-
成纤维细胞的增殖和活化引起的细胞外基质(extracellular matrix,ECM)的累积是肺纤维化的主要病理基础[1]。肺纤维化治疗难度大,且发展到晚期纤维化过程不可逆转,而一些药物的长期使用会提高肺纤维化的发生风险,对于这临床的一类并发症应格外重视。来氟米特(leflunomide,LEF)是治疗类风湿性关节炎的常用药物,但是有临床报道称,长期服用LEF可能提高肺纤维化的发生风险,但是也有研究认为LEF对肺纤维化影响不大[2]。微小RNA(microRNA,miRNA)长度约为18~22个核苷酸,虽然不具备编码功能,但是可通过识别和碱基配对的方式与靶基因信使RNA(message RNA,mRNA)的3'非翻译区(3'UTR)结合,从而参与基因表达的调控[3]。miR-449a具有抑制肿瘤细胞的增殖并诱导凋亡的作用[4],并且最新研究发现miR-449a可能与肺纤维化有关,在二氧化硅诱导的肺纤维化模型中,miR-449a可通过调节自噬缓解纤维化[5]。c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)是调节细胞增殖、凋亡和分化的重要蛋白,其磷酸化后可通过信号转导调控细胞生物学行为[6]。本文发现了miR-449a的过表达会显著缓解由LEF引起的肺成纤维细胞的增殖,而沉默miR-449a对细胞的影响相反,这可能是LEF引起肺纤维化的机制之一,报道如下。
-
人肺成纤维细胞MRC-5购自美国ATCC;LEF(苏州长征-欣凯制药有限公司,国药准字H20000550);RPMI-1640培养基以及血清购自美国Gibco公司;miR-449a mimic和inhibitor质粒由Genepharma公司构建;
LipofectamineTM 2000(美国Invitrogen公司);荧光显微镜(Olympus BX51);Model 680酶标仪(Bio-Rad,美国);流式细胞仪(BD FACScanto II,Becton Dickinson,美国)。CCK-8试剂盒(武汉华美公司);凋亡试剂盒(美国Thermo Fisher);PVDF膜(美国Bio-Rad公司);抗体购自美国Abcam公司,逆转录试剂盒TaKaRa和SYBR Prellix Ex TaqTM实时PCR试剂盒购自TaKaRa(日本)。
-
MRC-5细胞在RPMI-1640培养基中培养,温度为37 ℃,CO2浓度为5%。细胞被分为6组,即对照组、LEF组、LEF+mimic组、mimic组、LEF+inhibitor组和inhibitor组。其中LEF+mimic组和mimic组通过转染miR-449a mimic质粒过表达miR-449a的水平,LEF+inhibitor组和inhibitor组通过转染miR-449a inhibitor质粒使miR-449a的水平降低。对照组转染空载质粒。LEF组、LEF+mimic组和LEF+inhibitor组分别在5 mg/L LEF的条件下培养48 h。
-
将细胞裂解后收集总RNA并检测纯度,通过逆转录试剂盒合成cDNA,然后进行PCR反应,步骤如下:95 ℃下2 min,95 ℃下15 s,60 ℃下25 s和72 ℃下60 s,共进行40个循环。以U6作为内参,使用2-ΔΔCT法分析miR-449a水平。引物序列如下(5′-3′),miR-449a上游引物:TGCGGTGGCAGTGTATTGTTAGC,下游引物:CCAGTGCAGGGTCCGAGGT;U6上游引物:GGGCAGGAAGAGGGCCTAT,下游引物:TATGGCTAGCATGACTGGT。
-
将细胞调节至2×104个细胞/ml的密度,接种于96孔板中,100 μl/孔。再培养24、48和72 h后将10 μl的CCK-8试剂加入至每孔中,37 ℃下培养2 h。在酶标仪上测量450 nm处的吸光度(A),计算相对细胞活力。
-
分别将各组细胞200个细胞在6孔板中培养,每3天补充一次培养基,培养2周。用PBS洗涤细胞并加入甲醇固定15 min,加入使用结晶紫染色30 min,在显微镜下观察克隆形成的数目,≥50个细胞的集落为一个克隆形成。
-
将细胞洗涤后重悬于结合缓冲液中,使细胞浓度为2.5×105个/ml。根据试剂盒说明书将试剂加入细胞中,并通过流式细胞术分析细胞凋亡情况。
-
通过免疫荧光染色检测α平滑肌肌动蛋白(α smooth muscle actin,α-SMA)和胶质蛋白I(collagen I,Col I)的表达情况分析细胞的表型和细胞外基质。然后将细胞在4 ℃下使用α-SMA的抗体染色过夜,用异硫氰酸四甲基罗丹明的山羊抗兔抗体染色30 min。然后再于避光条件下利用4',6-二脒基-2-苯基吲哚(4',6-diamidino-2-phenylindole,DAPI)对细胞核染色10 min,通过荧光显微镜观察。
-
通过Western blot检测JNK和磷酸化JNK(p-JNK)蛋白的水平。将细胞裂解、离心收集总蛋白并检测蛋白浓度。使用10%的SDS-PAGE凝胶用于电泳分离蛋白,电泳后使用PVDF膜转膜并在室温下用5%无脂牛奶封闭2 h。分别加入一抗(稀释1:1 000)室温震荡2 h,后在4 ℃孵育过夜,加入二抗(稀释1:5 000),孵育3 h。通过Quantity One软件分析条带的灰度值并以GAPDH为参照计算目标蛋白质的表达量。
-
实验数据采用SPSS 19软件进行处理,实验结果以平均值±标准偏差(SD)表示,组间比较采用单因素方差分析和t检验,统计学显著性表示为P<0.05。
-
使用qPCR检测各组细胞中miR-449a表达水平。结果显示mimic组miR-449a水平显著高于对照组,inhibitor组的miR-449a表达水平显著低于对照组(P<0.05),说明转染实验成功。LEF组的miR-449a水平显著低于对照组(P<0.05),并且LEF+mimic组的miR-449a的表达水平显著高于LEF组,LEF+inhibitor组的miR-449a显著低于LEF组(P<0.05)。表明LEF可抑制人成纤维细胞中miR-449a的表达,见表1。
表 1 各组miR-449a表达水平比较
组别 miR-449a 对照组 1.16±0.08 LEF组 0.58±0.05* LEF+mimic组 2.04±0.16# mimic组 6.32±0.63* LEF+inhibitor组 0.41±0.06# inhibitor组 0.77±0.07* *P<0.05,与对照组比较;#P<0.05,与LEF组比较 -
使用CCK-8法检测各组细胞的相对细胞活力。结果显示在第48小时和第72小时,LEF组和inhibitor组的细胞活力显著高于对照组(P<0.05),而mimic组的细胞活力显著低于对照组(P<0.05)。此外,LEF+mimic组的细胞活力显著低于LEF组,LEF+inhibitor组的细胞活力显著高于LEF组(P<0.05),过表达miR-449a可部分逆转LEF对促进人成纤维细胞的细胞活力的作用,而降低miR-449a的水平会进一步促进细胞活力,见表2。
表 2 各组细胞的相对细胞活力比较(%)
组别 24 h 48 h 72 h 对照组 100.07±1.83 100.76±2.07 100.16±1.96 LEF组 103.67±2.06 110.83±2.15* 121.17±2.65* LEF+mimic组 99.98±2.14 98.57±2.11# 97.37±2.01# mimic组 97.54±1.97 91.79±2.35* 81.77±1.78* LEF+inhibitor组 107.68±2.08 118.67±3.07# 132.84±2.07# inhibitor组 104.31±1.79 111.38±2.67* 119.35±2.18* *P<0.05,与对照组比较;#P<0.05,与LEF组比较 -
LEF组和inhibitor组的克隆形成数目显著高于对照组而细胞凋亡率低于对照组(P<0.05),mimic组的克隆形成数目显著低于对照组而细胞凋亡率显著高于对照组(P<0.05)。此外,LEF+mimic组的克隆形成数目显著低于LEF组而细胞凋亡率显著高于LEF组(P<0.05),LEF+inhibitor组的克隆形成数目在LEF的基础上进一步升高而细胞凋亡率进一步降低(P<0.05)。过表达miR-449a可逆转LEF促进肺成纤维细胞增殖和抑制凋亡的作用,而低表达miR-449a会加剧LEF的作用,见表3。
表 3 各组细胞增殖和凋亡情况比较
组别 克隆形成数目(个) 细胞凋亡率(%) 对照组 54.32±4.36 5.53±0.94 LEF组 87.66±7.24* 3.11±0.76* LEF+mimic组 60.82±6.06# 6.73±1.26# mimic组 31.12±3.78* 17.32±3.28* LEF+inhibitor组 119.35±5.08# 2.14±0.62# inhibitor组 92.71±7.89* 3.45±0.83* *P<0.05,与对照组比较;#P<0.05,与LEF组比较 -
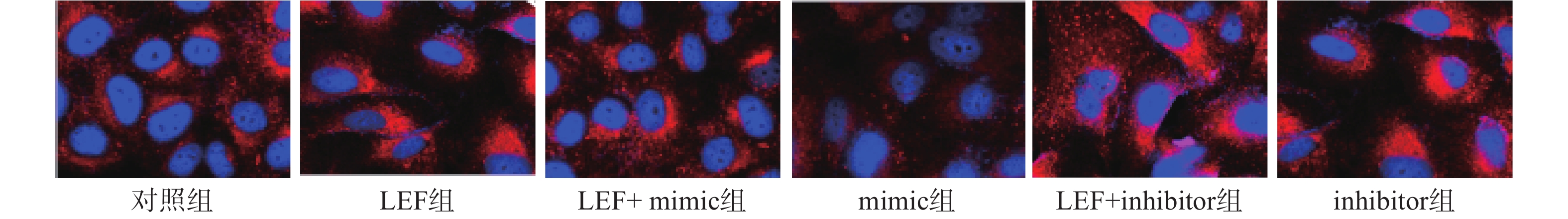
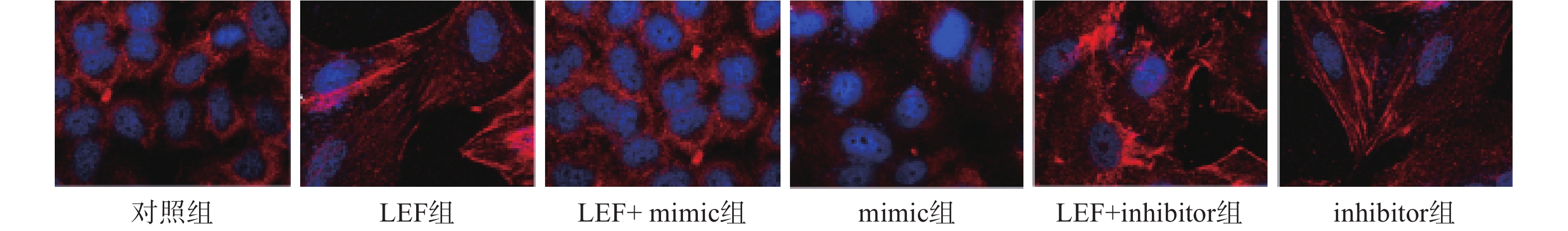
本次研究通过免疫荧光技术检测了各组α-SMA的水平来分析细胞向肌细胞转化情况,检测Col I的水平来分析ECM水平。其中蓝色荧光为细胞核,红色荧光为α-SMA或Col I蛋白。LEF组和inhibitor组的荧光强度显著高于对照组(P<0.05),而mimic组的相对荧光强度低于对照组(P<0.05)。此外,LEF+mimic组的相对荧光强度显著低于LEF组(P<0.05),LEF+inhibitor组的相对荧光强度显著高于LEF组(P<0.05)。过表达miR-449a可部分逆转LEF对促进人成纤维细胞α-SMA和Col I表达的促进作用,见图1、图2和表4。
表 4 各组细胞α-SMA相对荧光强度比较
组别 α-SMA Col I 对照组 1.02±0.11 1.24±0.14 LEF组 2.36±0.47* 2.57±0.38* LEF+mimic组 1.53±0.34# 1.89±0.25# mimic组 0.47±0.05* 0.45±0.06* LEF+inhibitor组 3.25±0.18# 4.13±0.54# inhibitor组 2.48±0.15* 3.11±0.39* *P<0.05,与对照组比较;#P<0.05,与LEF组比较 -
LEF组和inhibitor组的p-JNK/JNK水平高于对照组,mimic组的p-JNK/JNK水平显著低于对照组(P<0.05),并且LEF+mimic组中p-JNK/JNK水平显著低于LEF组(P<0.05),LEF+inhibitor组中p-JNK/JNK水平显著高于LEF组(P<0.05)。过表达miR-449a可逆转LEF促进JNK蛋白磷酸化的作用,见表5。
表 5 各组p-JNK/JNK相对水平比较
组别 p-JNK JNK p-JNK/JNK 对照组 2.04±0.18 2.16±0.16 0.94±0.09 LEF组 2.87±0.31 1.05±0.10 2.73±0.18* LEF+mimic组 1.67±0.19 2.24±0.21 0.75±0.07# mimic组 0.96±0.11 3.11±0.28 0.31±0.04* LEF+inhibitor组 3.04±0.24 1.10±0.10 2.76±0.25# inhibitor组 3.78±0.34 1.02±0.09 3.71±0.31* *P<0.05,与对照组比较;#P<0.05,与LEF组比较 -
肺纤维化是一种慢性进行性肺部疾病,但是临床上尚无治疗肺纤维化的特效方法和药物,目前用于进行性肺纤维化的唯一有效治疗方法是肺移植[7],若患者未接受肺移植,通常在诊断后的3至5年内出现肺功能丧失导致呼吸衰竭和死亡。肺纤维化的病理特征包括纤维增生和ECM沉积过多,但是这个过程较为漫长,并且在早期症状不明显,也缺乏相应的诊断手段,在患者确诊为肺纤维化时再采取治疗效果有限。因此虽然LEF是否会引起肺纤维化尚无定论,但是由于肺纤维化的恶性预后和致死率,LEF治疗过程中的肺纤维化风险仍是临床重点关注的问题。研究LEF促进肺纤维化的机制是寻找诊断和治疗肺纤维化新方法的重要途径。
LEF是一种调节免疫的药物,其作用机制通过抑制二氢乳清酸脱氢酶来抑制T淋巴细胞和其他类型细胞的细胞周期进程[8]。LEF引起的肺纤维化并导致患者死亡的病例随着LEF使用时间的增加而升高[9]。一项长期的调查随访报告指出,在5911例使用LEF治疗的患者中,共出现了80例间质性肺病,其中有27例患者死亡,并且结果判定其中有18例患者的死亡是由于LEF直接导致[10-11]。在肺纤维化的过程中,成纤维细胞向成肌纤维细胞转化和ECM的累积是两大特点[12]。因此本文主要分析了LEF对人肺成纤维细胞的影响,结果显示LEF可显著促进成纤维细胞的细胞活力和增殖,抑制其凋亡,并诱导细胞表达大量的α-SMA蛋白和ECM累积。α-SMA是上皮细胞向间质细胞转化的检测指标之一,也是体外研究肺纤维化的最常用指标,而Col I是ECM的主要成分[13]。作者提示了LEF可通过活化成纤维细胞和促进其增殖参与肺纤维化。
为进一步分析LEF调节肺成纤维细胞增殖和表达α-SMA的机制,我们检测了miR-449a在其中的作用。miR-449a是近年来新发现的一种miRNA,研究已经证实了其可通过靶向并诱导靶基因mRNA降解,抑制肺癌细胞的增殖、上皮间充质转化[14-15]。本次研究结果显示LEF可抑制miR-449a的表达水平,并且过表达miR-449a可抑制成纤维细胞的细胞活力、细胞增殖能力,抑制α-SMA和Col I蛋白的表达,并促进其凋亡。过表达miR-449a会逆转由LEF引起的细胞活化、增殖以及α-SMA和Col I蛋白的表达,而抑制miR-449a的水平会进一步加剧LEF的促纤维化作用。Zhang等[16]的研究结果也显示miR-449a具有调节α-SMA蛋白表达的作用。通过进一步的研究我们还发现LEF可促进JNK的磷酸化,过表达miR-449a会抑制JNK磷酸化水平并显著逆转LEF促进JNK磷酸化的作用。JNK的活化在促进肺癌发生和发展中的作用已经被广泛证实,此外,JNK可活化可能通过活化肝星状细胞引起肝纤维化[17]。Yang等[18]的研究结果显示阻断JNK途径可抑制成纤维细胞样滑膜细胞的活性,并抑制迁移和侵袭。Shingyochi等[19]的研究结果也显示了激活JNK通路会促进成纤维细胞的增殖和迁移。这提示LEF可能通过miR-499a促进JNK蛋白的磷酸化,从而促进肺成纤维细胞的活化和增殖,并促进细胞向肌纤维细胞转化和ECM的累积,进而引起肺纤维化。
综上所述,LEF可能通过抑制肺成纤维细胞中miR-449a的表达激活JNK途径,促进α-SMA的表达和ECM的累积,从而诱导成纤维细胞的活化和增殖,抑制其凋亡,从而引起肺纤维化。但是,关于LEF调节miR-449a的机制和miR-449a在JNK途径中的作用仍需要进一步的研究。
Mechanism of leflunomide in regulating pulmonary fibrosis by regulating miR-449a
-
摘要:
目的 探究来氟米特(leflunomide,LEF)通过调节微小RNA(microRNA,miR)-449a在肺纤维化中的机制研究。 方法 将人肺成纤维细胞MRC-5分为6组,即对照组、LEF组、LEF+mimic组、mimic组、LEF+inhibitor组和inhibitor组。通过质粒转染miR-449a mimic或inhibitor来过表达或沉默miR-449a,在5 mg/L LEF的条件下培养48 h。分别通过CCK-8法、克隆形成实验和流式细胞术检测各组细胞活力、细胞增殖能力和凋亡率。使用免疫荧光染色检测α平滑肌肌动蛋白(α smooth muscle actin,α-SMA)胶质蛋白I(collagen I,col I)。分别使用qPCR和Western blot检测miRNA和蛋白的水平。 结果 mimic组miR-449a水平显著高于对照组(P<0.05)。LEF组和inhibitor组的miR-449a水平显著低于对照组(P<0.05)。LEF+mimic组的miR-449a的表达水平显著高于LEF组,LEF+inhibitor组的miR-449a水平显著低于LEF组(P<0.05)。LEF组和inhibitor组的细胞活力和细胞增殖能力显著高于对照组(P<0.05)。mimic组的细胞活力和细胞增殖能力显著低于对照组(P<0.05)。LEF+mimic组的细胞活力和细胞增殖能力显著低于LEF组而LEF+inhibitor组的细胞活力显著高于LEF组(P<0.05)。LEF组和inhibitor组的细胞凋亡率低于对照组(P<0.05),mimic组的细胞凋亡率显著高于对照组(P<0.05)。LEF+mimic组的细胞凋亡率显著高于LEF组而LEF+inhibitor组的凋亡率显著低于LEF组(P<0.05)。LEF组和inhibitor组的α-SMA和Col I蛋白的荧光强度显著高于对照组(P<0.05),mimic组的相对荧光强度低于对照组(P<0.05)。LEF+mimic组的α-SMA和Col I蛋白相对荧光强度显著低于LEF组,LEF+inhibitor组的α-SMA和Col I蛋白相对荧光强度显著高于LEF组(P<0.05)。LEF组和inhibitor组的p-JNK/JNK水平高于对照组(P<0.05),mimic组的p-JNK/JNK水平显著低于对照组(P<0.05),LEF+mimic组中p-JNK/JNK水平显著低于LEF组而LEF+inhibitor组的p-JNK/JNK水平显著高于LEF组(P<0.05)。 结论 LEF可能通过抑制肺成纤维细胞中miR-449a的表达激活JNK途径,从而诱导成纤维细胞的活化和增殖,抑制其凋亡,从而引起肺纤维化。 -
关键词:
- 来氟米特 /
- 肺纤维化 /
- 成纤维细胞 /
- α平滑肌肌动蛋白 /
- 微小RNA-449a /
- c-Jun氨基末端激酶
Abstract:Objective To investigate the mechanism of leflunomide (LEF) in regulating pulmonary fibrosis by regulating microRNA (miR)-449a. Methods Human lung fibroblasts MRC-5 were divided into 6 groups: control group, LEF group, LEF+mimic group, mimic group, LEF+inhibitor group and inhibitor group. MiR-449a was overexpressed or silenced by plasmid transfection with miR-449a mimic or inhibitor and ncubate for 48 h at 5 mg / L LEF. The cell viability, cell proliferation ability and apoptotic rate of each group were measured by CCK-8 method, clone formation experiment and flow cytometry. Immunofluorescent staining was used to detect α smooth muscle actin (α-SMA) and collagen I (col I). The levels of miRNA and protein were detected using qPCR and Western blot, respectively. Results The miR-449a level in the mimic group was significantly higher than that in the control group (P<0.05). The level of miR-449a in LEF group and inhibitor group was significantly lower than that in control group (P<0.05). The expression level of miR-449a in LEF+mimic group was significantly higher than that in LEF group, and the level of miR-449a in LEF+inhibitor group was significantly lower than that in LEF group (P<0.05). The cell viability and cell proliferation ability of the LEF group and inhibitor group were significantly higher than those of the control group (P<0.05). The cell viability and cell proliferation ability of the mimic group were significantly lower than those of the control group (P<0.05). The cell viability and cell proliferation ability of the LEF+mimic group were significantly lower than those of the LEF group, while the cell viability of the LEF+inhibitor group was significantly higher than that of the LEF group (P<0.05). The apoptosis rate of LEF group and inhibitor group was lower than that of control group (P<0.05). The apoptosis rate of mimic group was significantly higher than that of control group (P<0.05). The apoptosis rate of LEF+mimic group was significantly higher than that of LEF group, while the apoptosis rate of LEF+inhibitor group was significantly lower than that of LEF group (P<0.05). The fluorescence intensity of α-SMA and Col I proteins in LEF group and inhibitor group were significantly higher than those in control group (P<0.05). The relative fluorescence intensity of mimic group was lower than that of control group (P<0.05). The relative fluorescence intensities of α-SMA and Col I proteins in LEF+mimic group were significantly lower than those in LEF group, while the relative fluorescence intensities of α-SMA and Col I protein in LEF+inhibitor group were significantly higher than those in LEF group (P<0.05). The levels of p-JNK / JNK in LEF group and inhibitor group were higher than those in control group (P<0.05). The p-JNK / JNK level in the mimic group was significantly lower than that in the control group (P<0.05). The level of p-JNK / JNK in LEF+mimic group was significantly lower than that in LEF group, while the level of p-JNK / JNK in LEF+inhibitor group was significantly higher than that in LEF group (P<0.05). Conclusion LEF may activate the JNK pathway by inhibiting the expression of miR-449a in lung fibroblasts, thereby inducing fibroblast activation and proliferation, inhibiting apoptosis, and causing pulmonary fibrosis. -
Key words:
- leflunomide /
- pulmonary fibrosis /
- fibroblasts /
- alpha smooth muscle actin /
- microRNA-449a /
- c-Jun N-terminal kinase
-
盐酸普萘洛尔(propranolol hydrochloride,PPL)是治疗婴幼儿血管瘤的一线和首选药物[1]。口服盐酸普萘洛尔疗效确切,但其存在首过效应强、生物利用度低、半衰期短等问题,且不良反应发生率高[2]。普通盐酸普萘洛尔外用制剂只对浅表型血管瘤有效,对深部型和复合型血管瘤的治疗仍需结合口服给药。诸多研究表明,立方液晶(cubosomes,Cubs)可显著提高经皮给药制剂的皮肤渗透性,且能提高其在皮肤尤其是皮肤真皮层的药物滞留量,有望能提高盐酸普萘洛尔外用制剂对深部型和复合型血管瘤的疗效[3~5]。因此,课题组拟基于立方液晶载药技术将盐酸普萘洛尔制备成一种纳米经皮给药制剂,以期能降低或避免口服给药带来的高不良反应发生率,提高盐酸普萘洛尔的治疗效果和患者依从性。前期实验中,课题组筛选了盐酸普萘洛尔立方液晶纳米粒(PPL-Cubs)的制备方法,并通过单因素考察结合星点设计效应面法优化了其最佳处方和制备工艺,结果制得的PPL-Cubs包封率低(约50%),远低于药典规定的80%。立方液晶为多层囊泡结构,类似于多囊脂质体,其可能与脂质体同样存在对水溶性化合物包封率较低的问题。鉴于前期研究表明,盐酸普萘洛尔在不同pH磷酸盐缓冲液下的溶解度存在极大差异,因此,本研究拟在立方液晶常规制备的基础上,引入“pH梯度法”的载药思路,制备PPL-Cubs,以期提高其包封率。
1. 仪器与试药
1.1 仪器
岛津LC-20AD型高效液相色谱仪(日本岛津公司);DV215CD型分析天平(美国奥豪斯公司);AL204型电子天平[梅特勒-托利多仪器(上海)有限公司];DF-101B集热式恒温加热磁力搅拌器(郑州长城科工贸有限公司);高压均质机(意大利NIRO-SAVI S.P.A.公司);NICOMP 380 ZLS激光粒度测定仪(美国PSS粒度仪公司);超滤离心管(100KD,Millipore)。
1.2 试药
盐酸普萘洛尔(含量99.9%,常州亚邦制药有限公司);单油酸甘油酯(法国GATTEFOSSé公司);泊洛沙姆407(德国BASF公司);甲醇、乙腈为色谱纯,水为超纯水,其余试剂为分析纯。
2. 方法与结果
2.1 包封率的测定
取PPL-Cubs适量,装入超滤离心管中,于4000 r/min离心10 min,收集离心液,采用课题组前期建立的盐酸普萘洛尔含量测定方法测定离心液中游离药物浓度C游离;取未透析的PPL-Cubs,测定药物浓度C总;根据公式EE(%)=[(C总−C游离)/ C总]×100%计算PPL-Cubs的包封率。
2.2 PPL-Cubs的制备
2.2.1 注入法
精密称取单油酸甘油酯9 g和泊洛沙姆407 1.5 g,加入10 ml无水乙醇,20 ℃水浴下搅拌溶解,为A相;精密称取盐酸普萘洛尔3.5 g,加入86 g纯化水,20 ℃水浴下搅拌溶解,为B相。于20 ℃水浴及600 r/min搅拌速度下,将A相缓慢地滴加至B相中,待磁力搅拌1 h后加入适量纯化水使总质量为100 g,再在800 bar压力下高压均质7次,得PPL-Cubs。
2.2.2 pH梯度法
精密称取单油酸甘油酯适量,40 ℃水浴加热使融化,为A相;精密称取泊洛沙姆407适量,加入适量纯化水,40 ℃水浴加热使溶解,并用1%磷酸溶液调节pH至酸性,为B相;于40 ℃水浴及600 r/min搅拌速度下,将A相缓慢滴加到B相中,待磁力搅拌30 min后,得空白立方液晶纳米粒粗品;取空白立方液晶纳米粒粗品,高压均质数次,得空白立方液晶纳米粒(B-Cubs)。取盐酸普萘洛尔溶解于适量纯化水中,得盐酸普萘洛尔水溶液;将盐酸普萘洛尔水溶液加入一定比例的B-Cubs中,搅拌均匀,并用氢氧化钠溶液调节pH至一定值,于一定温度下持续搅拌一定时间,再放置至室温,即得PPL-Cubs。
2.3 B-Cubs的制备工艺优化
前期试验结果表明,磁力搅拌速度、时间、温度、内水相pH值对B-Cubs的粒径基本无影响,高压均质压力及均质次数是影响其粒径的主要因素,故拟进一步优化高压均质压力和均质次数。
2.3.1 高压均质压力的考察
按照“2.2.2”项下方法,取空白立方液晶纳米粒粗品,分别在400、600、800、900、1000 bar下高压均质7次,测定制得B-Cubs的粒径及多分散指数(见表1)。结果表明,随高压均质压力的提高,制得B-Cubs的粒径和多分散指数均逐渐减小,当均质压力≥900 bar时,B-Cubs的粒径和多分散指数变化较小,故确定高压均质压力为900 bar。
表 1 高压均质压力的考察(n=3)压力(bar) 粒径(nm) 多分散指数 400 169.1±3.5 0.189±0.056 600 129.9±3.2 0.172±0.062 800 110.9±2.7 0.126±0.041 900 97.9±2.1 0.073±0.016 1000 96.4±1.9 0.057±0.009 2.3.2 高压均质次数的考察
按照“2.2.2”项下方法,取空白立方液晶纳米粒粗品,分别在900 bar下高压均质3、5、7、9次,测定制得B-Cubs的粒径及多分散指数(见表2)。结果表明,随高压均质次数的增加,制得B-Cubs的粒径和多分散指数均逐渐减小,当均质次数≥7次时,B-Cubs的粒径和多分散指数变化较小,故确定高压均质次数为7次。
表 2 高压均质次数的考察(n=3)次数(次) 粒径(nm) 多分散指数 3 160.4±4.6 0.173±0.052 5 129.2±3.8 0.140±0.037 7 97.9±2.1 0.073±0.016 9 93.3±1.7 0.067±0.011 2.4 B-Cubs的处方优化
2.4.1 单油酸甘油酯用量的考察
基于优化的B-Cubs最佳制备工艺,按照“2.2.2”项下方法,固定泊洛沙姆407用量为5%,内水相pH为3.0,考察单油酸甘油酯用量(15%、20%、25%、30%、35%)对制得B-Cubs粒径及多分散指数的影响(见表3)。结果表明,随单油酸甘油酯用量的增加,制得的B-Cubs粒径呈先减小后增大趋势,多分散指数则不断降低,当单油酸甘油酯用量为25%时,制得的B-Cubs具有最小的粒径和较适宜的多分散指数,故确定单油酸甘油酯用量为25%。
表 3 单油酸甘油酯用量的考察(n=3)用量(%) 粒径(nm) 多分散指数 15 137.8±3.4 0.160±0.033 20 119.2±2.9 0.147±0.032 25 97.9±2.1 0.073±0.016 30 118.3±3.5 0.024±0.015 35 150.8±5.4 0.026±0.011 2.4.2 泊洛沙姆407用量的考察
基于优化的B-Cubs最佳制备工艺,按照“2.2.2”项下方法,固定单油酸甘油酯用量为25%,内水相pH为3.0,考察泊洛沙姆407用量(3%、4%、5%、6%、7%)对制得B-Cubs粒径及多分散指数的影响(见表4)。结果表明,制得的B-Cubs粒径随泊洛沙姆407用量的增加逐渐降低,多分散指数变化无明显规律,但均较小(<0.1);当泊洛沙姆407用量≥5%时,粒径变化幅度降低,故确定泊洛沙姆407用量为5%。
表 4 泊洛沙姆407用量的考察(n=3)用量(%) 粒径(nm) 多分散指数 3 143.6±3.5 0.064±0.019 4 116.7±3.2 0.055±0.015 5 97.9±2.1 0.073±0.016 6 91.3±1.9 0.052±0.015 7 83.2±1.8 0.062±0.021 2.5 PPL-Cubs的包封率影响因素考察
2.5.1 外水相pH值的考察
根据前期盐酸普萘洛尔在不同pH的PBS中溶解度测定结果可知(见表5),盐酸普萘洛尔在pH≥8.5时溶解度显著下降。按照“2.2.2”项下方法,制备内水相pH为3.0的B-Cubs,并按载体/药物比(以单油酸甘油酯/盐酸普萘洛尔计)为6∶1的比例与B-Cubs和盐酸普萘洛尔水溶液进行混合,以10%氢氧化钠溶液分别调节外水相pH至7.5、8.0、8.5、9.0,于20 ℃水浴(载药温度)下600 r/min磁力搅拌15 min(载药时间),制得PPL-Cubs中药物浓度为1%,测定对包封率等参数影响(见表6)。结果表明,PPL-Cubs的包封率随外水相pH值的提高逐渐增加,当外水相pH值≥8.5时,包封率增加趋势渐小;外水相pH值对PPL-Cubs的粒径和多分散指数无明显影响。
表 5 盐酸普萘洛尔在不同pH PBS中的溶解度(n=3)pH 溶解度(mg/ml) 4.5 53.50±4.22 5.5 51.70±2.34 6.5 52.60±1.53 7.5 49.80±2.14 8.5 8.50±1.15 9.5 1.41±0.33 10.5 0.87±0.08 表 6 外水相pH值的考察(n=3)pH EE(%) 粒径(nm) 多分散指数 7.5 71.29±2.58 96.8±2.6 0.063±0.012 8.0 86.24±1.05 97.5±2.3 0.054±0.006 8.5 92.55±1.27 96.3±1.9 0.045±0.005 9.0 94.58±1.57 97.6±1.7 0.051±0.006 2.5.2 内水相pH值的考察
按照“2.5.1”项下方法,固定外水相pH为8.5时,分别考察内水相pH(3.0、4.0、5.0、6.0)对制得PPL-Cubs包封率等参数的影响(见表7)。结果表明,不同内水相pH的B-Cubs对制得的PPL-Cubs包封率无明显差异,对PPL-Cubs的粒径和多分散指数亦无明显影响。
表 7 内水相pH值的考察(n=3)pH EE(%) 粒径(nm) 多分散指数 3.0 92.55±1.27 96.3±1.9 0.045±0.005 4.0 91.85±1.05 97.5±2.3 0.054±0.006 5.0 91.62±1.27 96.3±1.9 0.045±0.005 6.0 89.33±1.57 97.6±1.7 0.051±0.006 2.5.3 载体/药物的考察
按照“2.5.1”项下方法,固定外水相pH为8.5时,分别考察载体/药物(5∶1、6∶1、7∶1、8∶1)对制得PPL-Cubs包封率等参数的影响(见表8)。结果表明,当载体/药物≥6时,PPL-Cubs的包封率不再增加;载体/药物比值对PPL-Cubs的粒径和多分散指数无明显影响。
表 8 载体/药物的考察(n=3)载体/药物 EE(%) 粒径(nm) 多分散指数 5∶1 90.93±1.52 98.5±2.7 0.076±0.015 6∶1 92.55±1.27 96.3±1.9 0.045±0.005 7∶1 92.06±2.37 97.5±2.1 0.077±0.015 8∶1 92.41±2.58 98.1±2.4 0.102±0.025 2.5.4 载药温度的考察
按照“2.5.1”项下方法,固定外水相pH为8.5时,分别考察载药温度(20、30、40、50 ℃)对制得PPL-Cubs包封率等参数的影响(见表9)。结果表明,载药温度对PPL-Cubs的包封率、粒径和多分散指数无明显影响。
表 9 载药温度的考察(n=3)载药温度(℃) EE(%) 粒径(nm) 多分散指数 20 92.55±1.27 96.3±1.9 0.045±0.005 30 91.05±1.95 96.9±2.3 0.068±0.021 40 91.38±2.08 97.1±2.6 0.066±0.012 50 90.55±1.75 97.2±2.1 0.053±0.018 2.5.5 载药时间的考察
按照“2.5.1”项下方法,固定外水相pH为8.5时,分别考察载药时间(15、30、45、60 min)对制得PPL-Cubs包封率等参数的影响(见表10)。结果表明,载药时间对PPL-Cubs的粒径和多分散指数无明显影响。
表 10 载药时间的考察(n=3)载药时间(min) EE(%) 粒径(nm) 多分散指数 15 92.55±1.27 96.3±1.9 0.045±0.005 30 92.09±1.54 97.2±2.4 0.071±0.013 45 92.01±2.01 97.5±1.6 0.065±0.024 60 91.86±1.86 98.1±1.9 0.075±0.026 2.5.6 B-Cubs粒径和多分散指数的考察
通过调整高压均质压力,制备不同粒径B-Cubs。按照“2.5.1”项下方法,固定外水相pH为8.5,分别考察B-Cubs粒径和多分散指数对制得PPL-Cubs包封率等参数的影响(见表11)。结果表明,B-Cubs的粒径和多分散指数不影响所制得PPL-Cubs的包封率,但B-Cubs的粒径和多分散指数基本决定了制得PPL-Cubs的粒径和多分散指数。
表 11 B-Cubs粒径和多分散指数的考察(n=3)载体 EE(%) PPL-Cubs 粒径(nm) 多分散指数 粒径(nm) 多分散指数 97.9±2.1 0.073±0.016 92.55±1.27 96.3±1.9 0.045±0.005 129.2±3.8 0.140±0.037 91.87±1.96 128.5±2.1 0.123±0.021 160.4±4.6 0.173±0.052 91.85±2.13 158.2±2.8 0.152±0.037 210.5±5.9 0.182±0.057 91.25±2.53 209.2±2.9 0.174±0.045 2.5.7 PPL-Cubs药物浓度的考察
按照“2.5.1”项下方法,固定外水相pH为8.5,分别考察药物浓度(0.1%、0.5%、1.0%、2.0%、3.0%)对制得PPL-Cubs包封率等参数的影响(见表12)。结果表明,随着PPL-Cubs中药物浓度的提高,包封率呈逐渐增加趋势,当药物浓度≥1%时,包封率增加趋势变慢。
表 12 PPL-Cubs中药物浓度的考察(n=3)浓度 EE(%) 粒径(nm) 多分散指数 0.1 51.83±3.17 97.2±2.4 0.057±0.013 0.5 81.87±2.12 96.3±2.1 0.062±0.012 1.0 92.55±1.27 96.3±1.9 0.045±0.005 2.0 94.42±1.37 96.3±1.9 0.045±0.005 3.0 95.87±1.28 97.8±2.5 0.042±0.007 2.6 pH梯度法制备PPL-Cubs的最优处方及制备工艺
精密称取单油酸甘油酯25.0 g,40 ℃水浴加热使融化,为A相;精密称取泊洛沙姆407 5.0 g,加入70 g纯化水,40 ℃水浴加热使溶解,并用1%磷酸溶液调节pH至3.0,为B相;于40 ℃水浴及600 r/min搅拌速度下,将A相缓慢滴加到B相中,待磁力搅拌30 min后,再在900 bar下高压均质7次,得B-Cubs。取盐酸普萘洛尔1 g,溶解于适量纯化水中,得盐酸普萘洛尔水溶液;将盐酸普萘洛尔水溶液加入24 g B-Cubs中,搅拌均匀,用10%氢氧化钠溶液调节pH至8.5,于20 ℃水浴持续搅拌15 min,再放置至室温,即得PPL-Cubs
3. 讨论
立方液晶纳米粒常用的制备方法包括注入法、熔融-分散法、热处理法、喷雾干燥法等[6]。试验前期以粒径、包封率等为评价指标筛选了最佳制备方法为注入法,并优化了其最佳处方制备工艺,结果制得的载药立方液晶纳米粒包封率较低(约50%)[7]。立方液晶是两亲性脂质分子分散在过量水中形成的含双连续水区和闭合脂质双分子层的蜂窝状液晶结构;水溶性分子被包封于立方液晶水道中,脂溶性分子被包封于脂质双层膜中,两亲性分子则贯穿其中。盐酸普萘洛尔在酸性环境下具有较高的溶解性,常规方法制得的盐酸普萘洛尔立方液晶纳米粒pH约为3.5,如何让其具有进入立方液晶载体内相的“动力”是提高载药立方液晶纳米粒包封率的关键。因此,本研究引入“pH梯度法”,通过创造高溶解度内环境(低pH值内水相)和低溶解度外环境(高pH值外水相),给盐酸普萘洛尔提供进入立方液晶载体内相的“动力”。离子化的盐酸普萘洛尔在调节pH的过程中逐渐变为分子形态的普萘洛尔而进入脂质区,脂质区的普萘洛尔分子接触内水相酸性环境而被离子化,内水相中离子化的盐酸普萘洛尔无法再通过脂质区而被捕获于内水相。结果表明,pH梯度法显著提高了PPL-Cubs的包封率,包封率达到90%。
-
表 1 各组miR-449a表达水平比较
组别 miR-449a 对照组 1.16±0.08 LEF组 0.58±0.05* LEF+mimic组 2.04±0.16# mimic组 6.32±0.63* LEF+inhibitor组 0.41±0.06# inhibitor组 0.77±0.07* *P<0.05,与对照组比较;#P<0.05,与LEF组比较 表 2 各组细胞的相对细胞活力比较(%)
组别 24 h 48 h 72 h 对照组 100.07±1.83 100.76±2.07 100.16±1.96 LEF组 103.67±2.06 110.83±2.15* 121.17±2.65* LEF+mimic组 99.98±2.14 98.57±2.11# 97.37±2.01# mimic组 97.54±1.97 91.79±2.35* 81.77±1.78* LEF+inhibitor组 107.68±2.08 118.67±3.07# 132.84±2.07# inhibitor组 104.31±1.79 111.38±2.67* 119.35±2.18* *P<0.05,与对照组比较;#P<0.05,与LEF组比较 表 3 各组细胞增殖和凋亡情况比较
组别 克隆形成数目(个) 细胞凋亡率(%) 对照组 54.32±4.36 5.53±0.94 LEF组 87.66±7.24* 3.11±0.76* LEF+mimic组 60.82±6.06# 6.73±1.26# mimic组 31.12±3.78* 17.32±3.28* LEF+inhibitor组 119.35±5.08# 2.14±0.62# inhibitor组 92.71±7.89* 3.45±0.83* *P<0.05,与对照组比较;#P<0.05,与LEF组比较 表 4 各组细胞α-SMA相对荧光强度比较
组别 α-SMA Col I 对照组 1.02±0.11 1.24±0.14 LEF组 2.36±0.47* 2.57±0.38* LEF+mimic组 1.53±0.34# 1.89±0.25# mimic组 0.47±0.05* 0.45±0.06* LEF+inhibitor组 3.25±0.18# 4.13±0.54# inhibitor组 2.48±0.15* 3.11±0.39* *P<0.05,与对照组比较;#P<0.05,与LEF组比较 表 5 各组p-JNK/JNK相对水平比较
组别 p-JNK JNK p-JNK/JNK 对照组 2.04±0.18 2.16±0.16 0.94±0.09 LEF组 2.87±0.31 1.05±0.10 2.73±0.18* LEF+mimic组 1.67±0.19 2.24±0.21 0.75±0.07# mimic组 0.96±0.11 3.11±0.28 0.31±0.04* LEF+inhibitor组 3.04±0.24 1.10±0.10 2.76±0.25# inhibitor组 3.78±0.34 1.02±0.09 3.71±0.31* *P<0.05,与对照组比较;#P<0.05,与LEF组比较 -
[1] JOSHI S, SINGH A R, WONG S S, et al. Rac2 is required for alternative macrophage activation and bleomycin induced pulmonary fibrosis; a macrophage autonomous phenotype[J]. PLoS One,2017,12(8):e0182851-e0182856. doi: 10.1371/journal.pone.0182851 [2] REN L M, LI R, CHEN L N, et al. Efficacy and safety of weekly leflunomide for the treatment of early rheumatoid arthritis: a randomized, multi-center study[J]. Int J Rheum Dis,2016,19(7):651-657. doi: 10.1111/1756-185X.12677 [3] BOCKHORN J, DALTON R, NWACHUKWU C, et al. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11[J]. Nat Commun,2013,4: 4(1):1393-1398. [4] LI J, LU M J, JIN J, et al. MiR-449a suppresses tamoxifen resistance in human breast cancer cells by targeting ADAM22[J]. Cell Physiol Biochem,2018,50(1):136-149. doi: 10.1159/000493964 [5] HAN R H, JI X M, RONG R, et al. MiR-449a regulates autophagy to inhibit silica-induced pulmonary fibrosis through targeting Bcl2[J]. J Mol Med,2016,94(11):1267-1279. doi: 10.1007/s00109-016-1441-0 [6] TANG Y N, GENG Q, CHEN D, et al. Germline proliferation is regulated by somatic endocytic genes via JNK and BMP signaling in Drosophila[J]. Genetics,2017,206(1):189-197. doi: 10.1534/genetics.116.196535 [7] HUANG Y, MA S F, ESPINDOLA M S, et al. Microbes are associated with host innate immune response in idiopathic pulmonary fibrosis[J]. Am J Respir Crit Care Med,2017,196(2):208-219. doi: 10.1164/rccm.201607-1525OC [8] DENG F, ZHANG P, FENG J, et al. Effect of leflunomideon inflammatory factors and immune function in rats with chronic glomerulonephritis[J]. J Sichuan Univ Med Sci Ed,2016,47(2):217-221. [9] PATEL A, ZHANG S J, PARAMAHAMSA M, et al. Leflunomide induces pulmonary and hepatic CYP1A enzymes via aryl hydrocarbon receptor[J]. Drug Metab Dispos,2015,43(12):1966-1970. doi: 10.1124/dmd.115.066084 [10] SCOTT D L. Interstitial lung disease and disease modifying anti-rheumatic drugs[J]. Lancet,2004,363(9416):1239-1240. [11] SAKAI F, NOMA S, KURIHARA Y, et al. Leflunomide-related lung injury in patients with rheumatoid arthritis: imaging features[J]. Mod Rheumatol,2005,15(3):173-179. doi: 10.3109/s10165-005-0387-9 [12] RYU C, SUN H X, GULATI M, et al. Extracellular mitochondrial DNA is generated by fibroblasts and predicts death in idiopathic pulmonary fibrosis[J]. Am J Respir Crit Care Med,2017,196(12):1571-1581. doi: 10.1164/rccm.201612-2480OC [13] ISHIDA Y, KIMURA A, NOSAKA M, et al. Essential involvement of the CX3CL1-CX3CR1 axis in bleomycin-induced pulmonary fibrosis via regulation of fibrocyte and M2 macrophage migration[J]. Sci Rep,2017,7(1):16833-16837. doi: 10.1038/s41598-017-17007-8 [14] LIANG L, HUIJUAN L, LIANJIANG D, et al. MiR-449a suppresses LDHA-mediated glycolysis to enhance the sensitivity of non-small cell lung cancer cells to ionizing radiation[J]. Oncol Res Feat Preclin Clin Cancer Therap,2017,26(4):547-556. [15] WU D D, LIU J, CHEN J L, et al. MiR-449a suppresses tumor growth, migration, and invasion in non-small cell lung cancer by targeting a HMGB1-mediated NF-κB signaling pathway[J]. Oncol Res,2019,27(2):227-235. doi: 10.3727/096504018X15213089759999 [16] ZHANG J, GAO F D, NI T J, et al. Linc-POU3F3 is overexpressed in in-stent restenosis patients and induces VSMC phenotypic transformation via POU3F3/miR-449a/KLF4 signaling pathway[J]. Am J Transl Res,2019,11(7):4481-4490. [17] SATO-MATSUBARA M, MATSUBARA T, DAIKOKU A, et al. Fibroblast growth factor 2(FGF2) regulates cytoglobin expression and activation of human hepatic stellate cells via JNK signaling[J]. J Biol Chem,2017,292(46):18961-18972. doi: 10.1074/jbc.M117.793794 [18] YANG Y L, YE Y J, QIU Q, et al. Triptolide inhibits the migration and invasion of rheumatoid fibroblast-like synoviocytes by blocking the activation of the JNK MAPK pathway[J]. Int Immunopharmacol,2016,41:8-16. doi: 10.1016/j.intimp.2016.10.005 [19] SHINGYOCHI Y, KANAZAWA S, TAJIMA S, et al. A low-level carbon dioxide laser promotes fibroblast proliferation and migration through activation of akt, ERK, and JNK[J]. PLoS One,2017,12(1):e0168937-e0168937. doi: 10.1371/journal.pone.0168937 -





 下载:
下载:
 下载:
下载: