-
肝纤维化(HF)是一种创伤愈合反应,其特征是细胞外基质(ECM)在肝内的过度沉积,导致肝功能丧失和肝脏结构破坏[1]。在正常肝脏中,肝星状细胞(HSC)是位于肝脏窦状隙的DISE腔内,负责储存维生素A。当肝脏受到损伤刺激时,HSC从静止状态转化为具有增殖、类维生素A消失、趋化性、收缩性、纤维生成等特性的肌成纤维细胞(MFs),参与肝纤维化进程[2-4]。
核糖体蛋白S5(RPS5)是核糖体40S小亚基的组成成分,是核糖体生物发生所必需的。越来越多的证据表明,一些核糖体蛋白除了参与蛋白质加工合成之外,还具有许多核糖体外功能[5-6]。例如,RPS5基因对于胚胎干细胞的分化和类胚体的形成至关重要[7];RPS5的β-发夹结构能够与HCV IRES相互作用,并介导HCV的翻译过程[8];RPS5在结肠癌中异常表达,并可能在癌变过程中作为检查点发挥重要作用[9]。此外,RPS5还参与小鼠红白血病细胞分化,其表达下调会影响细胞周期蛋白依赖激酶-2、-4和-6的蛋白水平,并延迟体外红细胞成熟,引起G1/G0细胞周期停滞[10-11]。
本课题组前期研究发现,在肝纤维化过程中,RPS5在静息和激活的HSC内存在差异表达[12-13]。体外实验证实RPS5参与HSC活化,体内研究表明,RPS5基因敲减可加重肝纤维化,RPS5过表达则减轻肝纤维化[13]。但是,我们并没有研究特异性敲减HSC内RPS5的表达对肝纤维化的影响[14-17]。为此,我们构建了GFa2(GFAP启动子)-驱动shRPS5腺病毒,并研究其靶向敲减HSC的RPS5表达对肝纤维化进展的影响。
-
高糖DMEM培养基(Gibco);Western及IP细胞裂解液液(Beyotime);BCA蛋白定量试剂盒(Thermo Fisher Scientific);RPS5抗体(Santa Cruz)、ALB、GAPDH抗体(Cell Signaling Technology)、GFAP、α-SMA、胶原蛋白 I抗体(Abcam);山羊(多克隆)抗兔 IgG(H+L)、山羊(多克隆)抗小鼠 IgG(H+L)(LI-COR Biosciences);Odyssey成像系统(LI-COR Biotechnology);SYBR Premix Ex TaqTM PCR 试剂盒(Takara)。
-
雄性Sprague-Dawley大鼠,清洁级,重约200 g,购于上海SLAC实验动物有限公司。
-
原代HSC和肝细胞从雄性SD大鼠分离,通过蛋白酶-胶原酶消化,单步Nycodenz梯度纯化[13]。分离的HSC和肝细胞在含有10%胎牛血清(FBS)DMEM培养基,5% CO2、37 ℃培养箱中培养。每隔1天更换1次培养基。通过GFAP免疫染色评估,HSC的纯度为90%~95%。本实验于分离后第3天在转分化的HSC上进行。HSC以感染复数(MOI)50感染腺病毒48 h。
-
根据AdEasy™ Adenoviral Vector System(AgilentStratagene, USA)的说明制备质粒 pShuttle-GFa2-shRPS5。将GFAP基因启动子(GFa2)、shRPS5序列和必需元件克隆到无启动子穿梭载体pShuttle中,穿梭载体pShuttle-GFa2驱动shRPS5的表达。重组AdGFa2-shRPS5和AdGFa2-shNC腺病毒在293细胞中扩增,通过氯化铯梯度超速离心纯化。使293细胞通过噬斑测定病毒原液的滴度。shRPS5和shNC序列见表1。
表 1 shRPS5和shNC序列
名称 序列 shRPS5 5′-GCTCATGACTGTACGAATTCTCGAGAATTCGTACAGTCAT GAGC-3′ shNC 5′-GCGCGCTTTGTAGGATTCGCTCGAGCGAATCCTACAAA GCGCGC-3′ -
将HSC细胞中的培养基吸弃,用PBS洗涤1次,加入Western及IP细胞裂解液冰上裂解20 min。通过BCA法测量蛋白浓度后将蛋白样品在95 ℃下加热5 min。制胶上样,将20 μg样品进行10% SDS-PAGE电泳,结束后将蛋白质转移到NC膜上。5%脱脂奶粉封闭1 h。分别加入RPS5、ALB、GAPDH、GFAP、α-SMA和I型胶原的单克隆抗体4 ℃孵育过夜,用PBST洗膜3次,每次10 min。山羊(多克隆)抗兔IgG(H+L)或山羊(多克隆)抗小鼠IgG(H+L)室温孵育2 h,PBST洗膜3次,每次10 min。Odyssey红外扫描成像系统对NC膜扫描,观察相关蛋白的表达情况。
-
用Trizol试剂盒提取总RNA。用SYBR Premix Ex TaqTM PCR试剂盒,合成cDNA,定量PCR检测基因mRNA表达。以管家基因β-肌动蛋白(β-actin)作为内部对照,并将基因特异性mRNA表达与β-actin表达进行归一化。引物序列见表2。
表 2 实时qPCR引物序列
名称 正向引物序列 反向引物序列 β-actin 5′-CCAT TGAACACGGCATTGTC-3′ 5′-TCATAGATGGGCACAC AGTG-3′ RPS5 5′-AAATGTGCAGGTGTTGACCA-3′ 5′-CACGCTCCTCCTGAAGAATC-3′ α-SMA 5′-CCGAGATCTCACCGACTACC-3′ 5′-TCCA GAGCGACATAGCACAG-3′ collagen I 5′-CCGTGACCTCAAGATG TGCC-3′ 5′-GCTCATACCTTCGCTT CCAA-3′ -
用二甲基亚硝胺(DMN)和胆管结扎术(BDL)的方法建立大鼠肝纤维化模型[13],尾静脉注射腺病毒(4×109pfu)特异性敲减肝内HSC的RPS5水平。
-
肝组织切片HE染色分析病理改变情况;羟脯氨酸(羟脯氨酸检测试剂盒)含量测定、切片天狼星红和 Masson染色评价胶原沉积情况;免疫组织化学染色检测α-SMA和RPS5的表达情况。
-
数据分析通过SPSS 21统计软件进行,采用t检验分析,实验结果用均数±标准差(
$\bar x $ ±s)表示。与对照组比较,P<0.05 则认为差异具有统计学意义。 -
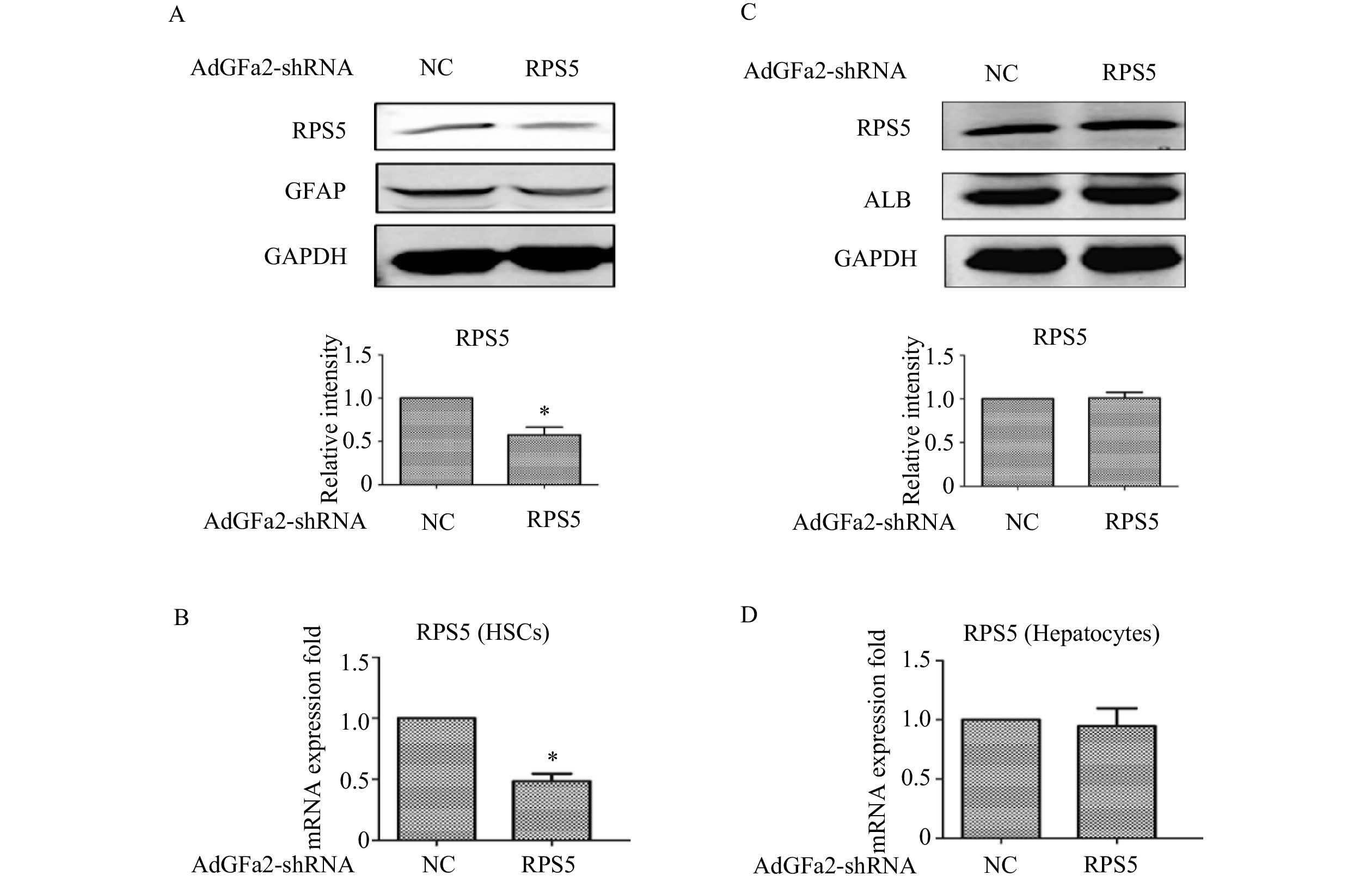
为了确定AdGFa2-shRPS5对RPS5表达的影响及对HSC的特异性,我们分别用AdGFa2-shRPS5和AdGFa2 shNC转染大鼠的原代HSC和肝细胞48 h,蛋白印迹法和实时PCR测定RPS5的表达情况。结果显示,RPS5 mRNA和蛋白质水平仅在原代HSC中降低(图1A、1B),在肝细胞中没有明显变化(图1C、1D)。
-
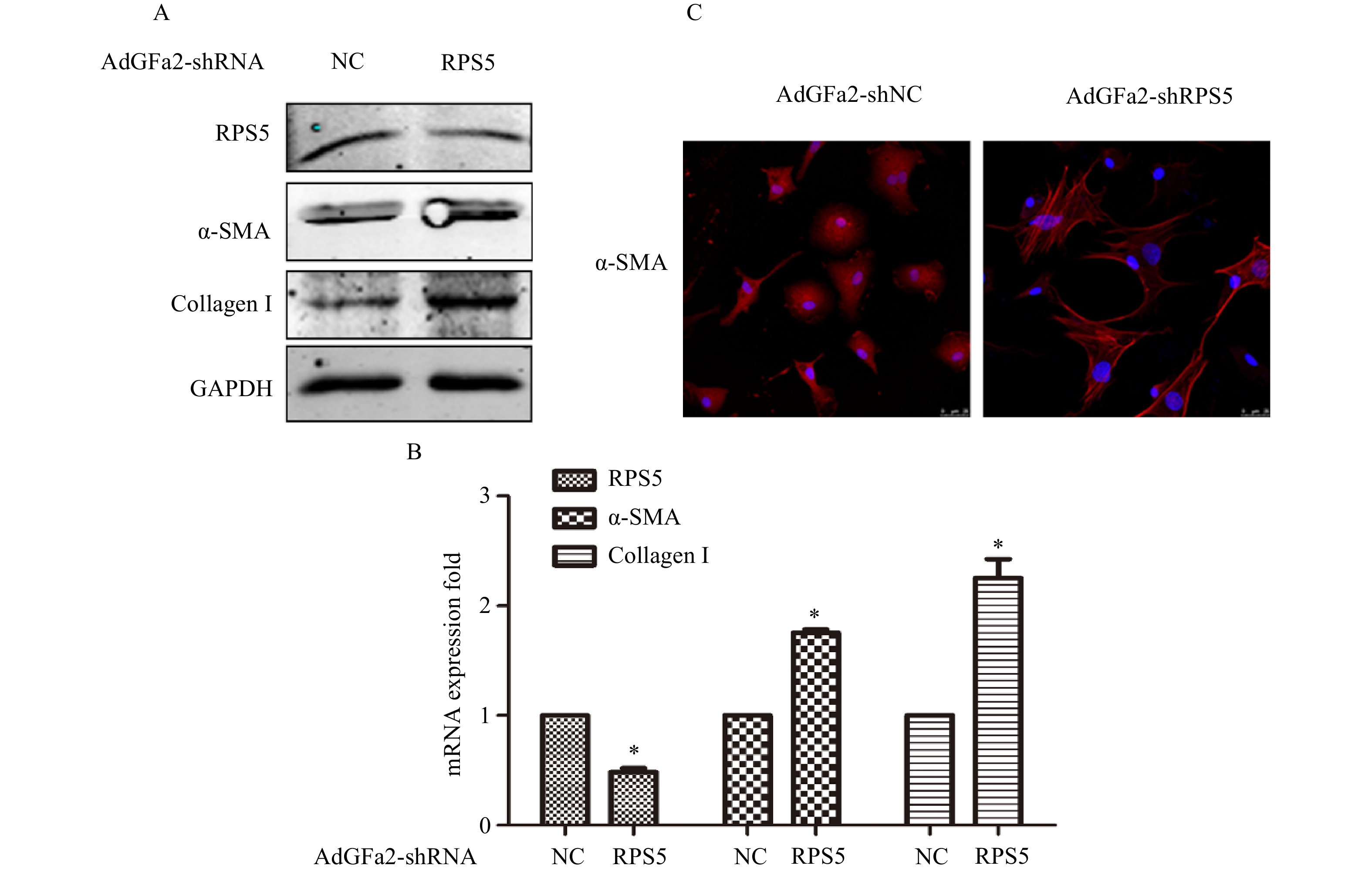
AdGFa2-shRPS5转染原代培养的HSC,蛋白印迹法和实时PCR分析α-SMA和I型胶原表达变化。结果表明,AdGFa2-shRPS5感染HSC后,α-SMA和I型胶原mRNA和蛋白质的表达显著增加(图2A、2B)。免疫荧光结果显示,转染AdGFa2-shRPS5的HSC形态明显改变,细胞更加扁平而延展(图2C)。以上结果表明RPS5敲减促进了HSC的活化。
-
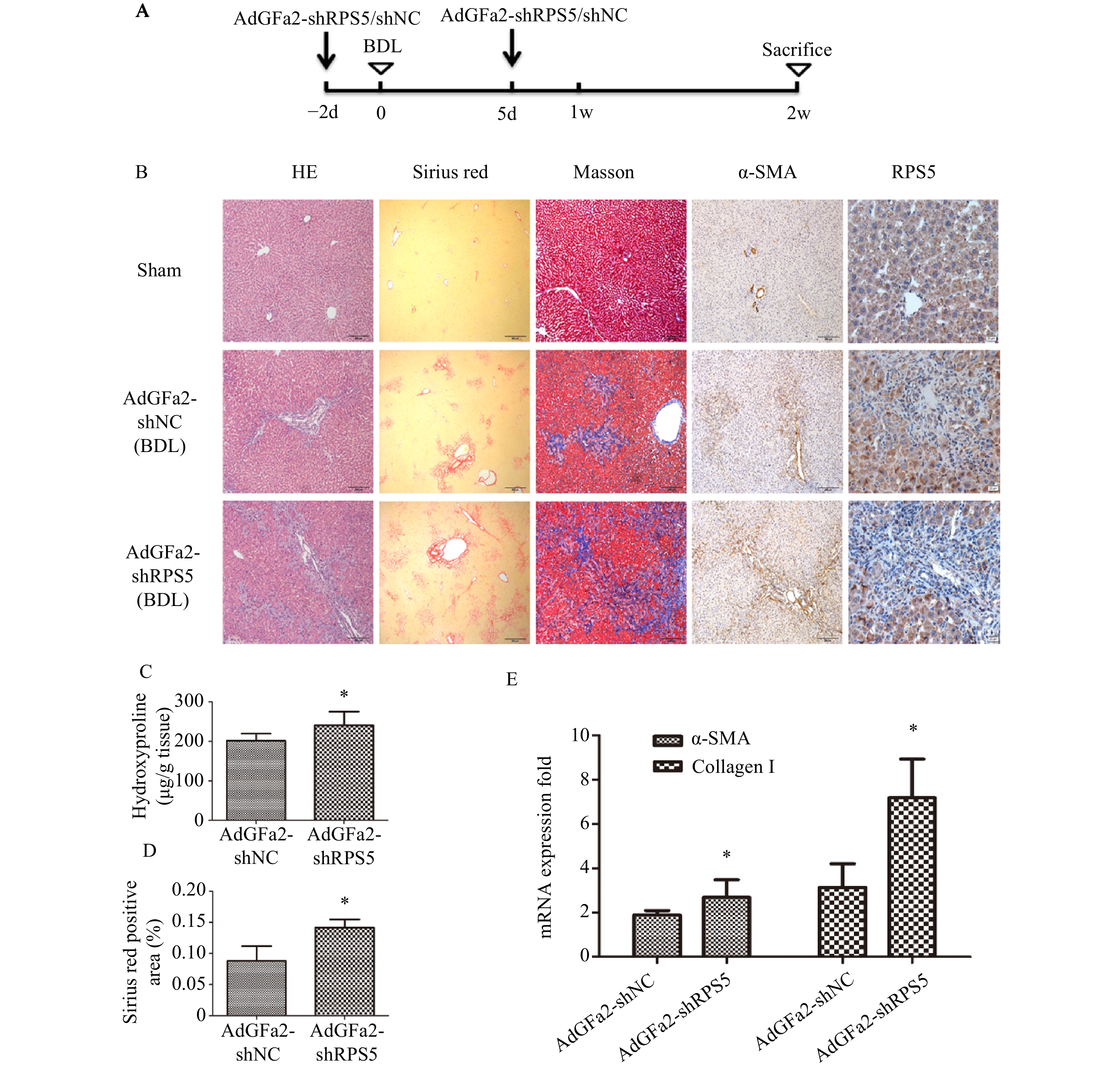
为了考察AdGFa2-shRPS5体内对肝纤维化的影响,我们构建了BDL肝纤维化模型。BDL手术前2 d和手术后5 d由尾静脉注射AdGFa2-shRPS5,2周后处死所有大鼠(图3A),评价肝纤维化情况。免疫组织化学染色结果所示,与AdGFa2-shNC组相比,AdGFa2-shRPS5降低了小鼠体内RPS5的表达,促进HSC的活化,α-SMA表达相应增加,表明肝纤维化程度加重。HE染色结果表明,在AdGFa2-shRPS5组中,反应性胆管周围有更广泛的胆管增生和有害的肝实质塌陷,α-SMA阳性表达增加。此外,天狼星红和Masson染色结果显示AdGFa2-shRPS5增加了胶原沉积(图3B)。天狼星染色半定量分析表明,AdGFa2-shRPS5处理的BDL大鼠纤维化面积增加了161%(P<0.05,图3C)。羟脯氨酸含量AdGFa2-shRPS5组明显高于AdGFa2 shNC组(P<0.05,图3D)。并且α-SMA和I型胶原mRNA的表达也与其蛋白质水平结果表达相一致(图3E)。
-
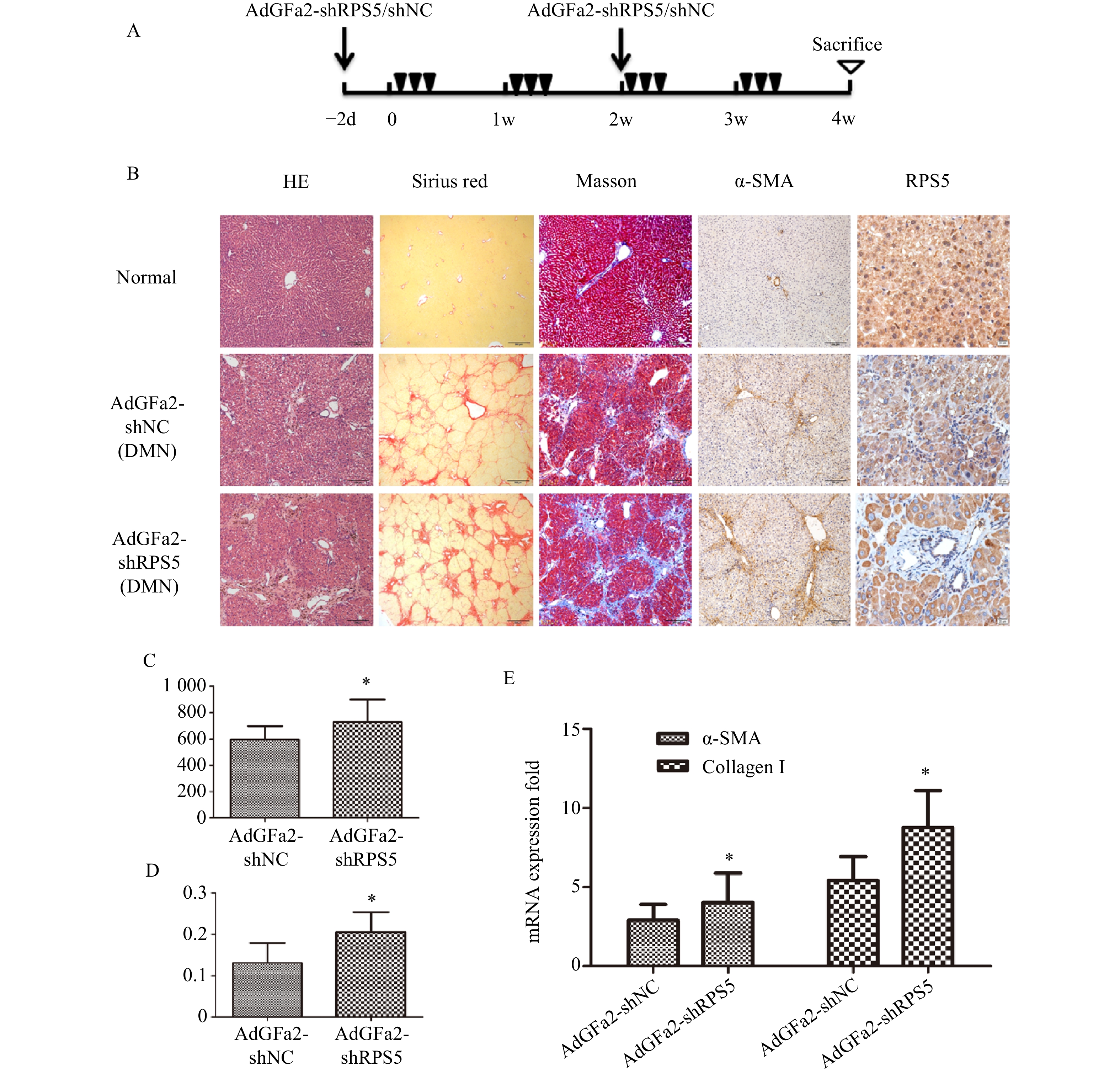
为了进一步证实AdGFa2-shRPS5对肝纤维化的作用,腹腔注射DMN制备纤维化模型。分别在第一次DMN注射前2天和后2周通过尾静脉向大鼠注射AdGFa2-shRPS5,在4周后处死所有大鼠(图4A)。AdGFa2-shRPS5抑制大鼠体内RPS5的表达,α-SMA在肝脏中的表达增加(图4B)。与BDL模型一致,HE、天狼星红、Masson、α-SMA染色及α-SMA RT-PCR分析证实(图4B,4E),AdGFa2-shRPS5也促进了DMN诱导的肝纤维化的发展。在DMN模型中,AdGFa2-shRPS5组的羟脯氨酸含量高于AdGFa2 shNC组(P<0.05,图4C)。天狼星红染色的半定量分析结果显示,AdGFa2-shRPS5使纤维化面积显著增加约157%(P<0.05,图4D)。综上结果证实特异性敲减HSC内的RPS5加剧了纤维化的进展。
-
肝纤维化是一个动态过程,其特征是慢性肝损伤导致ECM的过度沉积,当肝损伤发生时,HSC活化以获得成纤维能力,因此HSC是肝纤维化的主要生成细胞。文献表明,在转分化的HSC和人类肝硬化肝脏中RPS5显著降低[13]。RPS5的过表达使得体内肝纤维化得到改善,而RPS5的敲减促进了肝纤维化[13]。但究竟是哪一种细胞在这一过程中起主要作用仍值得进一步研究。因此,有必要仅针对肝脏中一个或有限细胞群进行研究。胶质纤维酸性蛋白GFAP是成熟星形胶质细胞中的主要中间丝状蛋白,但也在肝脏的HSCs中表达。因此,使用GFAP启动子GFa2来驱动RPS5敲减可能是靶向HSC的方法。在此研究中,我们使用GFAP启动子驱动shRNA系统来研究RPS5靶向HSCs对大鼠肝纤维化进展的影响。
本研究构建重组腺病毒AdGFa2-shRPS5,并证实AdGFa2-shRPS5可以特异性地将HSCs中RPS5的表达降低约50%,对肝细胞没有明显作用。研究证明RPS5的敲减促进HSCs的活化。体内研究结果表明,两种慢性肝损伤动物模型中纤维化的发展随着RPS5的特异性敲减而加重,并伴有显著的细胞外基质沉积。综上结果表明,RPS5的下调有助于HSC的激活,从而促进体内肝纤维化,表明RPS5可能是HSC激活的分子开关。
RPS5是核糖体40S小亚基的组成成分,是核糖体生物发生所必需的,并且具有许多核糖体外功能,如促进细胞的凋亡、DNA修复、细胞增殖和分化[5-6,10-11]。文献证实RPS5可抑制LPS诱导的巨噬细胞中的IL-6和NO释放,表明RPS5也参与炎症过程[18]。本研究结果表明,RPS5在调节HSC活化中起着重要作用,揭示了RPS5的新功能。由于GFa2启动子的活性较弱,我们没有研究过表达HSC中的RPS5对体内肝纤维化的影响。
本研究为敲减HSC中的RPS5对肝纤维化有促进作用提供了证据。使用AdGFa2-shRPS5的一个关键优势是靶向HSC,并且对正常肝细胞没有影响。结果表明,RPS5对肝纤维化的发生发展至关重要,可能是治疗肝纤维化的一个有前景的靶点。
Effects of specific knockdown of ribosomal protein S5 in hepatic stellate cells on liver fibrosis
-
摘要:
目的 观察特异性敲减肝星状细胞(HSC)内核糖体蛋白S5(RPS5)对大鼠肝纤维化的影响。 方法 构建胶质纤维酸性蛋白(GFAP)启动子驱动的RPS5 shRNA腺病毒,分别用AdGFa2-shRPS5及其对照AdGFa2 shNC转染大鼠原代HSC和肝细胞,通过蛋白印迹法和实时PCR测定RPS5、α-SMA和I型胶原表达情况;采用二甲基亚硝胺(DMN)和胆管结扎术(BDL)的方法建立大鼠肝纤维化模型,尾静脉注射腺病毒特异性敲减肝内HSC的RPS5水平。肝组织切片HE染色分析病理改变情况;羟脯氨酸含量测定、切片天狼星红和 Masson染色评价胶原沉积情况;免疫组织化学染色检测α-SMA和RPS5的表达情况。 结果 AdGFa2-shRPS5能够特异性敲减HSC中的RPS5表达水平,增加α-SMA和I型胶原在体外表达。体内研究结果表明,在两种慢性肝损伤动物模型中,特异性敲减HSC中RPS5表达水平能够促进HSC活化,增加细胞外基质的沉积,促进肝纤维化。 结论 RPS5对HSC激活和肝纤维化发生至关重要,可能是治疗肝纤维化的潜在靶点。 Abstract:Objective To observe the effect of specific knockdown of hepatic stellate cells (HSC) ribosomal protein S5 (RPS5) on liver fibrosis in rats. Methods The glial fibrillary acidic protein (GFAP) promoter-driven RPS5 shRNA adenovirus was established, and AdGFa2-shRPS5 and its control AdGFa2 shNC were used to transfect primary rat HSCs and hepatocytes, respectively. RPS5 was determined by Western-blot and Real Time PCR, α-SMA and type I collagen expression; the rat liver fibrosis model was established by dimethyl nitrosamine (DMN) and bile duct ligation (BDL), and intrahepatic HSC was specifically knocked down by tail vein injection of adenovirus of RPS5 levels. The pathological changes of liver tissue sections were analyzed by HE staining; the content of hydroxyproline, sections of Sirius red and Masson staining were used to evaluate collagen deposition; immunohistochemical staining was used to detect the expression of α-SMA and RPS5. Results AdGFa2-shRPS5 specifically knocked down the expression level of RPS5 in HSC and increased the expression of α-SMA and type I collagen in vitro. The in vivo results showed that in two animal models of chronic liver injury, specific knockdown of RPS5 expression in HSCs promoted HSC activation, increased the deposition of extracellular matrix, and promoted liver fibrosis. Conclusion RPS5 is essential for HSC activation and liver fibrosis, which could be a potential target for the treatment of liver fibrosis. -
肝纤维化(HF)是一种创伤愈合反应,其特征是细胞外基质(ECM)在肝内的过度沉积,导致肝功能丧失和肝脏结构破坏[1]。在正常肝脏中,肝星状细胞(HSC)是位于肝脏窦状隙的DISE腔内,负责储存维生素A。当肝脏受到损伤刺激时,HSC从静止状态转化为具有增殖、类维生素A消失、趋化性、收缩性、纤维生成等特性的肌成纤维细胞(MFs),参与肝纤维化进程[2-4]。
核糖体蛋白S5(RPS5)是核糖体40S小亚基的组成成分,是核糖体生物发生所必需的。越来越多的证据表明,一些核糖体蛋白除了参与蛋白质加工合成之外,还具有许多核糖体外功能[5-6]。例如,RPS5基因对于胚胎干细胞的分化和类胚体的形成至关重要[7];RPS5的β-发夹结构能够与HCV IRES相互作用,并介导HCV的翻译过程[8];RPS5在结肠癌中异常表达,并可能在癌变过程中作为检查点发挥重要作用[9]。此外,RPS5还参与小鼠红白血病细胞分化,其表达下调会影响细胞周期蛋白依赖激酶-2、-4和-6的蛋白水平,并延迟体外红细胞成熟,引起G1/G0细胞周期停滞[10-11]。
本课题组前期研究发现,在肝纤维化过程中,RPS5在静息和激活的HSC内存在差异表达[12-13]。体外实验证实RPS5参与HSC活化,体内研究表明,RPS5基因敲减可加重肝纤维化,RPS5过表达则减轻肝纤维化[13]。但是,我们并没有研究特异性敲减HSC内RPS5的表达对肝纤维化的影响[14-17]。为此,我们构建了GFa2(GFAP启动子)-驱动shRPS5腺病毒,并研究其靶向敲减HSC的RPS5表达对肝纤维化进展的影响。
1. 材料和方法
1.1 材料
1.1.1 实验试剂
高糖DMEM培养基(Gibco);Western及IP细胞裂解液液(Beyotime);BCA蛋白定量试剂盒(Thermo Fisher Scientific);RPS5抗体(Santa Cruz)、ALB、GAPDH抗体(Cell Signaling Technology)、GFAP、α-SMA、胶原蛋白 I抗体(Abcam);山羊(多克隆)抗兔 IgG(H+L)、山羊(多克隆)抗小鼠 IgG(H+L)(LI-COR Biosciences);Odyssey成像系统(LI-COR Biotechnology);SYBR Premix Ex TaqTM PCR 试剂盒(Takara)。
1.1.2 实验动物
雄性Sprague-Dawley大鼠,清洁级,重约200 g,购于上海SLAC实验动物有限公司。
1.2 方法
1.2.1 原代HSC和肝细胞提取
原代HSC和肝细胞从雄性SD大鼠分离,通过蛋白酶-胶原酶消化,单步Nycodenz梯度纯化[13]。分离的HSC和肝细胞在含有10%胎牛血清(FBS)DMEM培养基,5% CO2、37 ℃培养箱中培养。每隔1天更换1次培养基。通过GFAP免疫染色评估,HSC的纯度为90%~95%。本实验于分离后第3天在转分化的HSC上进行。HSC以感染复数(MOI)50感染腺病毒48 h。
1.2.2 构建重组腺病毒
根据AdEasy™ Adenoviral Vector System(AgilentStratagene, USA)的说明制备质粒 pShuttle-GFa2-shRPS5。将GFAP基因启动子(GFa2)、shRPS5序列和必需元件克隆到无启动子穿梭载体pShuttle中,穿梭载体pShuttle-GFa2驱动shRPS5的表达。重组AdGFa2-shRPS5和AdGFa2-shNC腺病毒在293细胞中扩增,通过氯化铯梯度超速离心纯化。使293细胞通过噬斑测定病毒原液的滴度。shRPS5和shNC序列见表1。
表 1 shRPS5和shNC序列名称 序列 shRPS5 5′-GCTCATGACTGTACGAATTCTCGAGAATTCGTACAGTCAT GAGC-3′ shNC 5′-GCGCGCTTTGTAGGATTCGCTCGAGCGAATCCTACAAA GCGCGC-3′ 1.2.3 蛋白印迹法(Western-blot)
将HSC细胞中的培养基吸弃,用PBS洗涤1次,加入Western及IP细胞裂解液冰上裂解20 min。通过BCA法测量蛋白浓度后将蛋白样品在95 ℃下加热5 min。制胶上样,将20 μg样品进行10% SDS-PAGE电泳,结束后将蛋白质转移到NC膜上。5%脱脂奶粉封闭1 h。分别加入RPS5、ALB、GAPDH、GFAP、α-SMA和I型胶原的单克隆抗体4 ℃孵育过夜,用PBST洗膜3次,每次10 min。山羊(多克隆)抗兔IgG(H+L)或山羊(多克隆)抗小鼠IgG(H+L)室温孵育2 h,PBST洗膜3次,每次10 min。Odyssey红外扫描成像系统对NC膜扫描,观察相关蛋白的表达情况。
1.2.4 实时PCR
用Trizol试剂盒提取总RNA。用SYBR Premix Ex TaqTM PCR试剂盒,合成cDNA,定量PCR检测基因mRNA表达。以管家基因β-肌动蛋白(β-actin)作为内部对照,并将基因特异性mRNA表达与β-actin表达进行归一化。引物序列见表2。
表 2 实时qPCR引物序列名称 正向引物序列 反向引物序列 β-actin 5′-CCAT TGAACACGGCATTGTC-3′ 5′-TCATAGATGGGCACAC AGTG-3′ RPS5 5′-AAATGTGCAGGTGTTGACCA-3′ 5′-CACGCTCCTCCTGAAGAATC-3′ α-SMA 5′-CCGAGATCTCACCGACTACC-3′ 5′-TCCA GAGCGACATAGCACAG-3′ collagen I 5′-CCGTGACCTCAAGATG TGCC-3′ 5′-GCTCATACCTTCGCTT CCAA-3′ 1.2.5 动物肝纤维化模型
用二甲基亚硝胺(DMN)和胆管结扎术(BDL)的方法建立大鼠肝纤维化模型[13],尾静脉注射腺病毒(4×109pfu)特异性敲减肝内HSC的RPS5水平。
1.2.6 组织标本采集及指标的检测
肝组织切片HE染色分析病理改变情况;羟脯氨酸(羟脯氨酸检测试剂盒)含量测定、切片天狼星红和 Masson染色评价胶原沉积情况;免疫组织化学染色检测α-SMA和RPS5的表达情况。
1.2.7 统计分析
数据分析通过SPSS 21统计软件进行,采用t检验分析,实验结果用均数±标准差(
$\bar x $ ±s)表示。与对照组比较,P<0.05 则认为差异具有统计学意义。2. 结果
2.1 AdGFa2-shRPS5对RPS5表达的抑制作用
为了确定AdGFa2-shRPS5对RPS5表达的影响及对HSC的特异性,我们分别用AdGFa2-shRPS5和AdGFa2 shNC转染大鼠的原代HSC和肝细胞48 h,蛋白印迹法和实时PCR测定RPS5的表达情况。结果显示,RPS5 mRNA和蛋白质水平仅在原代HSC中降低(图1A、1B),在肝细胞中没有明显变化(图1C、1D)。
2.2 AdGFa2-shRPS5对HSC活化的影响
AdGFa2-shRPS5转染原代培养的HSC,蛋白印迹法和实时PCR分析α-SMA和I型胶原表达变化。结果表明,AdGFa2-shRPS5感染HSC后,α-SMA和I型胶原mRNA和蛋白质的表达显著增加(图2A、2B)。免疫荧光结果显示,转染AdGFa2-shRPS5的HSC形态明显改变,细胞更加扁平而延展(图2C)。以上结果表明RPS5敲减促进了HSC的活化。
2.3 AdGFa2-shRPS5对BDL诱导的肝纤维化的影响
为了考察AdGFa2-shRPS5体内对肝纤维化的影响,我们构建了BDL肝纤维化模型。BDL手术前2 d和手术后5 d由尾静脉注射AdGFa2-shRPS5,2周后处死所有大鼠(图3A),评价肝纤维化情况。免疫组织化学染色结果所示,与AdGFa2-shNC组相比,AdGFa2-shRPS5降低了小鼠体内RPS5的表达,促进HSC的活化,α-SMA表达相应增加,表明肝纤维化程度加重。HE染色结果表明,在AdGFa2-shRPS5组中,反应性胆管周围有更广泛的胆管增生和有害的肝实质塌陷,α-SMA阳性表达增加。此外,天狼星红和Masson染色结果显示AdGFa2-shRPS5增加了胶原沉积(图3B)。天狼星染色半定量分析表明,AdGFa2-shRPS5处理的BDL大鼠纤维化面积增加了161%(P<0.05,图3C)。羟脯氨酸含量AdGFa2-shRPS5组明显高于AdGFa2 shNC组(P<0.05,图3D)。并且α-SMA和I型胶原mRNA的表达也与其蛋白质水平结果表达相一致(图3E)。
2.4 AdGFa2-shRPS5对DMN诱导的肝纤维化的影响
为了进一步证实AdGFa2-shRPS5对肝纤维化的作用,腹腔注射DMN制备纤维化模型。分别在第一次DMN注射前2天和后2周通过尾静脉向大鼠注射AdGFa2-shRPS5,在4周后处死所有大鼠(图4A)。AdGFa2-shRPS5抑制大鼠体内RPS5的表达,α-SMA在肝脏中的表达增加(图4B)。与BDL模型一致,HE、天狼星红、Masson、α-SMA染色及α-SMA RT-PCR分析证实(图4B,4E),AdGFa2-shRPS5也促进了DMN诱导的肝纤维化的发展。在DMN模型中,AdGFa2-shRPS5组的羟脯氨酸含量高于AdGFa2 shNC组(P<0.05,图4C)。天狼星红染色的半定量分析结果显示,AdGFa2-shRPS5使纤维化面积显著增加约157%(P<0.05,图4D)。综上结果证实特异性敲减HSC内的RPS5加剧了纤维化的进展。
3. 讨论
肝纤维化是一个动态过程,其特征是慢性肝损伤导致ECM的过度沉积,当肝损伤发生时,HSC活化以获得成纤维能力,因此HSC是肝纤维化的主要生成细胞。文献表明,在转分化的HSC和人类肝硬化肝脏中RPS5显著降低[13]。RPS5的过表达使得体内肝纤维化得到改善,而RPS5的敲减促进了肝纤维化[13]。但究竟是哪一种细胞在这一过程中起主要作用仍值得进一步研究。因此,有必要仅针对肝脏中一个或有限细胞群进行研究。胶质纤维酸性蛋白GFAP是成熟星形胶质细胞中的主要中间丝状蛋白,但也在肝脏的HSCs中表达。因此,使用GFAP启动子GFa2来驱动RPS5敲减可能是靶向HSC的方法。在此研究中,我们使用GFAP启动子驱动shRNA系统来研究RPS5靶向HSCs对大鼠肝纤维化进展的影响。
本研究构建重组腺病毒AdGFa2-shRPS5,并证实AdGFa2-shRPS5可以特异性地将HSCs中RPS5的表达降低约50%,对肝细胞没有明显作用。研究证明RPS5的敲减促进HSCs的活化。体内研究结果表明,两种慢性肝损伤动物模型中纤维化的发展随着RPS5的特异性敲减而加重,并伴有显著的细胞外基质沉积。综上结果表明,RPS5的下调有助于HSC的激活,从而促进体内肝纤维化,表明RPS5可能是HSC激活的分子开关。
RPS5是核糖体40S小亚基的组成成分,是核糖体生物发生所必需的,并且具有许多核糖体外功能,如促进细胞的凋亡、DNA修复、细胞增殖和分化[5-6,10-11]。文献证实RPS5可抑制LPS诱导的巨噬细胞中的IL-6和NO释放,表明RPS5也参与炎症过程[18]。本研究结果表明,RPS5在调节HSC活化中起着重要作用,揭示了RPS5的新功能。由于GFa2启动子的活性较弱,我们没有研究过表达HSC中的RPS5对体内肝纤维化的影响。
本研究为敲减HSC中的RPS5对肝纤维化有促进作用提供了证据。使用AdGFa2-shRPS5的一个关键优势是靶向HSC,并且对正常肝细胞没有影响。结果表明,RPS5对肝纤维化的发生发展至关重要,可能是治疗肝纤维化的一个有前景的靶点。
-
表 1 shRPS5和shNC序列
名称 序列 shRPS5 5′-GCTCATGACTGTACGAATTCTCGAGAATTCGTACAGTCAT GAGC-3′ shNC 5′-GCGCGCTTTGTAGGATTCGCTCGAGCGAATCCTACAAA GCGCGC-3′ 表 2 实时qPCR引物序列
名称 正向引物序列 反向引物序列 β-actin 5′-CCAT TGAACACGGCATTGTC-3′ 5′-TCATAGATGGGCACAC AGTG-3′ RPS5 5′-AAATGTGCAGGTGTTGACCA-3′ 5′-CACGCTCCTCCTGAAGAATC-3′ α-SMA 5′-CCGAGATCTCACCGACTACC-3′ 5′-TCCA GAGCGACATAGCACAG-3′ collagen I 5′-CCGTGACCTCAAGATG TGCC-3′ 5′-GCTCATACCTTCGCTT CCAA-3′ -
[1] BATALLER R, BRENNER D A. Liver fibrosis[J]. J Clin Invest,2005,115(2):209-218. doi: 10.1172/JCI24282 [2] FRIEDMAN S L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver[J]. Physiol Rev,2008,88(1):125-172. doi: 10.1152/physrev.00013.2007 [3] BATALLER R, BRENNER D A. Hepatic stellate cells as a target for the treatment of liver fibrosis[J]. Semin Liver Dis,2001,21(3):437-451. doi: 10.1055/s-2001-17558 [4] KINOSHITA K, IIMURO Y, FUJIMOTO J, et al. Targeted and regulable expression of transgenes in hepatic stellate cells and myofibroblasts in culture and in vivo using an adenoviral Cre/loxP system to antagonise hepatic fibrosis[J]. Gut,2007,56(3):396-404. doi: 10.1136/gut.2005.085704 [5] WARNER J R, MCINTOSH K B. How common are extraribosomal functions of ribosomal proteins[J]. Mol Cell,2009,34(1):3-11. doi: 10.1016/j.molcel.2009.03.006 [6] LINDSTRÖM M S. Emerging functions of ribosomal proteins in gene-specific transcription and translation[J]. Biochem Biophys Res Commun,2009,379(2):167-170. doi: 10.1016/j.bbrc.2008.12.083 [7] FORTIER S, MACRAE T, BILODEAU M, et al. Haploinsufficiency screen highlights two distinct groups of ribosomal protein genes essential for embryonic stem cell fate[J]. Proc Natl Acad Sci USA,2015,112(7):2127-2132. doi: 10.1073/pnas.1418845112 [8] BHAT P, SHWETHA S, SHARMA D K, et al. The beta hairpin structure within ribosomal protein S5 mediates interplay between domains II and IV and regulates HCV IRES function[J]. Nucleic Acids Res,2015,43(5):2888-2901. doi: 10.1093/nar/gkv110 [9] BANDRÉS E, MALUMBRES R, CUBEDO E, et al. A gene signature of 8 genes could identify the risk of recurrence and progression in Dukes' B colon cancer patients[J]. Oncol Rep,2007,17(5):1089-1094. [10] VIZIRIANAKIS I S, PAPPAS I S, GOUGOUMAS D, et al. Expression of ribosomal protein S5 cloned gene during differentiation and apoptosis in murine erythroleukemia (MEL) cells[J]. Oncol Res,1999,11(9):409-419. [11] MATRAGKOU C N, PAPACHRISTOU E T, TEZIAS S S, et al. The potential role of ribosomal protein S5 on cell cycle arrest and initiation of murine erythroleukemia cell differentiation[J]. J Cell Biochem,2008,104(4):1477-1490. doi: 10.1002/jcb.21722 [12] LIU X J, YANG L, LUO F M, et al. Association of differentially expressed genes with activation of mouse hepatic stellate cells by high-density cDNA microarray[J]. World J Gastroenterol,2004,10(11):1600-1607. doi: 10.3748/wjg.v10.i11.1600 [13] XU W H, HU H G, TIAN Y, et al. Bioactive compound reveals a novel function for ribosomal protein S5 in hepatic stellate cell activation and hepatic fibrosis[J]. Hepatology,2014,60(2):648-660. doi: 10.1002/hep.27138 [14] BACHEM M G, MEYER D, SCHÄFER W, et al. The response of rat liver perisinusoidal lipocytes to polypeptide growth regulator changes with their transdifferentiation into myofibroblast-like cells in culture[J]. J Hepatol,1993,18(1):40-52. doi: 10.1016/S0168-8278(05)80008-X [15] WEINER J A, CHEN A, DAVIS B H. Platelet-derived growth factor is a principal inductive factormodulating mannose 6-phosphate/insulin-like growth factor-II receptorgene expression via a distal E-box in activated hepatic stellate cells[J]. Biochem J, 2000, 345(Pt 2): 225-231. [16] YANG N N, MAHATO R I. GFAP promoter-driven RNA interference on TGF-β1 to treat liver fibrosis[J]. Pharm Res,2011,28(4):752-761. doi: 10.1007/s11095-011-0384-y [17] MAUBACH G, LIM M C C, ZHANG C Y, et al. GFAP promoter directs lacZ expression specifically in a rat hepatic stellate cell line[J]. World J Gastroenterol,2006,12(5):723-730. doi: 10.3748/wjg.v12.i5.723 [18] XU J, WANG K Q, XU W H, et al. The matrine derivate MASM prolongs survival, attenuates inflammation, and reduces organ injury in murine established lethal Sepsis[J]. J Infect Dis,2016,214(11):1762-1772. doi: 10.1093/infdis/jiw445 -





 下载:
下载:

 下载:
下载:


