel resis-tance in patients with ischemic cerebral infarction and the correlation with ABCB1 gene rs1045642 polymorphism[J]. Exp Ther Med, 2015, 9((1):): 267-271. doi: 10.3892/etm.2014.2058 [9] LI J, WANG Y, LI H, et al. Homocysteine level predicts response to dual antiplatelet in women with minor stroke or transient ischemic attack: subanalysis of the CHANCE trial[J]. Arterioscler Thromb Vasc Biol, 2020, 40(3): 839-846. doi: 10.1161/ATVBAHA.119.313741 [10] GALEAZZI R, OLIVIERI F, SPAZZAFUMO L, et al. Clustering of ABCB1 and CYP2C19 genetic variants predicts risk of major bleeding and thrombotic events in elderly patients with acute coronary syndrome receiving dual antiplatelet therapy with aspirin and clopidogrel[J]. Drugs Aging, 2018, 35(7): 649-656. doi: 10.1007/s40266-018-0555-1 [11] 刘亚巍, 赵小朗, 姚璞, 等. ABCB1、CYP2C19、CES1基因多态性与缺血性脑卒中患者氯吡格雷抵抗及疗效的关系[J]. 中国神经免疫学和神经病学杂志, 2021, 28(2): 146-151. [12] Bergmeijer T O, Reny J L, Pakyz R E, et al. Genome-wide and candidate gene approaches of clopidogrel efficacy using pharmacodynamic and clinical end points-Rationale and design of the International Clopidogrel Pharmacogenomics Consortium (ICPC)[J]. Am Heart J, 2018, 198 (10): 152-159. [13] Mansuy D, Dansette P M. Sulfenic acids as reactive interme-diates in xenobiotic metabolism[J]. [J]. Arch Biochem Biophys, 2011, 507((1):): 174-185. doi: 10.1016/j.abb.2010.09.015 [14] 钟迪, 张舒婷, 吴波. 《中国急性缺血性脑卒中诊治指南2018》解读[J]. 中国现代神经疾病杂志, 2019, 19(11): 897-901. [15] LE QUELLEC S, BORDET J C, NEGRIER C, et al. Comparison of current platelet functional tests for the assessment of aspirin and clopidogrel response. A review of the literature [J]. Thromb Haemost, 2016, 116(4): 638-650. [16] SHANKER J, GASPARYAN A Y, KITAS G D, et al. Platelet function and antiplatelet therapy in cardiovascular disease: implications of genetic polymorphisms[J]. Curr Vasc Pharmacol, 2011, 9(4): 479-489. doi: 10.2174/157016111796197224 [17] ZIEGLER S, SCHILLINGER M, FUNK M, et al. Association of a functional polymorphism in the clopidogrel target receptor gene, P2Y12, and the risk for ischemic cerebrovascular events in patients with peripheral artery disease [J]. Stroke, 2005, 36(7): 1394-1399. doi: 10.1161/01.STR.0000169922.79281.a5 [18] QURESHI Z, HOBSON A R. Clopidogrel “resistance”: where are we now? [J]. Cardiovasc Ther, 2013, 31(1): 3-11. doi: 10.1111/j.1755-5922.2011.00296.x [19] 杨雅薇, 李攀, 陈韬, 等. 四种氯吡格雷抗血小板功能检测方法的比较[J]. 第二军医大学学报, 2016, 37(8): 1002-1006. [20] 周红, 覃少东, 黄澄. 血栓弹力图在急性脑梗死患者诊疗中的应用进展[J]. 微量元素与健康研究, 2021, 38(1): 68-70. [21] SUN Y F, VENUGOPAL J, GUO C A, et al. Clopidogrel resis-tance in a murine model of diet-induced obesity is mediated by the interleukin-1 receptor and overcome with DT-678[J]. Arterioscler Thromb Vasc Biol, 2020, 40((6):): 1533-1542. doi: 10.1161/ATVBAHA.120.314146 [22] ZHOU M C, CAO S P, ZHONG H L, et al. Subacute stent thrombosis in a patient with type 2 diabetes and clopidogrel resistance: a case report[J]. Int J Clin Pharmacol Ther, 2020, 58(8): 449-453. doi: 10.5414/CP203547 [23] ANGIOLILLO D J, JAKUBOWSKI J A, FERREIRO J L, et al. Impaired responsiveness to the platelet P2Y12 receptor antago-nist clopidogrel in patients with type 2 diabetes and coronary artery disease[J]. J Am Coll Cardiol, 2014, 64((10):): 1005-1014. doi: 10.1016/j.jacc.2014.06.1170 [24] HU L, TANG Y, SHENG X H, et al. Anti-platelet therapy in mild cerebral infarction patients on the basis of CYP2C19 metabolizer status[J]. Cell Transplant, 2019, 28(8): 1039-1044. doi: 10.1177/0963689719851769 [25] ANGIOLILLO D J, BERNARDO E, ZANONI M, et al. Impact of insulin receptor substrate-1 genotypes on platelet reactivity and cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease[J]. J Am Coll Cardiol, 2011, 58(1): 30-39. doi: 10.1016/j.jacc.2011.02.040 [26] GATOPOULOU A, PAPANAS N, MALTEZOS E. Diabetic gastrointestinal autonomic neuropathy: current status and new achievements for everyday clinical practice [J]. Eur J Intern Med, 2012, 23(6): 499-505. doi: 10.1016/j.ejim.2012.03.001 [27] OSMANCIK P, PAULU P, TOUSEK P, et al. High leukocyte count and interleukin-10 predict high on-treatment-platelet-reactivity in patients treated with clopidogrel [J]. J Thromb Thrombolysis, 2012, 33(4): 349-354. doi: 10.1007/s11239-011-0659-5 [28] 谢文剑, 黄贝贝, 殷蒨, 等. lncRNA在冠心病氯吡格雷抵抗患者中的差异性表达[J]. 中南大学学报(医学版), 2019, 44(1): 9-13. [29] TUTTOLOMONDO A, DI RAIMONDO D, DI SCIACCA R, et al. Inflammatory cytokines in acute ischemic stroke [J]. Curr Pharm Des, 2008, 14(33): 3574-3589. doi: 10.2174/138161208786848739 [30] RAMAWAT B, SALUJA A, BHATACHARJEE J, et al. Acute ischemic stroke is associated with increased serum levels of pro-inflammatory cytokines [J]. Indian J Clin Biochem, 2021, 36(3): 380-381. doi: 10.1007/s12291-020-00892-8 -





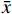
 下载:
下载:
 下载:
下载: 

