-
尽管现代医疗卫生条件有了很大改善,但心血管疾病仍然是世界上主要的健康问题。据2018年世界卫生组织数据统计,每年因心血管疾病导致死亡人数占总死亡人数超过30%。动脉粥样硬化(AS)是动脉壁的一种慢性疾病,是引起心血管疾病的重要因素,病理特征为动脉内膜炎症、坏死、纤维化和钙化,动脉特定部位斑块形成[1-2]。AS致病因素较为复杂,目前认为主要与血管内皮细胞损伤、炎症反应、脂质代谢紊乱、自噬与凋亡失衡等有关。近些年研究发现,肠道菌群紊乱,特别是肠道菌群代谢产物和AS发生发展有着密切联系[3]。现代医学在AS防治中常用的保守治疗药物主要为他汀类药物,临床上取得一定的疗效,但是疗效不足以及药物的副作用等局限性也慢慢呈现出来[4]。传统中草药作为中华文化的瑰宝,在中国的应用超过5 000年,疗效显著,传承至今仍展现出强大生命力。中药尤其是中药复方由于具有多成分、多靶点、多途径的整体协同作用特点,在心血管疾病等复杂疾病的治疗方面显示出独特的优势。越来越多的学者研究中药复方治疗AS的药效物质基础及其作用机制,为中医药走向现代化、国际化奠定基础。本文综述近5年中药复方抗AS的作用机制,为抗AS中药复方的深入系统研究提供参考。
-
高脂血症是动脉粥样硬化的独立危险因素,尤其是血液中低密度脂蛋白含量,如果长时间超过人体生理需要浓度,就会引起动脉粥样硬化的发生和发展[5]。刘传亮等[6]探讨复方丹参川芎中药配方颗粒在常规治疗基础上对老年颈动脉粥样硬化的影响及机制,发现和常规治疗组比较,加用复方丹参川芎中药配方颗粒能够显著降低患者血清总胆固醇(TC)、三酰甘油(TG)、低密度脂蛋白(LDL),有效改善血脂水平,缩小动脉粥样硬化斑块。梅琼等[7]研究发现,当归川芎组合能显著降低大鼠血清LDL、TC、TG水平,升高血清高密度脂蛋白(HDL)水平,显著降低冠状动脉粥样斑块面积指数(PAI),具有减轻冠状动脉粥样斑块的作用。张燕等[8]探讨不同剂量益气滋阴、活血通络复方对动脉粥样硬化患者LDL和HDL水平的影响,结果表明该方防治动脉粥样硬化的作用,与其能够降低LDL、升高HDL有关。王磊等[9]观察复方三七护脉汤,联合西医常规治疗心血瘀阻型冠心病稳定型心绞痛患者的临床疗效,发现复方三七护脉汤联合西医常规治疗6周后,血清TC、TG、LDL水平均明显下降,血清HDL水平明显升高,可明显改善患者的血脂水平,疗效优于西医常规治疗。
-
泡沫细胞是AS病变的关键成分,它由血管内皮下的巨噬细胞摄取大量脂质胆固醇转变而成[10]。泡沫细胞形成在AS发生发展中起着至关重要的作用,它主要与脂质内流与外排失衡有关。胆固醇逆向转运(RCT)是HDL将多余胆固醇转运至肝脏再循环或以胆酸形式排出体外的过程[11]。王晓宁[12]研究发现,化浊通脉方能够通过提高兔胆固醇跨膜逆转运关键蛋白小凹蛋白-1(cav-1)及基因水平、亲环素A的基因水平,上调三磷酸腺苷结合盒转运体A1(ATP-binding cassette transporters A1,ABCA1)蛋白表达,促进细胞内胆固醇流出,从而达到抗AS效果。祝骥等[13]探讨复方丹参片对颈AS兔PPAR-γ/LXR-α/ABCA1信号通路的影响,发现复方丹参片能够降低血脂,增加兔颈动脉中PPAR-γ、LXR-α及ABCA1 mRNA及蛋白质的表达,表明其可能通过激活PPAR-γ/LXR-α/ABCA1信号通路促进RCT而发挥抗AS作用。秦合伟等[14]探讨血管软化丸抗AS的作用机制,发现用药后小鼠主动脉的miR-33 a表达量降低,ABCA1基因和蛋白相对表达量均升高,体外实验发现血管软化丸含药血清呈浓度和时间依赖性下调miRNA33a表达,明显上调ABCA1 mRNA和蛋白的表达,但能被转染miRNA33 mimic抑制。表明血管软化丸通过调控miR-33a,进而影响下游信号ABCA1的表达,促进巨噬细胞内胆固醇流出,这可能是其抗AS作用机制之一。许丽婷等[15]研究发现黄连解毒汤含药血清各剂量组能够明显降低泡沫细胞内TC含量,明显升高ABCA1 mRNA表达,表明黄连解毒汤可能是通过上调泡沫细胞ABCA1的转录与表达,促进胆固醇外排来实现防治AS效果的。Deng等[16]研究发现中药复方丹蒌片提取物能够通过激活PPARα/ABCA1信号通路,促进RCT,改善巨噬细胞内脂质沉积,从而防治AS。
-
血管内皮细胞损伤被认为是动脉粥样硬化的起始环节,贯穿动脉粥样硬化发生发展的全过程。其发生机制可能与一氧化氮(NO)与活性氧化物(ROS)失衡,即氧化应激有关[17]。过量ROS产生是导致氧化应激重要因素,血管内皮细胞中ROS的产生可诱导LDL的氧化和ROS敏感炎症基因的表达[18]。近年来大量资料提示氧化应激及其产物,尤其是氧化低密度脂蛋白(Ox-LDL)是血管内皮损伤的主要因素,在动脉粥样硬化的发生、发展中发挥重要作用[19]。李红蓉等[20]研究通心络对Ox-LDL诱导损伤的血管内皮细胞的保护作用,对人脐静脉内皮细胞加Ox-LDL(终浓度为30 mg/L)造成血管内皮细胞氧化应激损伤模型,发现通心络组细胞线粒体膜电位、细胞培养液中NO含量、超过氧化物岐化酶(SOD)活力明显升高,表明通心络有较强的抗氧化能力,可减轻Ox-LDL对血管内皮细胞的损伤。汪玉成等[21]探讨泽泻汤在Ox-LDL诱导的血管平滑肌细胞(VSMC)增殖中的作用和机制,发现泽泻汤含药血清可显著抑制Ox-LDL诱导的VSMC增殖,机制可能与上调p27蛋白和抑制周期蛋白D1、周期蛋白E、PCNA表达有关。王红梅等[22]研究龙血通络胶囊对Ox-LDL致人脐静脉内皮细胞损伤的保护作用,发现龙血通络胶囊可促进Ox-LDL损伤的血管内皮细胞增殖,降低胞内丙二醛(MDA)含量,减少细胞乳酸脱氢酶(LDH)释放量,提高SOD活力及NO含量,提示龙血通络胶囊可通过抑制Ox-LDL对人脐静脉内皮细胞损伤来防治动脉粥样硬化。
-
炎症反应在动脉粥样硬化发展过程中扮演着重要角色。在动脉粥样硬化早期阶段,主要特征是血管内皮损伤,脂质代谢紊乱以及血流动力学改变,在动脉粥样硬化进展期,主要特征是内皮下炎症反应[23]。因此,改善炎症反应对防治动脉粥样硬化至关重要。
-
与动脉粥样硬化有关的炎症反应相关通路主要有核因子κB(NF-κB)信号通路、丝裂原活化蛋白激酶(MAPK)信号通路与AP-1,以及Janus激酶/信号传导及转录激活因子(JAK/STAT)信号通路等[24]。Cheng等[25]研究发现,银丹心脑通可抑制NF-κB转录活性,降低下游通路中肿瘤坏死因子α(TNF-α)、白介素1β(IL-1β)等相关炎症因子表达,延缓动脉粥样硬化进展,表明其抗动脉粥样硬化机制可能是通过NF-κB信号通路调控血管的炎症反应来实现的。刘叙阳等[26]以脂多糖诱导的血管内皮细胞为研究对象,探讨补阳还五汤(益气活血)、血府逐瘀汤(活血)、四君子汤(益气)含药血清对细胞Toll样受体4(TLR4)及下游信号转导通路主要元件的影响,发现补阳还五汤和血府逐瘀汤可抑制TLR4,下游髓样分化因子88(My D88),肿瘤坏死因子受体相关因子6(TRAF-6)及NF-κB的基因表达,改善炎症反应,从而防治动脉粥样硬化。罗永苗[27]以动脉粥样硬化患者为研究对象,探讨参七脉心通胶囊基于NF-κB信号通路抗动脉粥样硬化的作用机制,发现参七脉心通胶囊能够下调NF-κB表达,降低IL-6、TNF-α炎症因子水平,抑制NF-κB信号转导通路,改善炎症反应,从而达到防治动脉粥样硬化的作用。张冰冰等[28]研究益糖康对动脉粥样硬化兔血清中炎症因子以及NF-κB、MAPK信号通路的影响,发现其能有效抑制NF-κB、P38MAPK、JNK蛋白磷酸化水平,降低下游炎症因子IL-1β、IL-6、TNF-α的表达,表明其可能是通过NF-κB和MAPK通路防治动脉粥样硬化。
-
巨噬细胞极化在炎症反应中扮演重要作用。巨噬细胞表型主要分为M1型和M2型,M1型巨噬细胞通过分泌促炎因子(IL-6、IL-12、IL-1β、TNF-α等)来促进炎症反应,M2型巨噬细胞主要通过分泌抑炎因子(IL-10、TGF-β等)来抑制炎症反应[29-30]。秦合伟等[31]探讨血管软化丸抗动脉粥样硬化的分子机制时发现,血管软化丸含药血清可以诱导巨噬细胞高表达M2型巨噬细胞的标志CD206,使巨噬细胞由M1型向M2型极化,抑制炎症反应。Li等[32]研究发现,通心络能够抑制源于THP-1的Notch-1信号通路,阻止巨噬细胞向M1型转化,从而改善炎症反应来发挥抗AS疗效。
-
人体内肠道微生物组成复杂,肠道内菌群数量超过上千种,但是在全部细菌中,有30~40种的优势细菌却占比99%[33]。肠道微生物对人体消化功能和健康关系密切,肠道微生物组成改变可能会促进动脉粥样硬化进程,Menni等[34]指出肠道菌群的多样性和动脉粥样硬化进展呈负相关,也有研究[35]表明艾克曼菌能够通过恢复肠道屏障,改善代谢内毒素血症引起的炎症,减轻AS病变。除此之外,肠道微生物的代谢产物被认为是加速动脉粥样硬化发展的关键因素,尤其是三甲胺氧化物(TMAO)[36]。除TMAO外,肠道菌群代谢物短链脂肪酸丁酸能够抑制肠道胆固醇吸收,延缓动脉粥样硬化进展[37]。丙酸能够降低炎症水平,抑制动脉粥样斑块形成,从而减少心血管疾病发生[38]。
在中药复方通过肠道菌群防治动脉粥样硬化方面,Zhang等[39]研究发现,定心方4号能显著降低小鼠血脂,减少动脉斑块面积,小鼠肠道muribaculaceae和ruminococcaceae菌丰度增加,erysipelotrichaceae菌丰度降低,这些菌群的改变对脂代谢是有益的,表明定心方4号抗动脉粥样硬化机制可能与其改变肠道菌群组成有关。Zhu等[40]研究发现,中药复方泽泻汤明显改变小鼠肠道菌群组成,降低血清TMAO和肝脏黄素单加氧酶3(FMO3)水平,减少动脉粥样硬化斑块面积,表明其抗动脉粥样硬化作用可能是通过调节肠道菌群,进而调节TMA-FMO3-TMAO轴来实现的。Ji等[41]探讨中药复方通脉逐瘀汤通过调节肠道菌群抗动脉粥样硬化的作用机制,发现通脉逐瘀汤能显著改变雌性C57BL/6J小鼠肠道菌群组成,某些细菌的丰度比如肠杆菌、链球菌、梭菌等在给予通脉逐瘀汤处理后恢复正常水平。此外,通脉逐瘀汤能够降低小鼠血清TMAO水平,在抗生素处理来抑制肠道菌群的基础上再使用通脉逐瘀汤,TMAO水平未见明显改变,结果表明通脉逐瘀汤能够通过改变肠道菌群组成,调节相关肠道代谢通路效应,来发挥抗动脉粥样硬化作用。
靶向干预某些特定肠道菌群可能是防治AS或者其他心血管疾病的新途径[42],已有研究确定在LDLr-/-小鼠中定向重塑肠道微生物组以预防AS的发生和进展的可行性[43]。中医理论指出“心与小肠相表里”,这表里关系可能部分是通过调节肠道菌群途径来实现的。通过调节肠道菌群组成,进而改变肠道功能,影响与AS关系密切的肠道菌群代谢物,可能是中药复方通过肠-心轴防治AS的主要机制。目前,单味中药及其提取物通过调节肠道菌群抗AS的研究较多,中药复方通过调节肠道菌群抗AS的研究较少,AS和某类特定菌群之间的关系尚不十分确切,有待深入研究,中药复方调节肠道菌群的具体有效成分也有待进一步挖掘。
-
越来越多的学者研究传统中药治疗疾病药效物质基础及其作用机制,但是大部分研究基于单味中药,或者中药单体,涉及中药复方抗动脉粥样硬化作用机制的研究不多,通过调节肠道菌群作用机制研究中药复方抗动脉粥样硬化更少。中药复方具有多成分、多通路、多靶点的优势,但是目前研究基于药效方面多,具体作用机制网络通路研究较少,给中药复方现代研究带来机遇和挑战。中医理论指出,心与小肠相表里,这表里关系可能部分是通过调节肠道菌群这途径来实现的,这就为中药复方通过调节肠道菌群抗动脉粥样硬化提供了中医理论基础,目前16s RNA测序和宏基因组等技术的推广应用,更为肠道菌群的深入研究提供技术支撑,中药网络药理学的发展也为中药复方多种作用机制研究提供了可能。
Review of anti-atherosclerosis mechanism of a TCM formula
-
摘要: 心血管疾病在全球的发病率和致死率仍居高不下,动脉粥样硬化(AS)是心血管疾病的重要病理基础,其致病机制至今尚未完全明确。目前主要认为,AS与血管内皮细胞损伤、脂质代谢紊乱、炎症反应、自噬和凋亡失衡等因素有关。传统中草药特别是中药复方在防治AS中取得良好疗效,对中药复方抗AS的药效及作用机制研究也越来越多。通过检索近5年的中药复方研究文献,综述中药复方抗AS作用机制,为抗AS中药复方的深入研究提供参考。Abstract: The global morbidity and mortality of cardiovascular diseases remain high. Atherosclerosis is an important pathological basis of cardiovascular diseases, and its pathogenic mechanism has not been fully clarified. It was reported that pathogenic mechanism of atherosclerosis is related to vascular endothelial cell injury, lipid metabolism disorder, inflammatory reaction, imbalance between autophagy and apoptosis, et al. Traditional Chinese medicine(TCM) formula has shown good effects in the prevention and treatment of atherosclerosis. There are a lot of studies that showed the anti-atherosclerosis effect and the mechanism of TCM formula. In this paper, we reviewed the mechanism of anti-atherosclerosis action of TCM formula by summarizing the research literatures in the past five years, and provide reference for the further systematic study of anti-atherosclerosis effect of TCM formula.
-
Key words:
- TCM formula /
- atherosclerosis /
- mechanism /
- review
-
近红外光响应的肿瘤光热治疗因具有组织穿透性强、生物安全性好等优点,已引起广泛关注。作为新兴的光敏剂,八丁氧基酞菁钯具有成本低、易合成、毒性低等优点,在肿瘤光动力治疗(PDT)领域表现出巨大潜力。由于高渗透强滞留效应(EPR),脂质体作为药物载体可靶向集中于肿瘤组织,同时ROS可氧化破坏脂质双层膜,促进包载药物在肿瘤组织的精准释放。本实验以雷帕霉素为模型药物,采用课题组合成的八丁氧基酞菁钯为光敏剂,制备近红外光触发的ROS响应型雷帕霉素脂质体,并对其理化性质及体外释放特性进行考察。
1. 材料与方法
1.1 仪器
R206D旋转蒸发仪(上海申生科技有限公司);730 nm/1500 mW激光器(长春市亮丽光电有限公司);U3000高效液相色谱仪(Thermo Fisher公司);JY92.IIDN超声波细胞粉碎机(宁波新芝生物科技股份有限公司);马尔文激光粒度仪(英国Malvern公司)。
1.2 试剂
二硬脂酰磷脂酰胆碱(DSPC,Avanti公司);1,2-二亚油酰基-sn-甘油基-3-磷酸胆碱(DLPC,Avanti公司);二硬脂酰基磷脂酰乙醇胺-聚乙二醇2000(DSPE-mPEG2000,Lipoid 公司);胆固醇、雷帕霉素(MCE公司)、八丁氧基酞菁钯[PdPC(OBu)8,实验室自制];磷酸盐缓冲液(PBS,HyClone 公司)。
1.3 脂质体的制备[1]
采用薄膜分散法制备脂质体。精密称取DSPC、DLPC、胆固醇、DSPE-mPEG2000、雷帕霉素、八丁氧基酞菁钯置于250 ml圆底烧瓶中,加入适量氯仿溶解。65 ℃水浴,65 r/min减压旋转蒸发形成均匀薄膜,取PBS溶液4 ml水化。吸出脂质体,用PBS稀释至5 ml,200 W探头超声5 min,即得近红外光触发ROS响应型雷帕霉素脂质体。空白脂质体除不加雷帕霉素、八丁氧基酞菁钯外,制备方法相同。
1.4 脂质体处方筛选
以脂质体成膜及水化效果、药物包封率及载药量、包载药物光敏释放效率为指标,从DLPC、胆固醇、DSPE-mPEG2000处方量及PdPC(OBu)8投药量对光敏ROS脂质体处方进行单因素考察。
1.5 粒径、PDI及Zeta电位
取少量脂质体溶液,用去离子水稀释,使用马尔文激光粒度仪测量脂质体粒径、PDI及Zeta电位。
1.6 雷帕霉素含量测定[2]
1.6.1 色谱条件
色谱柱:Agilent TC-C18(2)(4.6 mm×250 mm,5 μm);流动相:甲醇-水(84∶16);流速1.0 ml/min;检测波长:278 nm;柱温:50 ℃;进样量:20 μl。
1.6.2 标准曲线绘制
精密称取雷帕霉素2.50 mg置于10 ml容量瓶中,用甲醇定容至刻度,得到浓度为250 μg/ml储备液。精密吸取储备液800、400、200、100、40、20、10、4 μl,分别于5 ml容量瓶中用甲醇定容。制成浓度为40、20、10、5、2、1、0.5、0.2 μg/ml的对照品溶液。HPLC仪检测并记录峰面积A,绘制出峰面积A与对照品质量浓度C的标准曲线并进行线性回归分析。
1.6.3 专属性考察
吸取雷帕霉素脂质体溶液500 μl加入5 ml容量瓶中,甲醇定容至刻度。超声20 min破乳后,以10 000 r/min进行超速离心10 min。取适量上清液过0.45 μm有机膜,制得供试品溶液。吸取空白脂质体按同法制备阴性对照品溶液。分别取对照品、阴性对照品、供试品按照“1.6.1”项下方法进样,记录HPLC图。
1.6.4 精密度
选取低、中、高3个浓度的对照品溶液,1 d内重复进样考察日内精密度,连续3 d每天测定一次,考察日间精密度。
1.6.5 提取回收率
吸取用于制备选定的低、中、高3个浓度对照品溶液的储备液于不同的5 ml容量瓶中,同一浓度共制备3个样品。向每个容量瓶中加入100 μl空白脂质体溶液,再用甲醇定容至刻度。超声20 min破乳后,进行超速离心,10 000 r/min,10 min。取适量上清液过0.45 μm有机膜,进行HPLC检测。
1.7 包封率及载药量
包封率与载药量是评价脂质体的重要指标。包封率是脂质体中包封药物量与脂质体中药物总量的百分比。载药量是脂质体中药物量与脂质体总量的百分比。
精密吸取脂质体500 μl置于5 ml容量瓶中,甲醇稀释至刻度。超声20 min破乳后,再进行超速离心,10 000 r/min,10 min。取适量上清液过0.45 μm有机膜,进行HPLC检测,计算得出脂质体中药物总量。
精密吸取适量脂质体溶液,先进行低速离心[3],3 000 r/min,15 min。取上层液体500 μl置于5 ml容量瓶中用甲醇稀释至刻度。超声20 min破乳后,再进行超速离心,10 000 r/min,10 min。取适量上清液过0.45 μm有机膜,进行HPLC检测,计算出脂质体中包封药物量。
1.8 体外释放实验[2]
采用反向透析法测定雷帕霉素脂质体光敏释放特性。以30 ml 20%乙醇为释放介质,加入50 ml离心管内;另吸取1 ml释放介质并加入透析袋内,将透析袋两端扎紧后放入离心管中;取300 μl雷帕霉素脂质体,用730 nm,1 W/cm2近红外光照射5 min后,转移至释放介质;将离心管移至摇床内,设置摇床条件为37 ℃,180 r/min。分别在1、2、4、8、12 h从透析袋内取样200 μl,用于样品测定和累积释放率的计算。取样后即补加新鲜释放介质200 μl,保持释放介质总量不变。以时间(t)为横坐标,以累积释放率(Q%)为纵坐标,绘制释放曲线。
2. 结果
2.1 雷帕霉素的含量测定
2.1.1 标准曲线的绘制
回归方程为A = 0.6240 C-0.1738 (r = 0.999 5)。雷帕霉素在0.2~40 μg/ml浓度范围内线性关系良好。
2.1.2 专属性考察
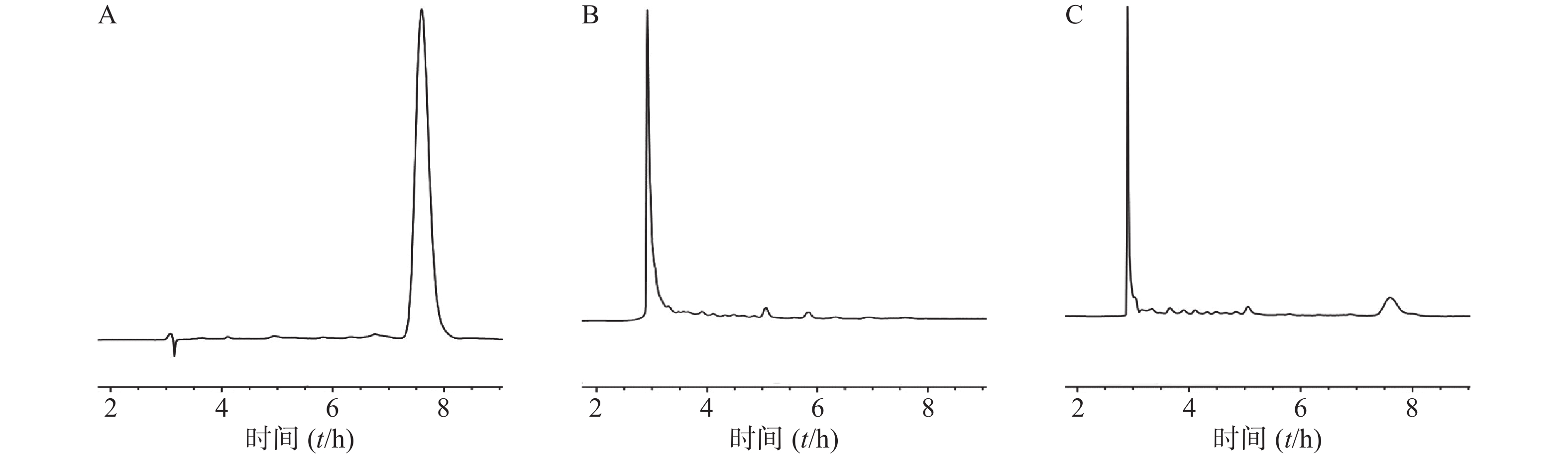
按照“1.6.1”项下色谱条件测定,雷帕霉素出峰良好,保留时间为7.8 min。表明此方法专属性良好(图1)。
2.1.3 精密度考察
由表1可知,雷帕霉素在低、中、高3个浓度都具有较好的准确度,日间精密度和日内精密度值均小于5%,表明该方法可用于雷帕霉素的含量测定。
表 1 雷帕霉素 HPLC 分析的精密度($\overline x \; $ ±s,n=3)浓度(μg/ml) 日内精密度 日间精密度 检测值(μg/ml) RSD(%) 检测值(μg/ml) RSD(%) 0.50 0.58±0.01 1.62 0.58±0.01 1.13 2.00 1.95±0.02 0.56 1.96±0.01 0.49 10.00 9.36±0.04 0.40 10.02±0.09 0.87 2.1.4 提取回收率
由表2可知,雷帕霉素在低、中、高3个浓度的回收率为97.64%~98.62%,符合95%~105%的范围,且RSD值均小于1%,表明该提取方法稳定可靠。
表 2 雷帕霉素 HPLC 分析的提取回收率($\overline x \; $ ±s,n=3)浓度(μg/ml) 检测值(μg/ml) 回收率(%) RSD(%) 0.50 0.57±1.54 113.47 1.69 2.00 1.93±0.02 96.48 0.82 10.00 9.82±0.05 98.21 0.52 2.2 脂质体处方单因素考察
2.2.1 DLPC用量
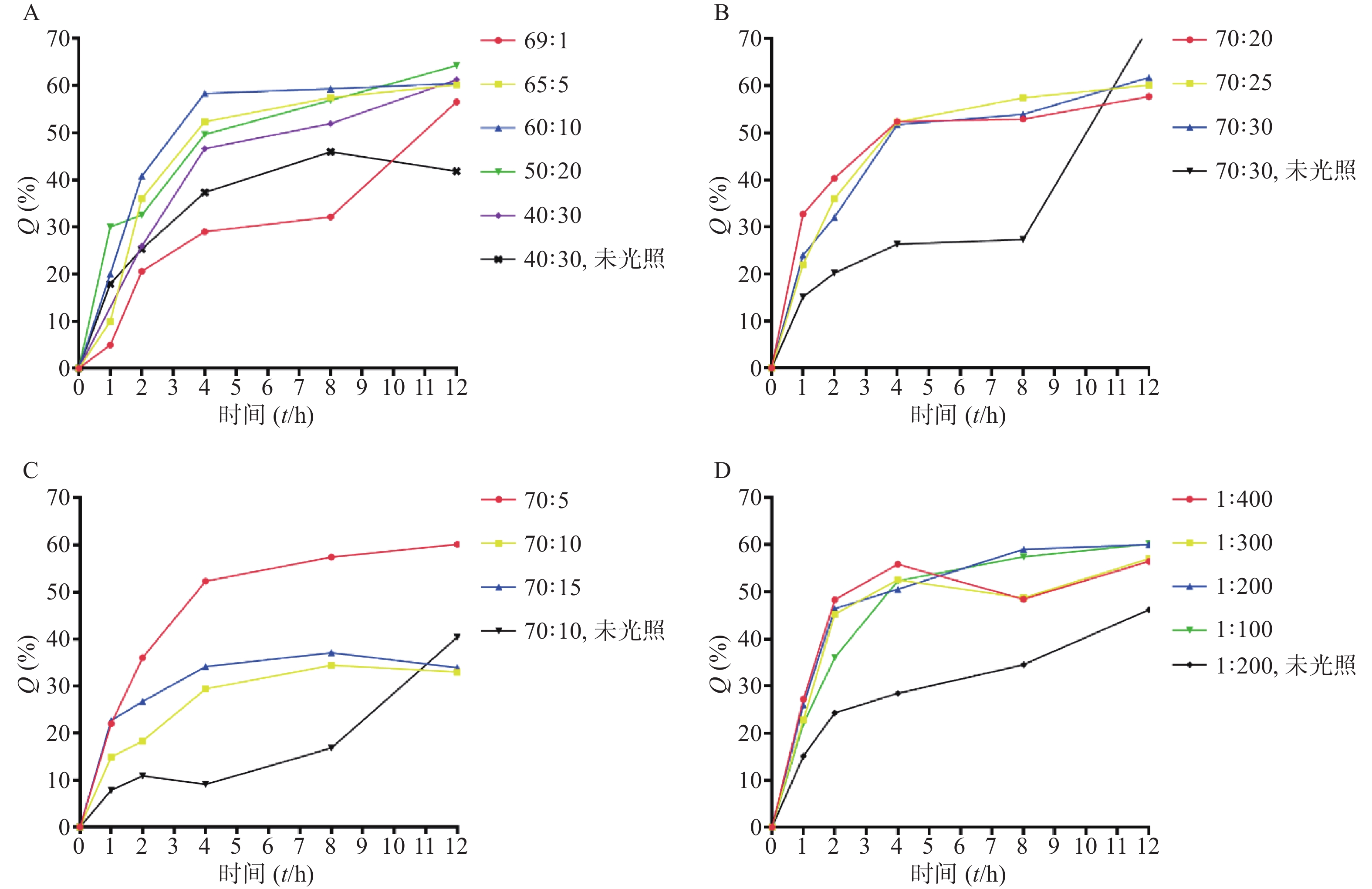
由表3可知,包含不同摩尔量DLPC的处方制得的脂质体,成膜及水化效果较好,粒径<200 nm,PDI<0.2,雷帕霉素包封率>90%,载药量>1%;脂质体体外释放试验,光照12 h后,测得雷帕霉素药物累积释放率>60%(图2A)。当DLPC含量较低时(DSPC∶DLPC=69∶1),雷帕霉素释放速率明显低于其他处方。随着DLPC含量的增加,雷帕霉素释放速率及12 h内累积释放率并无明显增加。
表 3 不同摩尔量DLPC脂质体的表征
DSPC:DLPC
(mol:mol)成膜 水化 粒径(l/nm) PDI Zeta电位(mV) 包封率(%) 载药量(%) 69∶1 均匀 无沉淀 139.7 0.051 −12.1 98.71 1.11 65∶5 均匀 无沉淀 173.2 0.194 −12.5 94.20 1.23 60∶10 均匀 无沉淀 143.4 0.035 −13.0 98.43 1.15 50∶20 均匀 无沉淀 126.7 0.165 −14.9 92.64 2.27 40∶30 均匀 无沉淀 120.5 0.173 −14.6 92.94 2.22 2.2.2 胆固醇用量
由表4可知,PC(DSPC+DLPC)与胆固醇之比过大(70∶10)或过小(70∶40),脂质体都不能成膜。当PC与胆固醇之比为70∶20、70∶25、70∶30时,所得脂质体成膜及水化效果较好,粒径<200 nm,PDI<0.2,雷帕霉素包封率>90%,载药量在1%左右。在进行脂质体体外释放实验中,测得12 h后雷帕霉素药物累积释放率>60%(图2B)。
表 4 不同摩尔量胆固醇脂质体的表征PC∶胆固醇
(mol∶mol)成膜 水化 粒径(l/nm) PDI Zeta电位(mV) 包封率(%) 载药量(%) 70∶10 不均匀 — — — — — — 70∶20 均匀 无沉淀 119.4 0.108 −11.0 96.69 0.96 70∶25 均匀 无沉淀 173.2 0.194 −12.5 94.20 1.23 70∶30 均匀 无沉淀 147.7 0.095 −12.7 94.56 0.88 70∶40 不成膜 — — — — — — 2.2.3 DSPE-mPEG2000的用量
由表5可知,当DSPE-PEG2000用量较小时,脂质体不能成膜。当PC与DSPE-PEG2000之比为70∶5、70∶10、70∶15时,所得脂质体成膜及水化效果较好,粒径<200 nm,PDI<0.2,雷帕霉素包封率>90%,载药量>1%。在进行的12 h脂质体体外释放实验中,摩尔比70∶5组测得雷帕霉素药物累积释放率>60%,其余两组药物累积释放率在33%左右(图2C)。
表 5 不同摩尔量DSPE-PEG2000脂质体的表征
PC: DSPE-PEG2000
(mol:mol)成膜 水化 粒径(l/nm) PDI Zeta电位(mV) 包封率(%) 载药量(%) 70∶0 不成膜 — — — — — — 70∶1 不成膜 — — — — — — 70∶5 均匀 无沉淀 173.2 0.194 −12.5 94.20 1.23 70∶10 均匀 无沉淀 136.2 0.144 −12.1 98.13 1.37 70∶15 均匀 无沉淀 108.9 0.197 −11.0 97.07 1.34 2.2.4 PdPC(OBu)8的用量
由表6可知,当PdPC(OBu)8用量较大(质量比1∶50),脂质体不能成膜。当PdPC(OBu)8与PC质量之比为1∶100、1∶200、1∶300、1∶400,测得粒径<200 nm,PDI<0.2,雷帕霉素包封率>90%,载药量>1%。在进行的12 h脂质体体外释放实验中,质量比1∶100、1∶200组释放效率高于其余两组,在60%左右(图2D)。
表 6 不同用量PdPC(OBu)8脂质体的表征
PdPC(OBu)8 :PC
(m:m)成膜 水化 粒径(l/nm) PDI Zeta电位(mV) 包封率(%) 载药量(%) 1∶400 均匀 无沉淀 179.3 0.140 −12.3 93.92 1.15 1∶300 均匀 无沉淀 157.4 0.143 −11.5 94.23 1.16 1∶200 均匀 无沉淀 145.9 0.142 −14.3 93.26 1.19 1∶100 均匀 无沉淀 136.2 0.144 −12.1 98.13 1.23 1∶50 不均匀 沉淀 — — — — — 3. 讨论
DLPC是一种人工合成的不饱和磷脂,在制成的脂质体中,DLPC将均匀分散在脂质双分子层膜上。当有ROS存在时,DLPC结构中的不饱和碳碳双键可被氧化,使脂质膜结构遭到破坏,促进包载药物的释放。光敏剂可在特定波长光照射下产生ROS,在肿瘤疾病的光动力治疗中得到广泛应用。但光敏剂自身潜在的光毒性和溶解性差是其突出缺陷。本文将光敏剂包载在脂质体中可以减少潜在的细胞毒性,同时可增加其溶解度。在研究脂质体中雷帕霉素释放的释放介质确定上,文献[2,4-5]中提供了多种选择。预实验显示,20%乙醇溶液作为释放介质时,不仅克服了脂溶性药物雷帕霉素在水中溶解性差的缺点,同时也使得在进行HPLC检测时干扰峰较少。综上所述,本研究成功制备了光敏ROS响应型雷帕霉素脂质体,表征结果较好。在短时间特定波长照射后,可实现脂溶性药物的快速释放。
-
[1] LIBBY P, RIDKER P M, HANSSON G K. Progress and challenges in translating the biology of atherosclerosis[J]. Nature,2011,473(7347):317-325. doi: 10.1038/nature10146 [2] BENTZON J F, OTSUKA F, VIRMANI R, et al. Mechanisms of plaque formation and rupture[J]. Circ Res,2014,114(12):1852-1866. doi: 10.1161/CIRCRESAHA.114.302721 [3] KOETH R A, WANG Z, LEVISON B S, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis[J]. Nat Med,2013,19(5):576-585. doi: 10.1038/nm.3145 [4] 杜文婷, 王臻楠, 顾耘. 动脉粥样硬化的中西医认识概况[J]. 中西医结合心脑血管病杂志, 2016, 14(22):2634-2637. doi: 10.3969/j.issn.1672-1349.2016.22.014 [5] LIBBY P, BURING J E, BADIMON L, et al. Atherosclero-sis[J]. Nat Rev Dis Primers,2019,5(1):56. doi: 10.1038/s41572-019-0106-z [6] 刘传亮, 陈国华, 李蕾. 复方丹参川芎中药配方颗粒干预老年颈动脉粥样硬化的研究[J]. 世界最新医学信息文摘, 2019, 19(82):15-16, 19. [7] 梅琼, 李全胜, 张静, 等. 当归川芎组合对血脂及冠状动脉组织结构影响的实验研究[J]. 湖北中医药大学学报, 2015, 17(5):47-49. doi: 10.3969/j.issn.1008-987x.2015.05.16 [8] 张燕, 李芳, 徐丽. 益气滋阴、活血通络复方对动脉粥样硬化患者LDL-C和HDL-C水平的影响[J]. 饮食保健, 2016, 3(10):72-73. [9] 王磊, 姚淮芳. 复方三七护脉汤联合西医常规治疗心血瘀阻型冠心病稳定型心绞痛20例临床观察[J]. 甘肃中医药大学学报, 2019, 36(3):48-51. [10] MAGUIRE E M, PEARCE S W A, XIAO Q. Foam cell formation: a new target for fighting atherosclerosis and cardiovascular disease[J]. Vascul Pharmacol,2019,112:54-71. doi: 10.1016/j.vph.2018.08.002 [11] 李杉杉, 申定珠, 陈川, 等. 以ABCA1为靶点的补肾中药复方防治动脉粥样硬化的思路探讨[J]. 中国中医急症, 2017, 26(5):834-837. doi: 10.3969/j.issn.1004-745X.2017.05.025 [12] 王晓宁. 化浊通脉方对动脉粥样硬化兔胆固醇逆向转运的影响[D]. 北京: 北京中医药大学, 2015. [13] 祝骥, 许颖龄, 卢德赵, 等. 复方丹参片对颈动脉粥样硬化兔PPAR-γ/LXR-α/ABCA1信号通路的影响[J]. 中国现代应用药学, 2016, 33(12):1503-1507. [14] 秦合伟, 李彦杰, 任锟, 等. 基于miR-33a调控ABCA1表达探讨血管软化丸抗动脉粥样硬化的作用机制[J]. 中医药信息, 2018, 35(6):1-7. [15] 许丽婷, 徐彬人, 盛蒙, 等. 黄连解毒汤含药血清对泡沫细胞ABCA1表达与胆固醇含量的影响[J]. 中国民族民间医药, 2020, 29(6):10-13. [16] HAO D, DANBIN W, MAOJUAN G, et al. Ethanol extracts of Danlou tablet attenuate atherosclerosis via inhibiting inflammation and promoting lipid effluent[J]. Pharmacol Res,2019,146:104306. doi: 10.1016/j.phrs.2019.104306 [17] HIGASHI Y, NOMA K, YOSHIZUMI M, et al. Endothelial function and oxidative stress in cardiovascular diseases[J]. Circ J,2009,73(3):411-418. doi: 10.1253/circj.CJ-08-1102 [18] KONDO T, HIROSE M, KAGEYAMA K. Roles of oxidative stress and redox regulation in atherosclerosis[J]. J Atheroscler Thromb,2009,16(5):532-538. doi: 10.5551/jat.1255 [19] GLIOZZI M, SCICCHITANO M, BOSCO F, et al. Modulation of nitric oxide synthases by oxidized ldls: role in vascular inflammation and atherosclerosis development[J]. International Journal of Molecular Sciences,2019,20(13):3294. doi: 10.3390/ijms20133294 [20] 李红蓉, 常成成, 郭勇英, 等. 通心络对氧化低密度脂蛋白损伤血管内皮细胞的保护作用[J]. 医学研究生学报, 2015, 28(11):1128-1132. [21] 汪玉成, 魏伟, 苏清平, 等. 泽泻汤对氧化型低密度脂蛋白诱导血管平滑肌细胞增殖的影响[J]. 中国动脉硬化杂志, 2016, 24(8):763-768. [22] 王红梅, 周建明, 吕耀中, 等. 龙血通络胶囊对氧化低密度脂蛋白损伤人脐静脉内皮细胞的保护作用[J]. 中国中药杂志, 2018, 43(6):1241-1246. [23] ZHU Y H, XIAN X M, WANG Z Z, et al. Research progress on the relationship between atherosclerosis and inflammation[J]. Biomolecules,2018,8(3):80. doi: 10.3390/biom8030080 [24] 肖安华, 李虹维, 颜春鲁, 等. 中药复方与有效成分调控NF-kB/MAPKs/JNK信号通路介导炎症反应抗AS的研究进展[J]. 中医药学报, 2019, 47(6):109-114. [25] CHENG L, PAN G F, ZHANG X D, et al. Yindanxinnaotong, a Chinese compound medicine, synergistically attenuates atherosclerosis progress[J]. Sci Rep,2015,5:12333. doi: 10.1038/srep12333 [26] 刘叙阳, 姜华. 3种不同治法的中药复方对Toll样受体4及下游信号转导通路主要元件的影响[J]. 中国实验方剂学杂志, 2016, 22(1):121-124. [27] 罗永苗. 基于NF-κB信号通路探讨参七脉心通胶囊抗动脉粥样硬化的作用机制[D]. 广州: 广州中医药大学, 2018. [28] 张冰冰, 石岩, 朱爱松. 中药复方益糖康对动脉粥样硬化兔核因子KB和丝裂原活化蛋白激酶信号通路的影响[J]. 时珍国医国药, 2018, 29(1):56-58. [29] COLIN S, CHINETTI-GBAGUIDI G, STAELS B. Macrophage phenotypes in atherosclerosis[J]. Immunol Rev,2014,262(1):153-166. doi: 10.1111/imr.12218 [30] DALL'ASTA M, DERLINDATI E, ARDIGÒ D, et al. Macrophage polarization: the answer to the diet/inflammation conundrum? Nutr Metab Cardiovasc Dis,2012,22(5):387-392. doi: 10.1016/j.numecd.2011.12.010 [31] 秦合伟, 李彦杰, 任锟, 等. 基于TLR3/TLR9介导巨噬细胞自噬/极化效应探讨血管软化丸抗AS的作用机制[J]. 辽宁中医杂志, 2019, 46(1):156-160, 225. [32] LI H R, CHANG L P, LIU Y J, et al. Effect of Tongxinluo on polarization of macrophages[J]. Chinese Pharmacological Bulletin,2017,33(4):577-580. [33] GUARNER F, MALAGELADA J R. Gut flora in health and disease[J]. Lancet,2003,361(9356):512-519. doi: 10.1016/S0140-6736(03)12489-0 [34] MENNI C, LIN C, CECELJA M, et al. Gut microbial diversity is associated with lower arterial stiffness in women[J]. Eur Heart J,2018,39(25):2390-2397. doi: 10.1093/eurheartj/ehy226 [35] LI J, LIN S Q, VANHOUTTE P M, et al. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in apoe-/- mice[J]. Circulation,2016,133(24):2434-2446. doi: 10.1161/CIRCULATIONAHA.115.019645 [36] PIECZYNSKA M D, YANG Y, PETRYKOWSKI S, et al. Gut microbiota and its metabolites in atherosclerosis develop-ment[J]. Molecules,2020,25(3):594. doi: 10.3390/molecules25030594 [37] CHEN Y, XU C, HUANG R, et al. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice[J]. J Nutr Biochem,2018,56:175-182. doi: 10.1016/j.jnutbio.2018.02.011 [38] BARTOLOMAEUS H, BALOGH A, YAKOUB M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage[J]. Circulation,2019,139(11):1407-1421. doi: 10.1161/CIRCULATIONAHA.118.036652 [39] ZHANG Y X, GU Y Y, CHEN Y H, et al. Dingxin Recipe IV attenuates atherosclerosis by regulating lipid metabolism through LXR-α/SREBP1 pathway and modulating the gut microbiota in ApoE-/- mice fed with HFD[J]. J Ethnopharmacol,2021,266:113436. doi: 10.1016/j.jep.2020.113436 [40] ZHU B, ZHAI Y, JI M, et al. Alisma orientalis beverage treats atherosclerosis by regulating gut microbiota in ApoE-/- mice[J]. Front Pharmacol,2020,11:570555. doi: 10.3389/fphar.2020.570555 [41] JI W Y, JIANG T, SUN Z, et al. The enhanced pharmacological effects of modified traditional Chinese medicine in attenuation of atherosclerosis is driven by modulation of gut microbiota[J]. Front Pharmacol,2020,11:546589. doi: 10.3389/fphar.2020.546589 [42] SUBRAMANIAN S, BLANTON L V, FRESE S A, et al. Cultivating healthy growth and nutrition through the gut microbiota[J]. Cell,2015,161(1):36-48. doi: 10.1016/j.cell.2015.03.013 [43] CHEN P B, BLACK A S, SOBEL A L, et al. Directed remodeling of the mouse gut microbiome inhibits the development of atherosclerosis[J]. Nat Biotechnol,2020,38(11):1288-1297. doi: 10.1038/s41587-020-0549-5 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 8651
- HTML全文浏览量: 2957
- PDF下载量: 70
- 被引次数: 0




 下载:
下载:
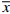
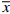

 下载:
下载:
