-
心肌梗死是一种严重的心血管疾病,由于冠状动脉血供急剧减少或中断,使心肌持续性缺血缺氧以致坏死,损害心功能且可能导致心律失常、休克以及心力衰竭等严重后果,已成为威胁人类生命健康的重大疾病之一[1-2]。近几十年来,随着医疗技术的进步,再灌注心肌治疗可显著改善心梗患者的生存率,但由于心梗发生发展及转归过程极其复杂,使其在临床治疗中仍然面临许多挑战[3],因此构建合适的动物模型对探究人心肌梗死的发病机制和病理过程、评价药物疗效以及探索新的治疗方法至关重要。通过结扎冠状动脉模拟心肌缺血过程来建立心肌梗死的动物模型是目前广泛应用的较为成熟的方法[4-5]。以往更倾向于选择较大动物构建模型,随着基因工程技术的发展,基因工程小鼠成为炙手可热的研究工具,因此建立简便有效的小鼠心梗模型对心肌梗死疾病的深入研究有重要意义。但目前国内关于小鼠心梗模型构建方法的报道较少,缺乏一种比较便捷的模型制作方法和无创评价手段。本文基于Gao等[6]报道的心梗模型构建方法结合实际操作总结了一些经验。
-
SPF级C57BL/6J 10~12周龄雄性小鼠,共29只,体重为(29.65±5.35)g,购于海西普尔-必凯实验动物有限公司。实验动物均饲养在具有IVC系统的动物房[人工照明12 h;温度(23±2)℃;相对湿度40%~60%;噪音≤60 dB],自由饮食进水,在正式实验之前需适应饲养环境至少1周。动物实验方案与操作均遵守动物福利及“3R”原则。
-
异氟烷(河北一品制药);4%水合氯醛、2%TTC染液(批号:E110BA0007、AC29BA0025,BBI Life Science);0.9%NaCl溶液;4%多聚甲醛组织固定液(批号:154608,博光生物)。气体麻醉机(上海曼普生物科技有限公司);恒温垫;彩色扫描仪(BenQ,K802);MPA血压与心率分析系统(上海奥尔科特生物科技有限公司);显微外科手术器械。缝线(3-0,用于肌肉、皮肤缝合)、缝针;带线缝针(7-0,用于结扎冠状动脉)。
-
所有手术器械使用前均高温蒸汽灭菌30 min。实验小鼠术前禁食12 h。按照麻醉机说明书连接好管路。将小鼠放进麻醉机的诱导箱,开启氧气调节气流量(0.25 MPa,1 L/min),调整麻醉药(异氟烷)浓度为5%,约1 min完成小鼠诱导麻醉,之后调整麻醉药浓度至2%,连接小鼠面罩并持续吸入。
-
小鼠仰卧位固定,左胸前手术区域剔除鼠毛并消毒。距胸骨左缘约1~2 mm皮肤处做长约1 cm纵向切口,切口处行垂直外翻褥式缝合预留缝线。逐层钝性分离胸壁肌肉,从第3或第4肋间隙快速进入胸腔,用止血钳撑开肋间隙,配合心脏跳动左手轻轻挤压使心脏从孔隙中弹出。在左心耳下缘1~2 mm、肺动脉圆锥旁0.5 mm处以7-0带线缝合针穿过冠状动脉前降支将其结扎,松紧适宜,控制进针深度(以隐约可见细针为宜)和行针宽度(2 mm左右)。由于不借助通气装置,要求开胸时间不要超过30 s,结扎操作尽量控制在10 s左右完成。结扎完成后轻柔地将心脏送回胸腔,挤压胸腔排出空气同时收紧结扎切口处预留缝线,完成手术。术中逐步调整麻药浓度至零,取下面罩后将小鼠置于恒温垫上约3~5 min即可复苏。
-
小鼠异氟烷吸入麻醉后,仰卧固定,按照仪器说明连接MPA系统心脏导联线,并将针电极分别固定在右上肢、左上肢,右下肢接地,避免周围磁场干扰。待基线平稳后,记录4~5个心动周期。实验中我们主要使用II导联观察心脏前下壁心梗发生的情况,与冠状动脉左前降支所供给的心肌区域相吻合。心电图导联的选择可以根据实验需求进行调整。
-
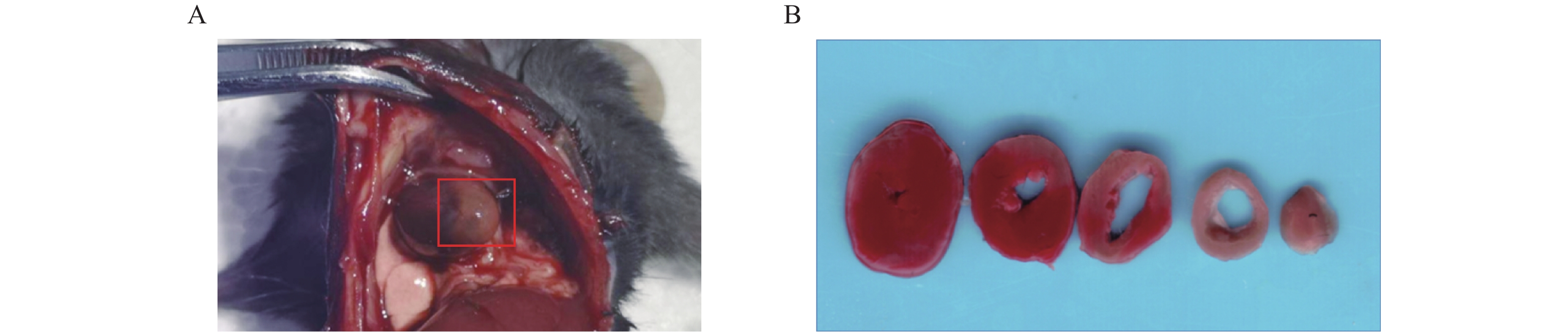
术后24 h对模型小鼠施行安乐死(4%水合氯醛,300 mg/kg),打开胸腔,肉眼观察心梗部位,并拍照记录。留取心脏标本置于生理盐水中,冲洗除去多余的血液,用滤纸吸干液体后修剪,仅保留全心室称重,记为M1(mg)。全心室心脏标本置于–20 ℃冷冻约30 min后,将心脏切成厚度为1 mm左右的薄片若干,经常规TTC染色后[7],进行扫描拍照。剪下白色梗死区域用滤纸吸干后称重,记为M2(mg),梗死区大小计算以百分比表示,即梗死大小(%)=(M2/M1)×100%。
-
数据均采用平均值±标准差(
$ {\rm{\bar X}} $ ±s)表示。 -
小鼠心梗模型总体成功率为79.3%(23/29)。其中,冠脉结扎术中死亡率为6.8%(2/29),1只由于穿刺过深导致心脏破裂出血,1只由于胸腔暴露时间过长导致气胸。术后早期(<4 h)死亡率为10.3%(3/29);2只解剖后发现胸腔内有大量积血,1只疑为发生不可逆转的致死型心律失常。此外,1只造模失败未发生心梗,术后24 h TTC染色未见明显梗死区,且术后即刻和术后4 h心电图均无ST段改变(表1)。
表 1 小鼠心梗模型失败原因分析及解决方法
失败时段 比例(%) 原因分析 只数 解决方法 术中死亡 6.8 术中心脏破裂致死 1 控制穿刺深度和宽度 术中气胸致死 1 穿刺结扎迅速,快速闭合胸腔 术后死亡 10.3 术后胸腔出血致死 2 保证视野开阔,用纱布保护胸壁及肺,避免刺破大血管和肺脏 术后致死型心律失常致死 1 结扎动作迅速轻柔,减少对心脏的刺激 其他 3.4 术后未发生心梗 1 (造模失败) -
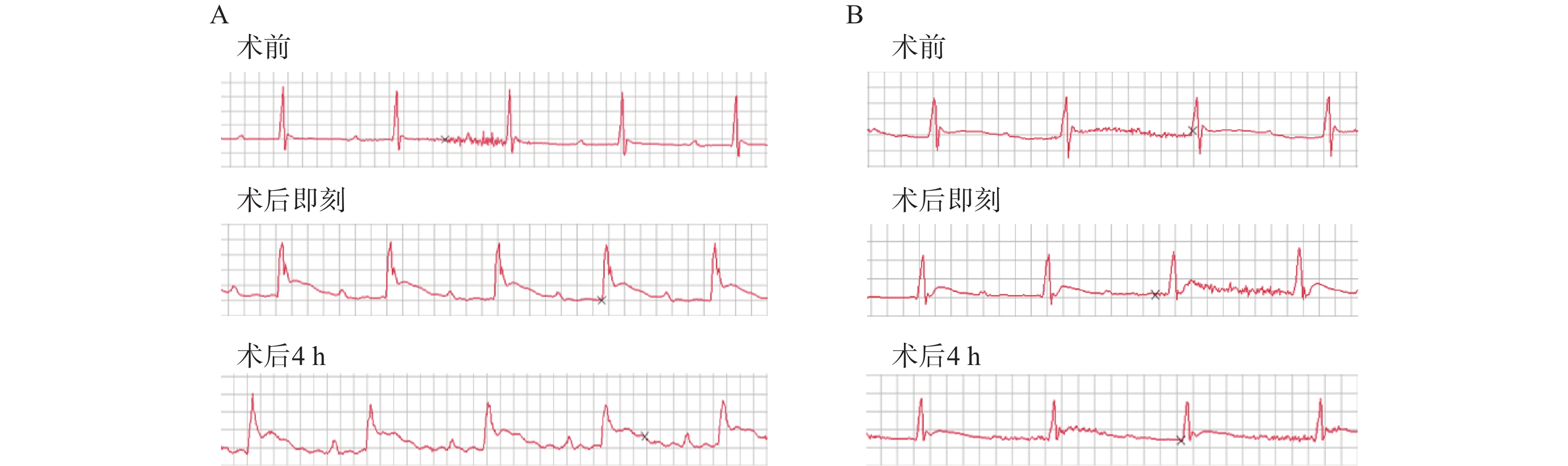
造模小鼠均施行术后即刻和术后4 h两次心电图检查,发现经24 h TTC染色后证实有心肌梗死发生的23只造模成功的小鼠中,两次心电图中均呈现出明显的ST段抬高以及变异T波(图1A),而造模失败的小鼠心电图则没有明显异常(图1B)。
-
术后24 h打开小鼠胸腔,肉眼可见左心室前下壁靠近心尖处呈灰白色(图2A)。取心脏标本经常规TTC染色后,可观察到梗死区域呈白色,而非梗死区域呈红色(图2B)。根据前文提到心梗区大小的计算方法,本实验中,心梗模型梗死区大小最大可达40%,几乎累及整个左心室;最小为17%,主要集中在心尖部位;梗死区平均大小为(28±6)%(见表2)。
表 2 23只小鼠心肌梗死区大小情况
项目 体重/g 全心室重量(M1)/mg 梗死心肌重量(M2)/mg 梗死大小(M2/M1)/(%) 总计($ {\rm{\bar X}} $±s) 29.65±5.35 76±13 21.91±7.06 28±6 -
建立疾病的实验动物模型常常是研究工作至关重要的一步,需要考虑多方面的因素,使疾病本身特征和研究目的与所建立的动物模型达到尽可能地一致。首先,实验动物的选择要考虑各实验室以及实验者自身的条件。就心梗模型而言,大动物(猪、犬、兔等)因其心脏在形态大小、解剖结构以及生理条件上与人类更为相似,构建模型成功率相对较高,通常用于病理生理过程以及药物治疗疗效等研究。小动物生命力弱、手术耐受性差,导致模型建立具有一定难度,但由于其能够建立相关基因工程动物形成较为一致的品系,对于深入研究心肌梗死病理及治疗相关机制有重要意义。目前心梗治疗研究不断深入,基于心肌细胞不可再生的特性,移植治疗、干细胞治疗、基因治疗等多种治疗手段及相关机制研究成为心梗治疗方案的新方向[8-10],建立一种稳定性强、成功率高的小鼠心梗模型是非常必要的。因此,笔者结合小鼠自身特点,在以往大动物模型的基础上,比较成功地建立了小鼠的心梗模型。
目前建立心肌梗死动物模型的方法有多种,包括:冠脉结扎法、药物法、球囊堵闭法、栓塞法以及血栓形成法等。其中药物法主要使用垂体后叶素等诱发血管痉挛促使心肌缺血梗死,但此法很难明确发生梗塞的动脉且效果具有不确定性;后三种方法可直接形成冠脉闭塞,但需要借助心导管技术和冠脉造影等影像学技术,且小鼠细小的冠状动脉给操作增加了难度[11]。而冠状动脉结扎法[12]操作简单、血管阻塞明确,比较符合心梗发生的病理过程,能较好的实现临床转化,因此本实验主要采用此法建立小鼠心梗模型。实验过程中,采用5%异氟烷诱导后联合2%异氟烷面罩持续吸入麻醉的方法,不同于传统的腹腔麻醉联合气管插管通气的方法[13],避免了气管插管和机械通气可能产生的组织损伤,同时也提高了麻醉的安全性和手术效率。但是,此法要求实验者在短时间内完成冠脉结扎术,尽快完成冠脉结扎并闭合胸壁,降低长时间暴露胸腔可能导致心律失常、气胸等致死风险。术中需要密切关注小鼠状况以便及时调整麻药浓度,从而促进小鼠的术后复苏。除了安全有效的麻醉方法之外,冠脉结扎位置的选择至关重要,由于小鼠血管细小,侧枝循环丰富且走行不易观察,因此要求实验者操作精确,既要保证能够引起足够面积的心肌梗死,又要避免梗死面积过大而导致死亡,尽量在同一高度结扎形成较为一致的模型;此外,小鼠心室壁较薄,操作者需要严格控制穿刺深度,避免穿刺过深直接刺破室壁造成大出血。
心梗模型的评价方法可根据实验动物的生存状态分为活体检测和尸检。前者主要包括冠状动脉造影荧光微粒注射、MRI、PET和超声心动图等[14-15],这些方法可以获得冠脉血供状况从而间接评估心肌梗死,但设备要求高、价格昂贵,检测操作复杂;后者则主要是染料染色法包括TTC、依文思蓝、Masson三色法等,可以获得心肌梗死的直接结果。心电图通常是临床心肌梗死病人首选的实验室检查。然而,在动物心梗模型的评价中,人们往往忽视这一最为简便快捷方法的应用。在本实验的模型构建中,笔者采用术后即刻和术后4 h心电图检查两个指标来评估小鼠心梗情况。首先,在术后立刻对模型鼠施行心电图检查有助于帮助我们判断冠脉结扎的初期效果,以及发现潜在的心律异常改变。其次,考虑到心肌梗死特异性标志物之一——肌钙蛋白(cTn),一般在心肌坏死后3~4 h开始升高[16],我们又选择术后4 h的心电图检查,再次对模型小鼠心梗发生情况进行判断,同时,排除由于手术对心脏刺激可能造成的假阳性结果,并利用术后24 h TTC病理染色来验证心电图评估心梗发生的准确情况。最终,发现心电图评价心梗发生的准确性较高,且具有操作简便、结果获取迅速、不受场地时间限制、经济成本低等特点,故而在模型制备结果的评价中较其他活体检测方法有明显优势。
本研究成功构建了小鼠心肌梗死模型并利用TTC染色法明确区域。同时观察到,心梗模型小鼠早期心电图呈现出明显ST段抬高可以作为心梗发生的可靠依据,即心电图可以作为小鼠心梗模型早期快速无创评价的可靠方法。因此,本实验建立的关于小鼠心肌梗死模型的高效制作方法和无创评价手段为确切研究心梗病理生理及治疗机制提供了良好选择。
Establishment of mouse myocardial infarction model and early electrocardio- gram assessment
-
摘要:
目的 构建并优化小鼠心肌梗死模型,联合使用冠脉结扎术后即刻和术后4 h的两次肢体导联心电图对心梗发生情况进行早期评价。 方法 C57BL/6J雄性小鼠29只,异氟烷吸入麻醉后,经左侧第3/4肋间进入胸腔,结扎冠状动脉左前降支建立模型,施行术后即刻和术后4 h肢体导联心电图评价心梗发生情况。术后24 h打开胸腔观察梗死情况,留取心脏标本进行TTC染色确定梗死区域并计算梗死面积。 结果 小鼠行冠状动脉结扎术,术中死亡率为6.8%(2/29),术后早期(<4 h)死亡率为10.3%(3/29),24 h存活率为82.8%(24/29);术后24 h TTC染色明确梗死发生,则心梗模型建立,造模成功率为79.3%(23/29),平均梗死区域大小(梗死心肌重量/全心室重量)为(28±6)%;成功建立模型的小鼠在术后4 h心电图可见明显ST-T改变,提示心梗已发生。 结论 成功建立小鼠心肌梗死模型,且联合使用术后即刻和术后4 h两次心电图可以作为小鼠心肌梗死模型快速无创的评价方法。 Abstract:Objective To establish and optimize a mouse myocardial infarction (MI) model, and to use twice limb lead ECGs immediately after coronary ligation and 4 h after surgery to evaluate the occurrence of myocardial infarction. Methods Twenty-nine male C57BL/6J mice were anesthetized with isoflurane. then a myocardial infarction model was established by ligating the left anterior descending (LAD) coronary artery through the third/fourth intercostal space of left anterior chest. Immediate and 4 h postoperative limb lead ECGs were performed. Twenty-four hours after surgery, the chest was opened and the occurrence of myocardial infarction was evaluated. The heart samples were taken for TTC staining to determine the infarct area and calculate the infarct area. Results During the mice underwent coronary artery ligation the intraoperative mortality was 6.8% (2/29), and the early postoperative (<4 h) mortality was 10.3% (3/29). The 24 h survival rate was 82.8% (24/29). 24 hours after TTC staining confirmed the occurrence of infarction, the myocardial infarction model was established. The success rate of the model was 79.3% (23/29), and the average infarct size (infarcted myocardial weight / whole ventricular weight) was (28 ± 6)%; The mice successfully established by the model showed obvious ST-T changes in the ECG at 4 hours after surgery, suggesting that a myocardial infarction has occurred. Conclusions The mouse myocardial infarction model was successfully established. The combined use of ECG immediately after surgery and 4 h after surgery could be used as a rapid and non-invasive evaluation method for mouse myocardial infarction. -
Key words:
- myocardial infarction /
- mouse /
- animal model /
- ECG assessment /
- TTC staining
-
中药挥发性成分(VOCs)是指中药中一类具有芳香气并易挥发的成分,其化学组成复杂,主要包括挥发油类以及其他分子量较小、易挥发的化合物,例如萜类、脂肪族、芳香族化合物等。VOCs具有发汗解表、芳香开窍、镇咳祛痰、理气、驱风、抑菌、镇痛、杀虫等多种功效。作为中药学研究的热点之一,高效率、高标准的检测药材中VOCs十分关键。因此,利用现代化方法来实现对中草药挥发性成分的细致分析,意义巨大。
VOCs常用气相色谱-质谱联用(GC-MS)方法进行分析,虽分离能力强,但样品需要预处理且分析时间长。气相色谱-离子迁移谱(GC-IMS)法结合了GC突出的分离能力和IMS快速响应、高灵敏度的特点,具有样品准备简便、高灵敏度、高分辨率等显著优势,结合化学计量学分析中药材VOCs所呈现图谱,可实现对药材VOCs无损、快速区分[1]。
1. 气相色谱–离子迁移谱联用技术原理
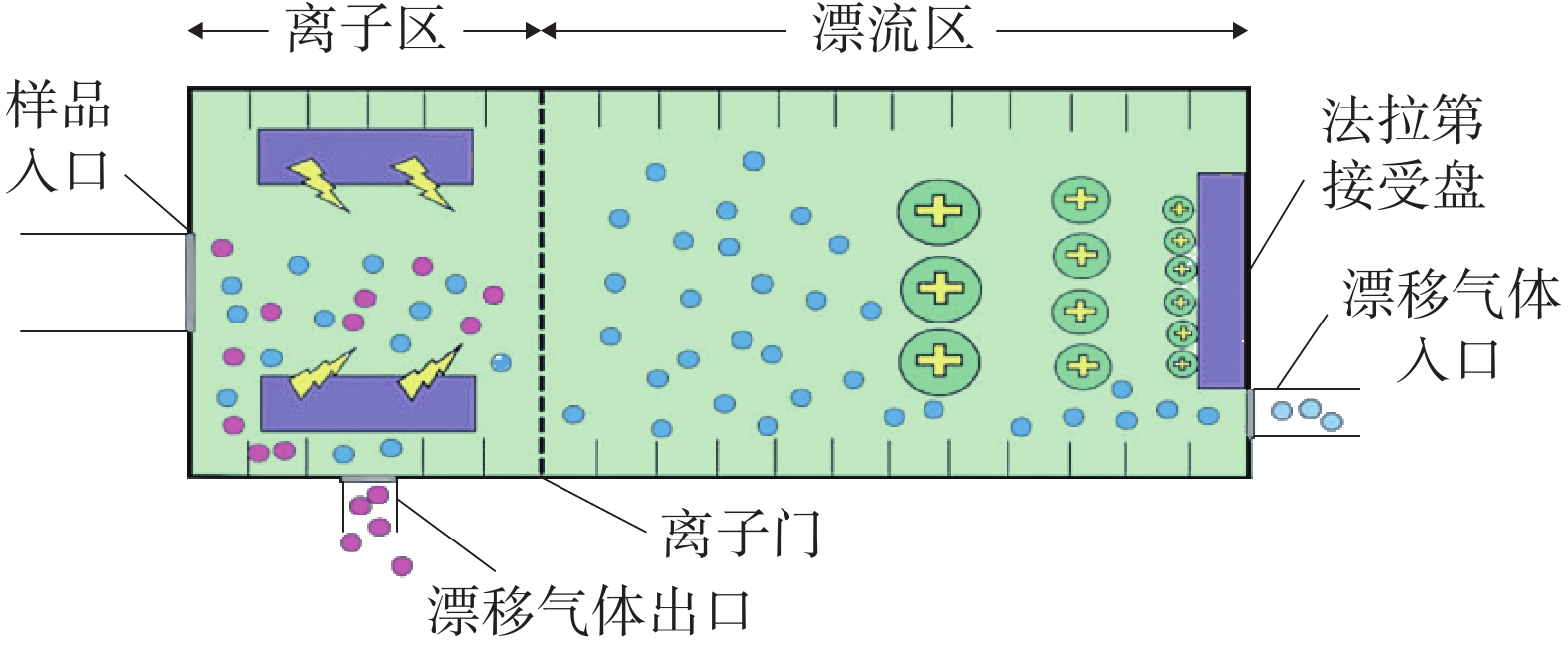
GC-IMS 技术的基本原理[2]是通过将样品混合物引进气相色谱仪进行分离,样品分子和载气分子在离子源放射性物质的作用下发生一系列反应形成产物离子,这些产物离子在不同的电场驱动下通过离子门进入迁移区,与逆向而来的中性迁移气体分子发生碰撞而损失能量。产物离子在电场中的迁移速率不同,到达检测器上的时间不同,从而使样品差异化分离(如图1),最后可得到一个包含有离子迁移时间(X轴),气相色谱保留时间(Y轴),离子强度(Z轴)的三维谱图(如图2)。
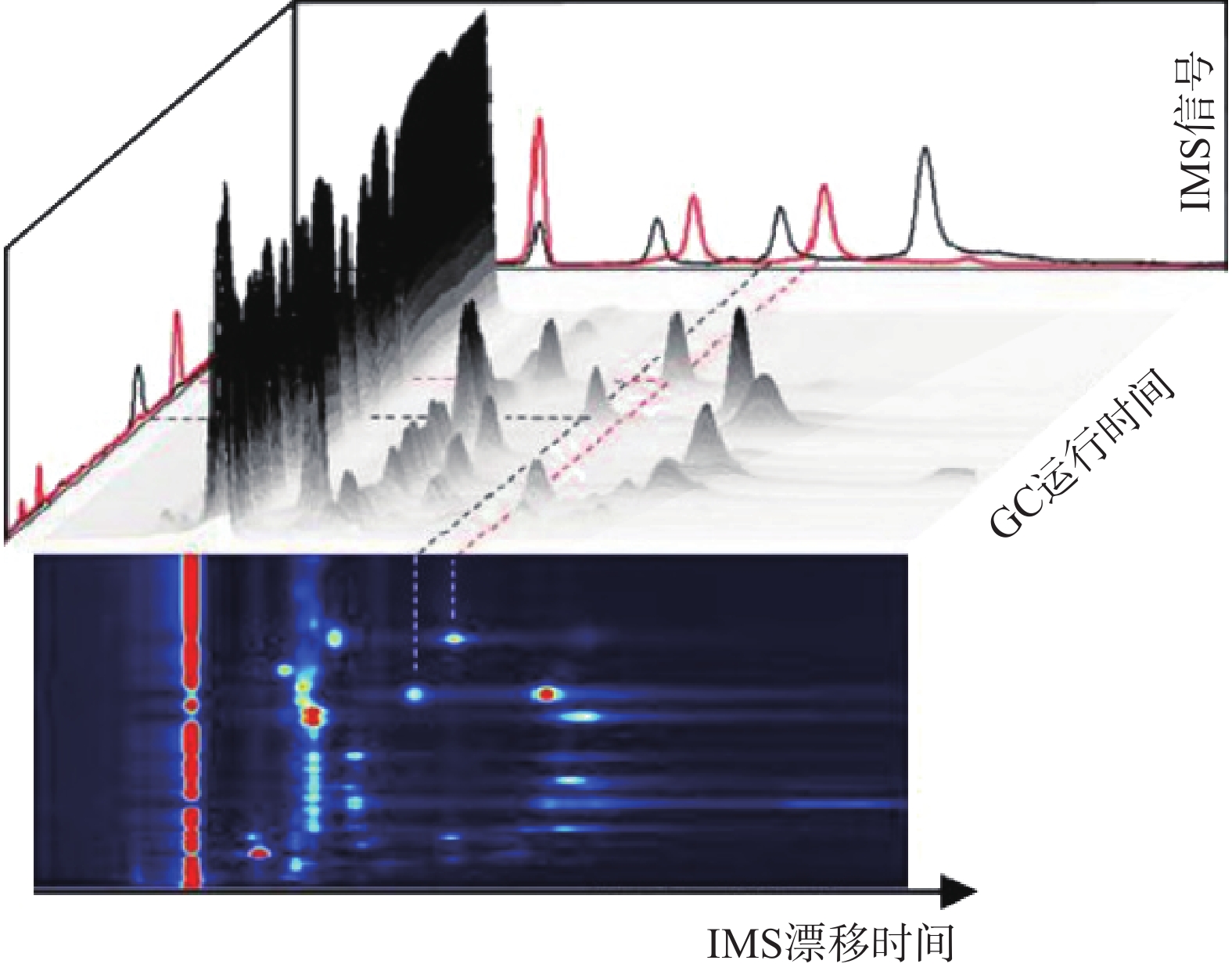 图 2 气相色谱-离子迁移谱联用技术结果三维谱图[3]
图 2 气相色谱-离子迁移谱联用技术结果三维谱图[3]顶空-气相色谱-离子迁移谱(HS-GC-IMS)是应用于检测药材中VOCs最为广泛的方式之一 [3]。其原理是首先将样品中的VOCs通过热孵育和振摇使之从药材中逸出,随后抽取顶空气体进行分析,避免复杂基质干扰,再使之进入气相色谱(GC)、离子迁移谱(IMS)中,得到相应谱图。HS-GC-IMS无需样品预处理,在分析复杂样品或需要快速检测场景方面更具优势。
自从1972年第一张经过GC分离的IMS谱图出现[2],早期主要用于军事领域(如化学战剂检测)和毒品筛查的先进技术逐渐面向市场,应用于研究(如图3),引进国内后广泛使用于中药研究。现阶段,通过GC-IMS捕捉到中药材中的微量VOCs,将其应用于区分中药的不同种类和产地、监控中药炮制过程以及中药复方组分分析等[4],为中药材的产地及真伪鉴别提供可靠的依据,同时也为质量控制和药效研究等方面提供数据支持。
2. 在中药鉴定中的应用
2.1 同属不同种的中药鉴别
同属不同种之间的中药材亲缘关系比较接近,在VOCs种类差异上不显著,利用GC-IMS技术善于捕捉挥发性化合物种类微小差异的特点,可对样品进行品种鉴别。
陈皮中主要含有三萜类、挥发油类等几百种成分,有平喘止咳、调节血管等药理作用[5-6],刘主洁等[7]和Lv等[8]采用HS-GC-IMS对陈皮和广陈皮样品中VOCs进行研究,分析二者的特征信号,以邻氨基苯甲酸二甲酯为广陈皮最明显的特征标记物,柠檬烯、癸醛等也可作为广陈皮的特征信号,利用这些特征信号可对陈皮和广陈皮进行区分。山莓是覆盆子的混淆品之一,为保证用药安全有效,有必要对两者进行区分[9],严爱娟等[10]采用GC-IMS技术检测山莓和覆盆子VOCs,明确鉴定出覆盆子特征成分较山莓多,其中癸醛、1-辛烯-3-醇等在覆盆子样品中的含量更高,苯甲醛、2-丁酮等在山莓样品中的含量更高,以此来实现山莓和覆盆子的区分。彭旭阳等[11]采用GC-IMS分析新疆和田地区“梭梭”和“红柳”肉苁蓉这两种不同寄主肉苁蓉挥发性物质之间的不同,找到了二者主要差异物质为苯甲醛、庚醛等。
2.2 中药不同产地鉴别
道地药材是指生长在特定的自然生态环境中,经过一系列技术培育和加工而成,且被公认为比其他地方生产的同种药材的质量和治疗效果好的药材[12]。但随着产地的迁移、品种的引入,在外观性状上对道地药材和其他产地的中药进行简单鉴别已经不能满足需求。
王振洲等[13]采用GC-IMS对来自不同产区的人参VOCs进行检测,发现2,5-二甲基吡嗪和2,6-二甲基吡嗪等VOCs在吉林集安四年生人参中含量较高,而吉林敦化四年生人参中含量相对较低,由此鉴别四年生吉林集安和敦化的人参。西洋参同属于五加科人参属,成功引种进入我国后,辽宁、吉林和山东是其主产区[14],王燕等[15]使用GC-IMS技术对美国、加拿大、山东等五个不同产地的西洋参进行研究,共鉴定出53种VOCs,其中美国西洋参中2-庚酮等成分的含量较高,而加拿大产α-蒎烯等成分的含量显著高于其它产区,中国产芳樟醇等成分的含量较高,其中吉林产地的含量是辽宁、山东产地的2.60和3.60倍,通过这些显著差异可对西洋参进行产地溯源和鉴别。
李曼祎等[16]使用GC-IMS技术对新疆、宁夏、内蒙古、青海四个主要产区的枸杞进行化学成分测定,分析出了四个地区含量差异较大的16种物质,发现内蒙古枸杞区别于另外三个产地特有的物质为正丁醇,同时筛选出了叶醇等五种标记性物质对枸杞产地进行区分。Li[17]等分别采用HS-SPME-GC-MS和HS-GC-IMS检测河北、河南、江苏、浙江、安徽以及山东6个不同地区的五味子,结果显示安徽的五味子萜类物质含量较高,同时该研究也证明了HS-GC-IMS对样品的分类效果优于HS-SPME-GC-MS。山东为瓜萎的道地产区[18],河北也是瓜萎的重要产区,不同产地的瓜萎可能在品质及所含成分上有所差别,从而对药效也会产生一定的影响[19-20]。张敏敏等[19]采用GC-IMS在瓜萎皮样品中共鉴定出醛类和醇类等88种VOCs,分析发现山东瓜蒌皮中2-庚酮、正壬醛等物质的含量低于河北瓜蒌皮,但1-己醇、糠醇等含量更高的结论,通过这些差异基本实现了两地区瓜萎皮的区分。Li等[21]采用HS-GC-IMS联合PCA建立松茸特征图谱,对分别来自云南和四川的松茸样品以及它们的菌盖和菌柄进行研究,发现虽然指纹图谱相似度较高,但各自也有其特征性挥发物:苯乙醛和糠醇等仅在云南产松茸的菌柄中被发现,戊烷仅在四川产松茸的菌柄中被发现,戊醛仅在云南产松茸的菌盖中检出,甲基吡嗪仅在四川产松茸的菌盖中检出,通过特征性挥发物的不同,可对分别产自云南和四川的松茸进行区分。
2.3 中药复方中主成分的鉴别
中药复方成分众多且复杂,确保其质量符合标准、疗效可靠、使用安全是关键,采用HPLC特征图谱进行分析是目前公认的良好方法之一[22]。GC-IMS检测灵敏度高、分离效果好,是对HPLC表征中药复方质量分析的有益补充。
Yuan等[23]设置了对照组、慢性不可预测轻度应激(CUMS)组和CUMS+百合鸡子黄汤组,采用HS-GC-IMS等方法对百合鸡子黄汤治疗CUMS大鼠粪便中挥发性化合物含量进行研究,鉴定出了11个生物标志物,找出了对照组大鼠粪便样品中甲硫醚含量较高,而CUMS组则较低,同时百合子鸡汤组抑郁表现出保护性干预作用;Yin等[24]采用HS-GC-IMS对开心散中的挥发性化合物进行分析,鉴别出β-细辛酮等十种VOCs可作为开心散的质量标记物,进一步为开心散质量控制及药效机制等的相关研究奠定基础;李巍等[25]利用HS-GC-IMS对清感秋饮中的VOCs进行定性定量分析,共鉴定出120种VOCs,其中,紫苏属酮、β-石竹烯等可能为其主要药效成分。
3. 在中药加工炮制中的应用
中药的加工炮制是提高临床疗效的重要手段,也是保证临床用药安全的重要措施[26]。应用不同的炮制方法可能会引起中药中的化学成分发生含量加减、成分转化与破坏等变化,采用GC-IMS技术对中药加工炮制过程中的VOCs进行动态监控,对于炮制工艺的规范、制定更优炮制方案等具有指导意义。
干燥是中药材和饮片加工制备过程中的重要且关键的环节之一,而中药中的VOCs易受干燥工艺的影响,对于富含VOCs的中药,准确控制干燥工艺有利于减少有效成分的损失。陈树鹏等[27]采用GC-IMS等技术确定烘干样品的整体香气属性优于晒干样品,这可能是由于烘干工艺对环境温度的调整使得烘干过程更有利于果香、柑橘香以及甜香香气保留。其中苯乙酸乙酯、乙酸乙酯等 12 种物质为晒干主要成分,2-甲基-1-丁醇、(E)-2-己烯-1-醇等为烘干主要成分;Wang等[24]在8S-GC-IMS技术的辅助下,了解了柑橘皮干燥以及不同条件下挥发性成分情况,研究柑橘皮各成分在不同干燥温度下的缺失,其中,70 ℃下干燥会导致2,2-苯基-1-苦基肼基和铁还原抗氧化能力显著降低。Yu等[29]通过GC-MS比较总离子色谱图中的峰面积与苯乙酸乙酯的峰面积,将VOCs的含量进行半定量再通过GC-IMS呈现图谱对结果进行比较,确定VOCs的身份,从而证实晒干有利于两个品种的网纹柑橘中萜醇类化合物的保存,热风干燥有利于脂肪族醛和倍半萜的保存,而冷冻干燥是保存酯类和酚类物质的最佳方法;Zhou等[30]对肉苁蓉进行酒制增效后粉碎、超微粉碎、醇提、水提等处理,采用HS-GC-IMS方法检测其VOCs并建立指纹图谱,发现增效处理的肉苁蓉VOCs的种类和含量有所减少,分析原因可能为各种化学物质之间在处理过程中会发生化学变化,而新鲜肉苁蓉则保存了更多种类的VOCs,超微粉碎处理和水提处理后的肉苁蓉挥发性化合物主要以醛类为主。除此之外,将其它中药基于GC-IMS技术在不同炮制方法中的应用汇总于表1。
表 1 GC-IMS技术在炮制研究方面的应用作者 药材及炮制方法 采用方式 实例 高以丹等 [31] 柴达木枸杞
冷冻干燥、自然阴干、热风烘干GC-IMS 从枸杞样品中鉴定出反-2-壬烯醛、2,4-庚二烯醛等52种VOCs,表明冷冻干燥法比自然阴干、热风烘干以及微波干燥更好,能够有效保留枸杞中的VOCs,使枸杞保持较高的品质。 时海燕等 [32] 六神曲
生品、炒品、焦品HS-GC-IMS 从六神曲生品、炒品和焦品中鉴别出60种化合物通过比较种类和差异,得出炒神曲比焦神曲健胃消食的效果更好。 林秀敏等 [33] 当归
酒洗、酒炙、酒浸GC-IMS 2-十一烯醛、丙酮等为酒洗与酒浸当归的主要差异性物质,2-十一烯醛、丙酮等为酒洗与酒炙当归的主要差异性物质,2-十一烯醛、辛酸乙酯等为酒浸与酒炙当归的主要差异性物质。 武旭等 [34] 胆南星
发酵炮制GC-IMS 发酵炮制有助于胆南星矫味矫臭 王雨晨等 [35] 太子参
常温晾干、晒干、热风干燥、
真空冷冻干燥GC-IMS 40 ℃热风干燥可以有效保留太子参样品中的VOCs,与晒干、晾干样品无差异,但真空冷冻干燥对太子参挥发性成分的影响较大,会造成挥发性成分以及风味的损失 焦焕然等 [36] 侧柏叶
常温晾干、晒干,热风干燥、
变温干燥GC-IMS 40 ℃和60 ℃热风干燥能够较好地保留瓜蒌样品中的核苷类和黄酮类成分 4. 与电子鼻联用
国内外也有许多采用GC-IMS与电子鼻联用对中药挥发性成分进行研究。电子鼻是一种通过模拟人嗅觉系统对检测物质进行品质评价的感官仪器,其原理是通过传感器阵列对气味分子进行检测和响应,将产生的信号经过预处理后送入模式识别系统,通过指纹图谱对挥发性成分或是气体进行定量或定性分析[37]。两种技术的联用为实验的结果研究提供了更高的准确度。
Feng等 [38]采用GC-IMS、GC-MS对不同地理标志的八种花椒的VOCs进行测定,证明了两种方法均可用于对不同花椒的分类,但相较之下GC-IMS操作时间更短,且有能够检测到含量很低物质,结果表明红花椒比青花椒能够释放出更多的萜烯、酯类和更少的醇类,同时该研究还与电子鼻联用表征花椒中的香气物质,W1W、W2W和W5S传感器对花椒样品VOCs的响应更强,说明花椒产品中可能含有更高丰度的萜烯、有机硫化物和氮氧化物。陈小爱等 [39]利用GC-MS、GC-IMS和电子鼻技术联用,分析老香黄在发酵期间的VOCs变化,GC-MS共鉴别出包括醇类等八个种类的46种VOCs,GC-IMS则检测出包括杂环类等九个类别的38种VOCs,同时电子鼻PCA有效区分了不同发酵时间的样品,发现发酵6个月后老香黄挥发性组分开始发生较大的变化,其中柠檬烯等14种是发酵期间含量较高且相对稳定的成分,发酵过程中产生的庚醛、糠醛等是构成老香黄特有气味的特征性成分。王世丽等 [40]通过电子鼻辨识南北柴胡气味特征物质与GC-IMS检测其挥发性成分,发现南北柴胡中短链烷烃、醛类等物质差异较大,癸醛、异戊烯醛等可作为南柴胡的特征物质,2-甲基丙酸、3-甲基丁醇可作为北柴胡的特征物质,此外乙酸、乙酸甲酯等成分在北柴胡中显著高于南柴胡。
5. 总结和展望
GC-IMS在中药研究中的应用前景非常广阔,不仅可以对同属不同种、不同产地来源、不同采收期以及不同贮存时间的中药VOCs进行分析鉴别,还可以帮助分析炮制前后中药VOCs含量变化以及在复方中寻找质量标志物,为药物质量控制与药效研究提供帮助。另外,随着技术的不断进步和中药现代化需求的增加,GC-IMS可以与特征图谱相结合,构建特征指纹图谱;也可以与电子鼻等其他分析手段融合,发挥出新的效果,让其所能提供的信息更加全面。但该项技术作为新兴科技仍需解决许多问题,比如应探索融合数据库的体系架构[41]。目前,GC-IMS通常使用的数据库为NIST出版的标准质谱图,对于中药VOCs的专业数据库搭建还不全面,部分VOCs需要自行判断建立文档保存入库,对实验进程造成不便。由于中药挥发性成分复杂,GC-IMS可能因峰重叠导致部分成分无法准确定性,例如分析复方丹参片时,GC-IMS仅能明确鉴定其中60%的化合物,需GC-MS辅助验证。而且GC-IMS对象单一,无法检测多糖、生物碱等非挥发性成分,难以全面评价中药质量。
总的来说,GC-IMS技术为中药研究提供了一种新的科学工具,有利于推动中药科学研究深入,也为中药产业的发展走向国际化和标准化提供支持。
-
表 1 小鼠心梗模型失败原因分析及解决方法
失败时段 比例(%) 原因分析 只数 解决方法 术中死亡 6.8 术中心脏破裂致死 1 控制穿刺深度和宽度 术中气胸致死 1 穿刺结扎迅速,快速闭合胸腔 术后死亡 10.3 术后胸腔出血致死 2 保证视野开阔,用纱布保护胸壁及肺,避免刺破大血管和肺脏 术后致死型心律失常致死 1 结扎动作迅速轻柔,减少对心脏的刺激 其他 3.4 术后未发生心梗 1 (造模失败) 表 2 23只小鼠心肌梗死区大小情况
项目 体重/g 全心室重量(M1)/mg 梗死心肌重量(M2)/mg 梗死大小(M2/M1)/(%) 总计( $ {\rm{\bar X}} $ ±s)29.65±5.35 76±13 21.91±7.06 28±6 -
[1] THYGESEN K, ALPERT J S, JAFFE A S, et al. Third universal definition of myocardial infarction[J]. Glob Heart,2012,7(4):275-295. doi: 10.1016/j.gheart.2012.08.001 [2] EMELIA J. BENJAMIN, MICHAEL J. BLAHA, STEPHANIE E. CHIUVE. Heart disease and stroke statistics—2017 Update: A Report From the American Heart Association[J]. Circulation,2017,135(10):e146-e603. [3] HEUSCH G, GERSH B J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge[J]. Eur Heart J,2016:ehw224. doi: 10.1093/eurheartj/ehw224 [4] KUMAR M, KASALA E R, BODDULURU L N, et al. Animal models of myocardial infarction: Mainstay in clinical translation[J]. Regul Toxicol Pharmacol,2016,76:221-230. doi: 10.1016/j.yrtph.2016.03.005 [5] TANG Y P, LIU Y, FAN Y J, et al. To develop a novel animal model of myocardial infarction: a research imperative[J]. Anim Models Exp Med,2018,1(1):36-39. doi: 10.1002/ame2.12010 [6] GAO E H, LEI Y H, SHANG X Y, et al. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse[J]. Circ Res,2010,107(12):1445-1453. doi: 10.1161/CIRCRESAHA.110.223925 [7] GUO Y R, WU W J, QIU Y M, et al. Demonstration of an early and a late phase of ischemic preconditioning in mice[J]. Am J Physiol-Heart Circ Physiol,1998,275(4):H1375-H1387. doi: 10.1152/ajpheart.1998.275.4.H1375 [8] GAO F, KATAOKA M, LIU N, et al. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction[J]. Nat Commun,2019,10:1802. doi: 10.1038/s41467-019-09530-1 [9] ROJAS S V, KENSAH G, ROTAERMEL A, et al. Transplantation of purified iPSC-derived cardiomyocytes in myocardial infarction[J]. PLoS One,2017,12(5):e0173222. doi: 10.1371/journal.pone.0173222 [10] CAHILL T J, CHOUDHURY R P, RILEY P R. Heart regeneration and repair after myocardial infarction: translational opportunities for novel therapeutics[J]. Nat Rev Drug Discov,2017,16(10):699-717. doi: 10.1038/nrd.2017.106 [11] TAO B, GAO H K, ZHENG M W, et al. Preclinical modeling and multimodality imaging of chronic myocardial infarction in minipigs induced by novel interventional embolization technique[J]. EJNMMI Res,2016,6(1):59. doi: 10.1186/s13550-016-0214-7 [12] KOLK M V V, MEYBERG D, DEUSE T, et al. LAD-ligation: a murine model of myocardial infarction[J]. J Vis Exp,2009,14(32):1438. [13] MICHAEL L H, ENTMAN M L, HARTLEY C J, et al. Myocardial ischemia and reperfusion: a murine model[J]. Am J Physiol-Heart Circ Physiol,1995,269(6):H2147-H2154. doi: 10.1152/ajpheart.1995.269.6.H2147 [14] KATHERINE C. WU, JOãO A.C. LIMA. Noninvasive imaging of myocardial viability: current techniques and future developments[J]. Circ Res,2003,93(12):1146-58. doi: 10.1161/01.RES.0000103863.40055.E8 [15] GRAY G A, WHITE C I, THOMSON A, et al. Imaging the healing murine myocardial infarctin vivo: ultrasound, magnetic resonance imaging and fluorescence molecular tomography[J]. Exp Physiol,2013,98(3):606-613. doi: 10.1113/expphysiol.2012.064741 [16] CUMMINS B, AUCKLAND M L, CUMMINS P. Cardiac-specific troponin-l radioimmunoassay in the diagnosis of acute myocardial infarction[J]. Am Heart J,1987,113(6):1333-1344. doi: 10.1016/0002-8703(87)90645-4 -





 下载:
下载:
 下载:
下载: