-
克罗恩病(Crohn’s disease, CD)是一种病因未明的炎症性肠病(inflammatory bowel disease, IBD)。临床以腹痛、腹泻、肠梗阻等为特点,常伴有肠外表现,并发症多,致残率高,发作与缓解交替出现,治疗难度较大。英夫利昔单抗(infliximab, IFX)是IBD治疗中最早使用的生物制剂,但是随着目前国内广泛应用,有部分IBD患者对IFX表现为失应答。临床药师通过血药浓度监测对1例CD合并低蛋白血症患者出现失应答的原因进行分析,考虑该患者血清白蛋白水平影响药物药动学过程,协助医生共同解决用药问题,以期提高IFX的应答,并对患者进行药学监护。
-
患者,女,53岁,身高165 cm,体重48 kg,体重指数(BMI)为17.63,于2017年7月无明显诱因出现黏液血便,每日4~5次,伴脐周痛,于当地医院对症治疗,症状好转,之后症状间断出现。曾就诊于外院提示溃疡性结肠炎,予口服美沙拉嗪1 g tid,联合美沙拉嗪灌肠液4 g qn,灌肠治疗,症状有所缓解,但无法达到完全缓解。2019-01-21就诊于长海医院,经肛小肠镜检查:回肠多发溃疡,病理:(回肠下段)见固有层较多淋巴结,浆、中性粒细胞浸润,局灶见肉芽肿,符合克罗恩病改变。依据《世界卫生组织克罗恩病诊断标准》,符合节段性病变及全壁性炎性反应,内镜回肠至肛周多发节段性纵行溃疡伴狭窄,确诊克罗恩病,予IFX 300 mg静脉滴注治疗,出院后在进少渣软食的同时口服肠内营养制剂增加营养素的摄入。至2020-03-26期间规律行IFX 300 mg维持治疗,2020-04-23日因患者出现腹痛症状反复,故将IFX剂量调整至400 mg。
患者于2020-06-17再次就诊我院,仍有腹痛腹胀症状,结肠镜:克罗恩病,回肠末端病理示:固有层较多淋巴细胞、浆细胞、中性粒细胞浸润,未见肉芽肿。查炎症因子水平:(白介素)IL-6 23.9 pg/ml,TNFα 133 pg/ml,粪便钙卫蛋白(FC)>1800 μg/g,IFX血药浓度<0.4 μg/ml,抗体血清浓度<4 ng/ml,由于IFX血药浓度低于下限,临床药师与医师分析后,考虑药物剂量不足引起继发性失应答,缩短注射间隔,本次输注IFX后间隔4周返院行IFX治疗。患者白蛋白25 g/L,住院期间输注20%人血白蛋白50 ml,临床药师建议医师对该患者进行全肠内营养支持,每日口服肠内营养粉剂(TP)(荷兰Abbott.Lab.B.V.,批准文号:H20130320)1罐,短肽型肠内营养剂(德国MilupaGmbH,批准文号:H20170170)1盒。患者出院4周后(2020-07-18)返院行IFX 400 mg治疗,查炎症因子水平:IL-6 14.9 pg/ml,CRP 21.4 mg/L,红细胞沉降率 41 mm/H,TNFα 56.3 pg/ml,查炎症因子与炎症指标较前下降,症状有所缓解,疾病活动度下降。
-
患者为中年女性,IFX用药期间临床症状反复,处于疾病活动期,且IFX血清谷浓度<0.4 μg/ml,低于有效的谷浓度范围(3~7 μg/ml),抗体浓度测定<4 ng/ml,未产生抗药抗体。有研究表明,IFX谷浓度水平可预测其治疗CD的疗效[1]。Hibi等认为达到临床反应的谷浓度阈值约1.0 μg/ml [2],在韩国人群中的研究显示基于疾病活动度的谷浓度截断值为0.68 μg/ml[3]。有研究发现,在对IFX治疗最初有应答的IBD患者中,最终也有高达40%的患者由于药物暴露不佳、副作用或其他原因不明的机制而失去反应[4]。结合该患者临床症状及实验室指标,考虑该患者为IFX剂量不足从而引起继发性失应答。
对继发性失应答的患者进行治疗药物监测(therapeutic drug monitoring,TDM)可指导治疗方案的调整 [5]。针对TDM结果,临床药师利用药动学、药效学、临床药物治疗学等知识,综合分析产生该结果的原因,评估该结果对药物治疗效果、安全性及用药依从性等方面的影响,为临床医师确定药物治疗方案、药师实施药物治疗管理及患者自我管理提供参考[6]。针对该患者的TDM结果,临床药师提出以下建议:该患者谷浓度低,抗体阴性,现用剂量400 mg,患者体重48 kg,已经达到8 mg/kg,IFX最高剂量为10 mg/kg,同时考虑免疫抑制剂如硫唑嘌呤可导致严重的骨髓抑制、肝功能异常等剂量依赖性不良反应,建议采取缩短注射间隔,由8周缩短为间隔4周,后期若患者应答情况差,加用硫唑嘌呤联合治疗或更换同类别或跨类别转换治疗。医师采纳,嘱患者出院后4周返院行IFX治疗,后期随访炎症因子与炎症指标较前下降,活动度下降。
-
虽然关于失应答的确切原因还不明确,但是目前研究观点认为导致IFX药物失效可能是由于以下两个关键机制:①药物免疫原性。由于IFX是人鼠嵌合型抗体,存在鼠源成分,因此有可能引发人体免疫系统产生抗药抗体,识别并中和体内药物,增加药物清除率,导致药物浓度的降低。②药动学变化。受疾病严重程度、血清蛋白水平和体重指数等多个因素的影响,体内药物代谢和清除速率发生变化,导致药物浓度偏低。
与传统化学药物不同,IFX是大分子药物,由于其分子质量较大,使其呈现出不同于小分子药物的独特药代特征,几乎不存在经肾清除,一般包括:①通过抗体与Fcγ受体结合,被网状内皮系统的吞噬细胞进行蛋白分解代谢,然后被细胞内的溶酶体降解成肽段和氨基酸;②通过FcRn介导的循环[7]。所以影响这些机制的参数就会导致更多的清除和较低的药物浓度。
患者此次入院,血清白蛋白25 g/L,查既往病史资料,自2020-03-26起血清白蛋白水平一直<30 g/L,处于低蛋白血症的状态,是否血清白蛋白水平影响药物清除及应答?临床药师查阅文献后认为,血清白蛋白水平低会导致外源的IgG即IFX分解代谢增加,清除率增加,从而降低了IFX药物暴露,产生失应答。一项真实世界探索影响IBD患者IFX药动学因素的大型队列研究发现,高滴度ATIs、高体重和低血清白蛋白水平是影响IFX清除的独立因素[8]。有研究利用ACCENT I和REACH试验数据建立非线性混合效应模型,发现当CD患者白蛋白降低时清除率是增加的[9]。Dotan等也发现IFX清除率与白蛋白、体重和ATI等患者因素显著相关,建议对低蛋白血症者缩短给药间隔,体重较低者应给予较高的初始剂量[10]。有研究提出FcRn共同挽救IgG和白蛋白的机制在解释血清白蛋白水平与IFX药动学之间的关系中起重要作用,虽然可能需要进一步的研究来阐明FcRn的作用[11]。该患者住院期间及时静脉输注人血白蛋白纠正低白蛋白水平是合理的。
-
临床药师对该患者进行营养筛查,NRS2002总评分≥3分,同时对当前营养状况进行评估:血生化评价营养指标血清白蛋白25 g/L,前白蛋白115 mg/L,明显偏低,提示患者属于营养状况不良,需加强营养支持。有研究表明,肠内营养不仅可以改善营养状态,还能诱导和维持CD缓解,可能机制主要是:下调促炎因子,发挥抗炎作用,促进肠道黏膜上皮愈合,降低肠道渗透性,且使机体脂肪和蛋白质等大分子致病抗原的含量大大降低, 进而起到减少肠道黏膜抗原暴露的目的,使肠道休息,还可调整肠道菌群[12]。并且不同的配方成分在诱导克罗恩病患者缓解时的疗效无显著差异,蛋白质类型均不影响肠内营养效果[13]。该患者前期一直服用整蛋白型肠内营养制剂,药师认为营养成分完整、接近正常饮食组成、价廉、口感好的整蛋白型肠内营养制剂是适宜的。短肽肠内营养制剂可直接吸收,不依赖于消化酶可快速纠正低蛋白状态,且低脂配方不增加肠道负担。联合使用可以及时补充患者蛋白质,有助于肠道黏膜对蛋白质的吸收,提高血浆前蛋白、白蛋白水平,临床药师建议患者联合使用。2006年欧洲肠外肠内营养学会(ESPEN)推荐缓解期克罗恩病患者:25~30 kcal/(kg·d),活动期CD患者:约高出缓解期8%~10%,该患者进行全肠内营养,联合使用短肽型和整蛋白型制剂,肠内营养粉剂(TP)400 g(1800 kcal)每日一罐,肠内营养粉剂(短肽型)125 g(500 kcal)每日一袋,一日总热量为2300 kcal,是合理的。
-
该患者接受IFX治疗过程中,容易诱发机会性感染,如真菌感染、病毒感染等,这些感染会导致各种感染性疾病,本次治疗前检查无机会感染发生,继续加强监护。针对该患者肠内营养支持的监护,首先要加强营养状况监测,定期筛查营养风险,定期检查微量营养素缺乏情况,如维生素D、B12、叶酸等;其次,进行用药教育增加患者用药依从性和意识,全肠内营养不仅是营养支持,更是一种治疗,能够诱导CD缓解,并可能有助于维持缓解,延缓复发,促进肠黏膜溃疡愈合;最后,密切监测相关并发症,如肠道并发症(腹泻、腹胀、恶心、呕吐等)、代谢并发症(水电解质平衡异常、血糖波动等)。
-
克罗恩病是一种慢性炎症性病变,属于需要长期治疗的疾病。临床药师对患者进行全程药学监护,特别是结合患者合并低蛋白血症的病情,查阅相关文献,分析IFX血药浓度较低的原因,协助医师优化治疗方案,较好地发挥了临床药师进行药学服务实践的作用。该病例提示我们,临床患者使用IFX是具有个体差异的,特别是对于复杂病情的患者,我们应该提高警惕,尤其是要关注血清白蛋白水平对IFX消除以及疗效的影响。
Pharmaceutical care for a Crohn's disease patient with hypoalbuminemia and non-response to infliximab
-
摘要:
目的 探讨临床药师在克罗恩病患者出现英夫利昔单抗继发性失应答的个体化治疗和用药监护中的作用。 方法 临床药师参与1例克罗恩病合并低蛋白血症患者的药学实践过程,及时查阅文献对英夫利昔单抗血药浓度检测结果进行解读,分析该药的药动学过程,高度怀疑患者血清白蛋白水平降低致消除加快,浓度降低引起继发性失应答。 结果 临床药师协助医生调整药物治疗方案,患者经治疗后病情趋于好转。 结论 临床药师充分了解药物药动学变化,对治疗药物监测结果进行解读,可协助临床发现药物治疗相关问题,有利于建立个体化治疗方案,提高患者生物制剂用药的安全性及有效性。 Abstract:Objective To investigate the role of clinical pharmacists in individualized treatment and pharmaceutical care for a Crohn’s disease patient with non-response to infliximab. Methods The clinical pharmacist participated in the pharmaceutical care for a Crohn’s disease patient with hypoalbuminemia. Clinical pharmacists interpreted the blood concentration results of infliximab based on literature review, analyzed the pharmacokinetic process of drugs, and suggested that low serum albumin levels may cause the accelerated drug elimination and resulted in reduced drug concentration and secondary non-response. Results Clinical pharmacists assisted clinician adjusting the medication regimen and the patient recovered well after the new treatment plan. Conclusion With good understanding in medication pharmacokinetics and the blood test results, clinical pharmacists can help to solve the drug therapy related problems and establish an individual treatment plan to improve the safety and effectiveness of the biological medications. -
结直肠癌,又称大肠癌,好发于大肠黏膜,是常见消化系统恶性肿瘤,早期症状不明显不易发现,晚期则表现贫血、体重减轻等。由于现代生活节奏加快及各种不良饮食、作息、环境和遗传等因素的影响,使得我国的发病率逐年增加[1]。据《中国肿瘤登记年报》内容显示:2015年我国肿瘤登记地区结直肠癌发病率和病死率分别为17.1/10万和7.9/10万,发病率男女性别比和城乡比分别为1.5和1.4,病死率分别为1.6和1.4。与年报数据接近的《中国死因监测数据集》显示,2017年我国结直肠癌病死率为6.9/10万[2]。虽然,目前已有各种化疗、外科手术、中医治疗等诊治方法,但是仍然存在早期诊查率低、预后差等问题,患者术后5年的生存率仍没有得到较大的改善,故针对结直肠癌的诊疗研究亦成为现在的热点[3]。
卡培他滨(Cap)是结直肠癌辅助化疗及一线治疗药物,通常与多西他赛、奥沙利铂、爱必妥等联合应用,常见结直肠癌化疗方案是联合奥沙利铂。Cap是前体药,体内转化成氟尿嘧啶,并在肿瘤组织中代谢为5-氟尿嘧啶,从而抑制核苷酸的合成,发挥抗肿瘤作用。肿瘤组织中5-氟尿嘧啶的浓度是血液中的100倍以上,靶向性好,不良反应轻微,且大部分患者都可耐受[4]。手足综合征(HFS)是服用Cap后出现的常见药物不良反应,多为1~2级,少数达到3级。临床主要表现为进展性症状变化,早期症状主要发生在手掌和足底,出现不同程度的瘙痒,指尖、手掌和足底充血,之后会持续发展为手掌和足底的暗红和肿胀,随后产生水泡,最终发展为脱皮,极大影响患者用药依从性及生存质量,成为后期持续治疗效果不佳的严重因素之一[5]。Cap导致HFS的发病机制尚不清楚,但病理特征表现为不同程度的细胞点片坏死、轻度的海绵状水肿、血管扩张、表皮与真皮交界处有炎性渗出。有研究表明[6-8],显微镜镜下观察到血管舒张和水肿,类似于炎症反应,而炎症发生时,活跃的炎症因子主要有白介素类中的IL-1β、IL-6、IL-10、IL-12、IL-17、IL-23,肿瘤坏死因子(TNF-α)、干扰素(IFN-γ)、C-反应蛋白(CPR)以及趋化性细胞因子(CCL-5)。因此,本研究对出现HFS的结直肠癌患者血浆中的主要炎症因子进行考察,推测其中涉及到的炎症因子,建立炎症因子含量变化与HFS发生的相关性,为Cap发生HFS的防治提供一定参考。
1. 材料和方法
1.1 一般资料
选取2018年9月至2019年2月海军军医大学附属长征医院普外科接受卡培他滨化疗方案的35例结直肠癌患者作为本次研究对象。纳入标准:①年龄≥18周岁;②经临床确诊为结直肠癌的患者;③接受了以Cap为基础的化疗;④预计生存期≥3个月,无主要器官的功能障碍;⑤有化疗指证,包括心肝肾等脏器功能正常,骨髓造血功能正常;⑥治疗前病情评估应有完整的体格检查和实验室检查,包括全血细胞计数、生化功能检查等;⑦生活质量:首次出现先写中文名(KPS)评分≥60分;⑧自愿签署知情同意书。排除标准:①孕、哺乳期患者;②5年内患过其他恶性肿瘤者;③经临床确定,对FU类药物过敏或严重代谢不良的患者;④有严重感染的患者;⑤经临床确定,患有其他会影响实验结果的恶性疾病;⑥不符合纳入标准,未按规定用药,无法判断疗效,或资料不全等影响疗效或安全性判断者。HFS事件评价标准:根据美国卫生及公共服务部2009年出版的常见不良反应事件评价标准4.0(CTCAE v4.0)分级,1级:轻微皮肤改变或皮肤炎(红斑、水肿、角化过度、不痛);2级:皮肤改变(剥落、水泡、出血、肿胀、角化过度),疼痛,影响工具性日常生活活动;3级:重度皮肤改变(剥落、水泡、出血、水肿、角化过度),疼痛,个人自理能力受限。自患者服用Cap后进行临床观察,直到患者出现HFS后停止,收集患者出现HFS时的血液样本,采用EDTA-3K抗凝管采集血样,储存于−80 ℃冰箱冻存。本研究经过海军军医大学附属长征医院伦理委员会批准,35例患者基本信息及HFS信息见表1。
表 1 患者一般资料及HFS分级情况例数 性别 年龄(岁) 肿瘤部位 HFS分级 男 女 均值 中位数 直肠 结肠 未发生 1级 2级 3级 35 23 12 55.9 61 14 21 12 16 3 4 (65.7%) (34.3%) — — (29.9%) (70.1%) (34.2%) (45.7%) (8.5%) (11.4%) 1.2 仪器和耗材
Human IL-6 ELISA kit试剂盒、 Human IL-1β ELISA kit试剂盒、Human IL-10 ELISA kit试剂盒、Human IL-12p70 ELISA kit试剂盒、Human IL-17/IL-17A ELISA kit试剂盒、Human IL-23 ELISA kit试剂盒、Human IFN-γ ELISA kit试剂盒、Human CRP ELISA kit试剂盒、Human TNF-α ELISA kit试剂盒以及Human RANTES ELISA kit试剂盒均购自国润医疗供应链服务(上海)有限公司。酶标仪(Biotek,型号:800TSI)购自美国伯腾仪器(北京代表处)有限公司。37 ℃孵箱(型号:FYL-YS-151L,温度:0 ℃~100 ℃)由北京福意电器有限公司提供。
1.3 炎症因子指标检测方法
参照试剂盒说明书测定35例结直肠癌患者服用卡培他滨后血浆中各炎症因子包括白介素类中的IL-1β、IL-6、IL-10、IL-12、IL-17、IL-23, TNF-α,IFN-γ,CPR和CCL-5含量水平。将血液样本4 000 r/min离心15 min, 收集血浆样本,按照试剂盒说明书测定各炎症因子的含量。
1.4 统计学方法
使用 Microsoft Excel 软件进行统计结果分析,采用Graphpad Prism 8.3.0作图。
2. 结果
2.1 标准曲线
IL-23标准曲线的浓度为2 000、1 000、500、250、125、62.5、31.25 pg/ml;IFN-γ、IL-17、TNF-α、IL-12标准曲线的浓度为1 000、500、250、125、62.5、31.25、15.6 pg/ml;CCL-5、IL-1β的标准曲线的浓度为500、250、125、62.5、31.25、15.6、7.8 pg/ml;IL-6、CRP的标准曲线的标准品浓度为200、150、50、25、12.5、6.25、3.125 pg/ml;IL-10的标准曲线的浓度为50、25、12.5、6.25、3.125、1.56、0.78 pg/ml。各炎症因子标准曲线呈现良好线性关系,可为样本的检测提供阳性对照,见表2。
表 2 炎症因子线性范围及标准曲线炎症因子 线性范围pg/ml 标准曲线 r IL-23 0~2 000 Y=594.66X−23.268 0.994 8 IFN-γ 0~1 000 Y=384.31X+7.9709 0.999 3 IL-17 Y=1379X−12.849 0.997 9 TNF-α Y=352.02X+5.7229 0.999 7 IL-12 Y=448.55X+8.5172 0.994 6 CCL-5 0~500 Y=168.42X−12.215 0.997 2 IL-1β Y=206.82X−4.5823 0.999 6 IL-6 0~200 Y=67.278X−6.6851 0.996 7 CRP Y=90.523X -3.228 0.990 5 IL-10 0~50 Y=27.541X−0.403 0.997 5 2.2 样本实测
35例服用Cap后未发生与发生各级HFS的两类结直肠癌患者,其血浆中各炎症因子含量水平见表3。结果显示,不同级别的HFS各炎症因子含量存在一定的差异性,提示Cap诱发HFS会导致血浆中不同炎症因子相互作用发生级联反应。其中,TNF-α 的含量水平在发生HPS呈整体下调状态,且明显低于未发生者的含量水平。其余炎症因子含量水平变化则无规律可循(图1)。
表 3 不同分级HFS患者中的各炎症因子含量测定结果(pg/ml,$\bar x $ ±s)炎症因子 未发生 1级 2级 3级 浓度平均值 标准差 浓度平均值 标准差 浓度平均值 标准差 浓度平均值 标准差 IL-1β 83.64 203.67 60.98 180.17 8.96 5.03 303.17 303.50 IL-6 19.57 52.35 12.29 31.84 4.68 0.34 22.60 25.96 IL-10 8.280 17.19 10.53 26.67 0.17 0.11 0.10 0.03 IL-12 67.80 148.40 99.40 282.18 22.87 9.42 195.11 250.89 IL-17 316.96 807.82 413.09 1118.61 76.79 46.93 905.52 1361.64 IL-23 218.06 566.46 190.55 542.33 12.11 5.95 663.67 911.80 IFN-γ 89.87 248.25 77.82 209.39 17.36 5.01 274.05 392.16 CRP 52.99 85.03 65.77 91.81 6.91 3.49 157.98 114.55 TNF-α 221.01 461.14 58.98 186.95 2.67 2.51 2.64 1.54 CCL-5 98.11 30.01 87.84 20.60 101.78 19.20 85.93 15.49 注:未发生HFS 12例;1级HFS 16例;2级HFS 3例;3级HFS 4例 3. 讨论
随着结直肠癌发病率的上升,Cap的临床应用越来越广泛,其带来的不良反应也急剧增加,其中,HFS严重影响患者生活质量以及用药依从性,更甚者则终止治疗,故必须对Cap诱发HFS进行药学监护[9-10]。临床常见的监护策略主要有减少给药剂量、中断药物治疗以及使用其他药物(如塞来昔布胶囊200 mg,po,bid +尿素乳膏涂抹患处,tid)干预HFS严重程度等方法。肿瘤坏死因子(TNF-α)是机体重要的炎性细胞因子之一,具有多生物学效应及生理病理性功能,且TNF-α在HFS发生的不同程度时血浆中含量有显著变化,推测TNF-α 血浆中含量水平越低发生HFS的程度越严重,并在进行药学监护时具有一定参考价值。
本实验以HFS为主要研究方向,对患者血浆中炎症因子进行检测,结果发现Cap诱发不同程度HFS血浆中的炎症因子含量有一定的差异性。由于临床化疗治疗医生很少让患者的HFS进展到2、3级再进行处理,所以患者例数较少,数据不能支撑比较。但对未发生与1级HFS比较发现,1级HFS患者血浆中IL-1β、IL-6、IL-23、IFN-γ、TNF-α、CCL-5的含量明显低于未发生HFS患者;而IL-10、IL-12、IL-17、CRP血浆中含量明显高于未发生HFS患者,且TNF-α浓度水平变化较为明显。目前,Cap诱发HFS的发生机制尚不清楚,可能是多途径炎症因子相互作用的结果。因此,关注定血浆炎症因子含量水平变化,在一定程度上可以反应Cap诱导HFS的严重程度,通过测定血浆中炎症因子含量的变化,有利于防治Cap诱发HFS情况,并对临床有重要的指导意义。
-
[1] BORTLIK M, DURICOVA D, MALICKOVA K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease[J]. J Crohns Colitis,2013,7(9):736-743. [2] HIBI T, SAKURABA A, WATANABE M, et al. Retrieval of serum infliximab level by shortening the maintenance infusion interval is correlated with clinical efficacy in crohnʼs disease[J]. Inflamm Bowel Dis,2012,18(8):1480-1487. [3] OH E H, KO D H, SEO H, et al. Clinical correlations of infliximab trough levels and antibodies to infliximab in South Korean patients with Crohn's disease[J]. World J Gastroenterol,2017,23(8):1489-1496. [4] NAKASE H. Optimizing the use of current treatments and emerging therapeutic approaches to achieve therapeutic success in patients with inflammatory bowel disease[J]. Gut Liver,2020,14(1):7-19. [5] 中华医学会消化病学分会炎症性肠病学组. 中国炎症性肠病治疗药物监测专家共识意见[J]. 中华消化杂志, 2018, 38(11):721-727. [6] Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society;Hospital Pharmacy Committee of Chinese Pharmaceutical Association;Evidence-Based Pharmacy Committee of Chinese Pharmaceutical Association;Chinese Pharmacists Association Therapeutic Drug Monitoring Pharmacists Branch;Chinese Pharmacists Association Home-Based Pharmaceutical Care Pharmacists Branch;Writing Group of the Expert Consensus on the Interpretation of Therapeutic Drug Monitoring;. 治疗药物监测结果解读专家共识[J]. 中国医院药学杂志, 2020, 40(23):2389-2395. [7] KEIZER R J, HUITEMA A D, SCHELLENS J H, et al. Clinical pharmacokinetics of therapeutic monoclonal antibodies[J]. Clin Pharmacokinet,2010,49(8):493-507. [8] BRANDSE J F, MOULD D, SMEEKES O, et al. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease[J]. Inflamm Bowel Dis,2017,23(4):650-660. [9] FASANMADE A A, ADEDOKUN O J, BLANK M, et al. Pharmacokinetic properties of infliximab in children and adults with Crohn's disease: a retrospective analysis of data from 2 phase III clinical trials[J]. Clin Ther,2011,33(7):946-964. [10] DOTAN I, RON Y, YANAI H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study[J]. Inflamm Bowel Dis,2014,20(12):2247-2259. [11] FASANMADE A A, ADEDOKUN O J, OLSON A, et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis[J]. Int J Clin Pharmacol Ther,2010,48(5):297-308. [12] HARTMAN C, ELIAKIM R, SHAMIR R. Nutritional status and nutritional therapy in inflammatory bowel diseases[J]. World J Gastroenterol,2009,15(21):2570-2578. [13] ZACHOS M, TONDEUR M, GRIFFITHS A M. Enteral nutritional therapy for induction of remission in Crohn's disease[J]. Cochrane Database Syst Rev,2007(1):CD000542. -

 点击查看大图
点击查看大图
计量
- 文章访问数: 3065
- HTML全文浏览量: 1700
- PDF下载量: 24
- 被引次数: 0




 下载:
下载:
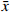

 下载:
下载:
