-
据2019年国际糖尿病联盟发布的全球糖尿病地图,我国成人糖尿病患者数量位居世界第一,已达1.164亿人[1]。与非糖尿病人群相比,2型糖尿病(T2DM)患者发生心血管疾病的风险可增加2~4倍,其中心肌梗死和卒中是T2DM患者死亡的主要原因[2]。美国FDA和欧洲药品管理局要求加强对现有降糖药物的心血管安全性监测,同时所有新型降糖药物上市前必须通过心血管安全性研究(CVOT)[3]。2015年EMPA-REG OUTCOME试验结果揭晓了恩格列净对T2DM患者有显著的心血管保护作用,随后LEADER、CANVAS试验相继证实了利拉鲁肽、卡格列净等的心血管益处。目前,国内外对钠-葡萄糖共转运蛋白2(SGLT-2)抑制剂、胰高血糖素样多肽-1(GLP-1)受体激动剂的心血管保护作用开展了广泛的研究,现就SGLT-2抑制剂、GLP-1受体激动剂的心血管安全性研究进展做一综述。
-
肾脏重吸收葡萄糖主要依赖SGLT-2,其主要分布于近曲小管S1、S2段管腔侧细胞膜上,介导90%葡萄糖的重吸收[4]。SGLT-2抑制剂通过抑制肾脏肾小管中的SGLT-2,抑制葡萄糖重吸收,降低肾糖阈,促进尿葡萄糖排泄,起降低血液循环中葡萄糖水平的作用。目前,FDA批准上市的SGLT-2抑制剂有:恩格列净、卡格列净、达格列净、埃格列净。在我国被批准上市的有:恩格列净、卡格列净和达格列净。
-
恩格列净、卡格列净、达格列净、埃格列净的心血管安全性试验分别称为EMPA-REG OUTCOME、CANVAS、DECLARE-TIMI 58、VERTIS-CV试验,均为大型、双盲、随机、安慰剂对照的前瞻性试验。试验均以心血管死亡、非致死性心肌梗死和非致死性卒中的主要心血管事件(MACE)为终点,评价SGLT-2抑制剂对T2DM患者的心血管作用。试验结果(见表1)显示,与安慰剂相比,恩格列净、卡格列净均可降低T2DM患者心血管事件的发生风险(P=0.038和0.02),而达格列净在心血管事件的风险与安慰剂相比无统计学差异;同时研究显示,恩格列净可降低T2DM患者的心血管死亡、全因死亡及心力衰竭住院风险(P<0.001、P<0.001、P=0.002),卡格列净、达格列净可降低T2DM患者心衰住院风险[5-7]。在心肌梗死和卒中风险事件,恩格列净与安慰剂相比无统计学差异;卡格列净与安慰剂相比在心血管死亡、非致死性心肌梗死或非致死性卒中事件无统计学差异。VERTIS-CV试验结果未发布,埃格列净对T2DM患者的心血管作用尚不明确。
表 1 SGLT-2抑制剂的心血管安全性研究
试验项目 EMPA-REG OUTCOME CANVAS DECLARE-TIMI 58 干预措施 恩格列净(10~25 mg/d) 卡格列净(100~300 mg/d) 达格列净(10 mg/d) 样本量(例,T/C①) 4687/2333 5795/4347 8582/ 8578 平均年龄(年) 63.1 63.3 64.0 中位随访期(年) 3.1 2.4 4.2 合并ASCVD(例,%) 7020(100%) 6656(65.6%) 6974(40.6%) 主要事件(HR,95%CI) 0.86(0.74~0.99) 0.86(0.75~0.97) 0.93(0.84~1.03) 心肌梗死 0.87(0.70~1.09) 0.85(0.69~1.05) NA 缺血性卒中 1.18(0.89~1.56) 0.90(0.71~1.15) 1.01(0.84~1.21) 心血管死亡 0.62(0.49~0.77) 0.87(0.72~1.06) 0.98(0.82~1.17) 全因死亡 0.68(0.57~0.82) 0.87(0.74~1.01) 0.93(0.82~1.04) 心力衰竭住院 0.65(0.50~0.85) 0.67(0.52~0.87) 0.73(0.61~0.88) 注:T/C:试验组/对照组;ASCVD,动脉粥样硬化性心血管疾病;NA,无数据。 -
纳入SGLT-2抑制剂的EMPA-REG OUTCOME、CANVAS、DECLARE-TIMI 58试验进行Meta分析,共34322例患者,其中60.2%合并动脉粥样硬化性心血管疾病(ASCVD),疗效包括:心血管事件,心血管死亡、心力衰竭住院和肾病进展的复合事件[8]。研究表明,SGLT-2抑制剂可降低T2DM患者发生心血管事件的风险(HR 0.89, 95% CI 0.83~0.96, P=0.0014),但结果仅在合并ASCVD的T2DM人群中具有统计学差异(HR 0.86, 95% CI 0.80~0.93),对伴有ASCVD高危因素的T2DM患者,SGLT-2抑制剂与安慰剂相比在主要结果无统计学差异(HR 1.00, 95% CI 0.87~1.16)。同时研究显示,在合并或伴有ASCVD高危因素,以及合并/无心力衰竭T2DM人群中,SGLT-2抑制剂均可降低心血管死亡或心力衰竭住院风险(HR 0.77, 95% CI 0.71~0.84, P<0.0001)[8]。另一项纳入了上述四项试验的Meta分析结果显示,SGLT-2抑制剂可降低合并或伴有ASCVD高危因素的T2DM患者心血管事件的发生风险(HR 0.88, 95% CI 0.82~0.94, P<0.001)[9]。此外,另一项Meta分析提示SGLT-2抑制剂可降低T2DM合并慢性肾脏病(CKD)患者心血管死亡、非致死性心肌梗死、非致死性卒中的复合事件发生风险(RR 0.81, 95% CI 0.70~0.94),心力衰竭住院风险可降低39%(95% CI 0.48~0.78)[10]。
-
CVD-REAL是一项研究SGLT-2抑制剂对T2DM患者的心血管益处,共纳入309 056名T2DM患者,其中87%的患者无心血管疾病史。结果显示,SGLT-2抑制剂可降低T2DM患者的心衰住院风险(HR 0.61, 95% CI 0.51~0.73, P<0.001),降低全因死亡风险(HR 0.49, 95% CI 0.41~0.57, P<0.001),心衰住院和全因死亡事件的复合事件风险降低46%(95% CI 0.48~0.60, P<0.001)[11]。同时CVD-REAL试验的亚组分析提示,与其他降糖药物相比,SGLT-2抑制剂可降低T2DM患者的心肌梗死和卒中风险(HR 0.85, 95% CI 0.72~1.00, P=0.05; HR 0.83, 95% CI 0.71~0.97, P =0.02)[12]。OBSERVE-4D研究纳入了来自4个美国大型医保报销数据库的1 060 449例T2DM患者,头对头地比较卡格列净与其他SGLT-2抑制剂或其他非SGLT-2抑制剂,对T2DM患者心力衰竭住院的影响。研究结果显示,与非SGLT-2抑制剂的降糖药相比,卡格列净降低T2DM患者因心力衰竭住院的风险(HR 0.39, 95% CI 0.26~0.60),卡格列净与其他SGLT-2抑制剂对T2DM患者心力衰竭住院风险的影响无统计学差异(HR 0.90, 95% CI 0.71~1.13);对于合并心血管疾病的T2DM人群,卡格列净可降低心力衰竭住院风险(HR 0.44, 95% CI 0.36~0.54),但与其他SGLT-2抑制剂相比,研究结果无统计学差异(HR 0.70, 95% CI 0.30~1.63)[13]。恩格列净的EMPRISE试验结果显示,与西格列汀相比,恩格列净可降低T2DM患者心力衰竭风险(HR 0.50, 95% CI 0.28~0.91)[14]。
-
GLP-1是一种肠促胰素,分布于胰腺、心脏、肺、脑和胃肠道等区域,通过葡萄糖浓度依赖性的方式促进胰岛β细胞分泌胰岛素,抑制胰岛α细胞释放胰高血糖素[15-16]。GLP-1受体激动剂通过提高T2DM患者体内GLP-1受体活性,促进胰岛素分泌,抑制胰高血糖素释放,延缓胃排空,中枢性食欲抑制等多种机制调控机体血糖稳态。目前FDA批准上市的GLP-1受体激动剂有利拉鲁肽、索马鲁肽、艾塞那肽、利司那肽、度拉糖肽、阿必鲁肽。中国食品药品监督管理总局批准上市的GLP-1受体激动剂有利拉鲁肽、艾塞那肽、度拉糖肽、贝那鲁肽和洛塞那肽。
-
GLP-1受体激动剂相关的大型心血管安全性试验有LEADER、SUSTAIN-6、EXSCEL、ELIXA试验,分别研究了利拉鲁肽、索马鲁肽、艾塞那肽和利司那肽对T2DM患者心血管疾病的影响,其中LEADER、SUSTAIN-6、EXSCEL均以心血管事件为主要结局,ELIXA以心血管死亡、非致死性心肌梗死、非致死性卒中和不稳定性心绞痛住院的复合终点为主要结局[17-20]。试验结果(见表2)显示,利拉鲁肽、索马鲁肽可降低心血管事件发生风险(P=0.01、P=0.02);此外,利拉鲁肽可降低T2DM患者发生心血管死亡和全因死亡的风险(P=0.007、P=0.02),索马鲁肽可降低非致死性卒中风险(P=0.04);利拉鲁肽与安慰剂在非致死性心肌梗死、非致死性卒中和心力衰竭住院事件方面无统计学差异;在心肌梗死、全因死亡、心血管死亡及心衰住院终点方面,索马鲁肽与安慰剂无明显统计学差异[17-18]。与安慰剂相比,艾塞那肽和利司那肽对T2DM合并心血管疾病的患者具有非劣效性,两者在主要终点处均无统计学差异(P=0.81、P=0.06)[19-20]。
表 2 GLP-1受体激动剂的心血管安全性研究
试验项目 EXSCEL SUSTAIN-6 LEADER ELIXA 干预措施 艾塞那肽(2 mg/w) 索马鲁肽(0.5~1.0 mg/w) 利拉鲁肽(0.6~1.8 mg/d) 利司那肽(10~20 μg/d) 样本量(例,T/C①) 7256/7396 1648/1649 4668/4672 3034/3034 中位随访期(年) 3.2 2.1 3.5 2.1 合并ASCVD(例,%) 10782(73.1%) 2735(83%) 6764(72.4%) 6068(100%) 主要终点(HR,95%CI) 0.91 (0.83~1.00) 0.74(0.58~0.95) 0.87 (0.78~0.97) 1.02 (0.89~1.17) 心肌梗死 0.97 (0.85~1.10) 0.74 (0.51~1.08) 0.86 (0.73~1.00) 1.03 (0.87~1.22) 缺血性卒中 0.85 (0.70~1.03) 0.61 (0.38~0.99) 0.86 (0.71~1.06) 1.11 (0.47~2.62) 心血管死亡 0.88 (0.76~1.02) 0.98 (0.65~1.48) 0.78 (0.66~0.93) 0.98 (0.78~1.22) 全因死亡 0.86 (0.77~0.97) 1.05 (0.74~1.50) 0.85 (0.74~0.97) 0.94 (0.78~1.13) 心力衰竭住院 0.94 (0.78~1.13) 1.11 (0.77~1.61) 0.87 (0.73~1.05) 0.96 (0.75~1.23) 注:T/C:试验组/对照组。 -
GLP-1受体激动剂的心血管安全性试验结果不一,为验证GLP-1受体激动剂对心血管的保护作用,Bethel等[21]将上述四项大型CVOT试验纳入Meta分析,结果显示,GLP-1受体激动剂可降低心血管事件发生风险(HR 0.90, 95%CI 0.82~0.99, P=0.033),同时心血管死亡风险降低13%(95%CI 0.76~0.96, P=0.007),全因死亡风险降低12%(95%CI 0.81~0.95, P=0.002);但在心肌梗死、卒中、不稳定性心绞痛住院或心力衰竭住院方面,GLP-1受体激动剂与安慰剂相比均无统计学差异。Kristensen等[22]根据现有临床试验证据进行Meta分析和系统回顾,结果显示,GLP-1受体激动剂可降低T2DM患者心血管事件的发生风险(HR 0.88, 95% CI 0.82~0.94, P<0.0001),其中,心血管死亡风险降低12%(95% CI 0.81~0.96, P=0.003),卒中风险降低12%(95% CI 0.83~0.95, P=0.001),心力衰竭住院风险可降低9%(95% CI 0.83~0.99, P=0.028)。
-
一项为期36个月的利拉鲁肽研究[23]共纳入307例患者,其中19.9%的患者合并心血管疾病,研究显示心血管事件的发生率低于LEADER试验(2.59比3.4 /100人·年),但合并心血管疾病的T2DM患者再发心血管不良事件的风险明显增高。另一项基于人群的开放性队列研究[24]显示,接受GLP-1治疗的T2DM患者较未经GLP-1受体激动剂治疗的T2DM患者全因病死率降低(RR 0.64, 95%CI 0.56~0.74, P<0.0001)。
-
EMPA-REG OUTCOME、CANVAS、LEADER、SUSTAIN-6试验,分别为恩格列净、卡格列净、利拉鲁肽、索马鲁肽具有心血管保护作用提供了可靠的临床证据。因此《2020ADA/EASD糖尿病诊疗标准》推荐,无论糖化血红蛋白水平如何,对于合并ASCVD、伴有ASCVD高危因素、合并糖尿病肾病或合并心力衰竭的T2DM患者,建议将具有明确心血管益处的SGLT-2抑制剂作为降糖治疗方案的药物之一,同时推荐具有明确心血管益处的GLP-1受体激动剂作为合并或伴有ASCVD高危因素的T2DM患者治疗药物之一[25]。但指南未明确推荐SGLT-2抑制剂和GLP-1受体激动剂的具体药品和用法用量,同时具有心血管保护作用的新型降糖药物之间缺乏头对头的随机对照试验,治疗方案之间的有效性和安全性差异尚不明确。因此,有研究通过网状Meta分析,间接比较T2DM患者应用SGLT-2抑制剂和GLP-1受体激动剂的心血管益处。
-
Täger等[26]根据卡格列净、达格列净、恩格列净和埃格列净的大型临床随机对照试验,以全因死亡为主要终点,心血管死亡和心力衰竭恶化为次要终点,通过网状Meta分析研究,间接比较了4种SGLT-2抑制剂对T2DM患者的心血管安全性。试验结果显示,与安慰剂相比,卡格列净和恩格列净可改善T2DM患者的全因死亡风险(RR 0.85, 95% CI 0.75~0.97; RR 0.67, 95% CI 0.55~0.80),降低心血管死亡风险(RR 0.85, 95% CI 0.73~0.99; RR 0.61, 95% CI 0.49~0.77),减少心力衰竭恶化的风险(RR 0.62, 95% CI 0.52~0.75; RR 0.66, 95% CI 0.50~0.86)。达格列净可降低T2DM患者心力衰竭恶化的风险(RR 0.75, 95% CI 0.62~0.88),与卡格列净和达格列净相比,恩格列净对降低T2DM患者的全因死亡和心血管死亡风险有明显优势。埃格列净与安慰剂或其他SGLT-2抑制剂相比,在主要结果和次要结果方面均无统计学差异。
-
一项纳入了LEADER、SUSTAIN-6、EXSCEL、ELIXA试验的网状Meta分析[27]比较了GLP-1受体激动剂利拉鲁肽、索马鲁肽、艾塞那肽和利司那肽对T2DM患者的心血管安全性。试验结果显示,与安慰剂相比,GLP-1受体激动剂可降低T2DM患者的心血管死亡风险(RR 0.87, 95% CI 0.78~0.96),其中利拉鲁肽可减少T2DM患者心血管死亡风险21%。在心血管死亡、心力衰竭住院、非致死性心肌梗死、非致死性卒中方面,利拉鲁肽、索马鲁肽、艾塞那肽和利司那肽分别与其他3种GLP-1受体激动剂相比均无统计学差异。但研究显示,与其他3种GLP-1受体激动剂相比,利拉鲁肽是降低T2DM患者心血管死亡和心衰住院风险的首选药物,索马鲁肽对降低T2DM患者的非致死性卒中和非致死性心肌梗死具有明显优势。
-
对SGLT-2抑制剂和GLP-1受体激动剂的14个双盲、随机对照试验进行网状Meta分析,比较两者对T2DM患者发生心血管不良事件的影响[28]。试验共纳入121 047例患者,主要结果为MACE,次要结果为非致死性卒中、非致死性心肌梗死、心血管死亡、全因死亡、心衰住院等[28]。结果显示,SGLT-2抑制剂与安慰剂相比可降低T2DM患者的心血管死亡风险(OR 0.82, 95% CI 0.73~0.93)和全因死亡风险(OR 0.84, 95% CI 0.77~0.92),SGLT-2抑制剂和GLP-1受体激动剂均可降低MACE的发生风险(OR 0.88, 95% CI 0.82~0.95; OR 0.87, 95% CI 0.82~0.93),降低心衰住院风险(OR 0.68, 95% CI 0.61~0.77; OR 0.87, 95% CI 0.82~0.93),SGLT-2抑制剂降低T2DM患者心衰住院风险的优势高于GLP-1受体激动剂(OR 0.79, 95% CI 0.69~0.90),而GLP-1受体激动剂可降低T2DM患者的非致死性卒中风险(OR 0.88, 95% CI 0.77~0.99),SGLT-2抑制剂在非致死性卒中风险方面与安慰剂相比无统计学差异[28]。
一项以全因死亡率为主要结果的网状Meta分析[29]提示,SGLT-2抑制剂和GLP-1受体激动剂均可降低T2DM患者的全因病死率(HR 0.80, 95% CI 0.71~0.89; HR 0.88, 95% CI 0.81~0.94),减少T2DM患者的心血管死亡风险(HR 0.79, 95% CI 0.69~0.91; HR 0.85, 95% CI 0.74~0.94)。与对照组相比,SGLT-2抑制剂可减少T2DM患者的心衰住院风险(HR 0.62, 95% CI 0.54~0.72)和卒中风险(HR 0.86, 95% CI 0.77~0.97)。
-
2型糖尿病患者心血管事件发生率明显高于普通人群,与微血管并发症不同,研究显示严格控制血糖对其降低动脉粥样硬化性心血管疾病发生风险的效果一般[30]。自2008年以来,多项心血管安全性试验逐渐证实了SGLT-2抑制剂、GLP-1受体激动剂不增加2型糖尿病患者发生心血管事件的风险,其中研究证实恩格列净、卡格列净、利拉鲁肽、索马鲁肽对T2DM患者具有心血管获益,为2型糖尿病治疗策略提供了新的方向。对现有的临床证据进行Meta分析,结果提示,SGLT-2抑制剂可降低心血管事件的发生风险,在合并或伴有ASCVD高危因素的T2DM人群中结果一致;GLP-1受体激动剂对于合并或伴有ASCVD高危因素的T2DM患者也具有一定的心血管保护作用。但达格列净、艾塞那肽和利司那肽在CVOT中未显示有心血管益处,具有心血管保护作用的恩格列净、卡格列净、利拉鲁肽和索马鲁肽也缺乏头对头的研究,SGLT-2抑制剂和GLP-1受体激动剂的疗效优势尚不明确,需要进一步研究。目前,多种新型降糖药物正在进行心血管安全性试验,其研究结果将陆续公布,相信未来有更多降糖药物对2型糖尿病患者具有心血管保护作用。
Advances in cardiovascular safety of SGLT-2 inhibitors and GLP-1 receptor agonists
-
摘要: 2型糖尿病是动脉粥样硬化性心血管疾病发病的高危因素。研究发现,钠-葡萄糖共转运蛋白2(SGLT-2)抑制剂、胰高血糖素样多肽-1(GLP-1)受体激动剂对2型糖尿病合并心血管疾病的患者具有心血管保护作用。故从心血管安全性试验及其Meta分析、网状Meta分析方面,对SGLT-2抑制剂、GLP-1受体激动剂的心血管安全性研究进展进行归纳和总结。
-
关键词:
- SGLT-2抑制剂 /
- GLP-1受体激动剂 /
- 2型糖尿病 /
- 心血管安全性
Abstract: Type 2 diabetes is a high risk factor for atherosclerotic cardiovascular disease. Studies have found that SGLT-2 inhibitor and GLP-1 receptor agonists have cardiovascular protective effects in patients with type 2 diabetes and cardiovascular disease. Therefore, from the aspects of cardiovascular safety test and its Meta-analysis and net-like Meta-analysis, the research progress of cardiovascular safety of SGLT-2 inhibitors and GLP-1 receptor agonists is summarized.-
Key words:
- SGLT-2 inhibitors /
- GLP-1 receptor agonists /
- type 2 diabetes /
- cardiovascular safety
-
补体系统是人体重要的免疫防御系统之一,是由30多种广泛存在于血清、组织液和细胞膜表面的蛋白质组成的,具有精密调控机制的蛋白质反应系统,其主要通过3种途径激活:经典途径、旁路途径和甘露糖结合凝集素途径。补体系统正常激活,可在靶细胞上形成膜攻击复合物,导致靶细胞的溶解,补体的这一功能在机体的免疫系统中起重要的防御和免疫监视作用,对抵御外来微生物的入侵和维持机体平衡有重要的作用。然而该系统的过度激活将释放炎性过敏毒素C3a和C5a,具有化学诱导作用的C5a能趋化嗜中性粒细胞、中核细胞和嗜酸性粒细胞,这些细胞释放蛋白酶和具有趋化作用细胞因子,进一步聚集T、B淋巴细胞和其他炎性细胞,从而促进炎症反应的发生,引起系统性红斑狼疮、类风湿性关节炎、动脉粥样硬化、肾小球肾炎等[1-2]。近年来已有研究表明[3],补体系统的激活是类风湿性关节炎中慢性滑膜炎的发病因素之一。因此,抑制补体系统的过度激活可能是治疗类风湿性关节炎的重要机制之一。
三色片为复旦大学附属中山医院的院内制剂,由雷公藤、黄芪和丹参三味药材按1∶1∶1的比例配伍组成,在临床上用于治疗类风湿性关节炎、系统性红斑狼疮、银屑病和湿疹等结缔组织疾病。我院临床医生在长期的医疗实践中总结出来的经验方,效果显著[4]。组方中雷公藤,性味辛寒,有大毒,归肝、肾经,具有清热解毒、活血化瘀、通络止痛、杀虫止痒等功效。现代研究表明,雷公藤内酯醇对大鼠脑皮质内注射β-淀粉酶后补体C1q和C3的表达有抑制作用,表明雷公藤对补体系统有抑制作用,目前临床上广泛用于治疗类风湿性关节炎、系统性红斑狼疮、银屑病和湿疹等结缔组织疾病[5]。组方中的黄芪用于脾肺气血或中气下陷之症、卫气虚所致表虚自汗、气虚血滞导致的肢体麻木、关节痹痛等症,可联合治疗类风湿性关节炎[6]。黄芪在治疗2型糖尿病大鼠的研究中发现其能降低补体C3的水平,表明其对补体系统具有一定的调节作用[7-8]。丹参是最常用的活血化瘀中药之一,具有祛瘀止痛,养血安神的功效,现代药理学研究表明其还具有保护肝脏的功能[9],可拮抗雷公藤的肝毒性。本研究通过经典途径抗补体活性测定方法筛选出三色片醇提物的乙酸乙酯部位抗补体活性最佳,并采用UPLC-Q-TOF-MS法对该部位的化学成分进行结构表征,为三色片抗补体活性药效物质基础及治疗补体过度激活相关疾病提供科学依据。
1. 仪器、试剂与材料
Tripie TOF5600+型四级杆-飞行时间串联质谱仪,配备电喷雾电离源和CDS自动校正系统(美国Applied Biosystems公司);Peak view2.2和Master view1.1数据处理系统(美国Applied Biosystems公司);LC-30A超高效液相色谱仪,包括高压输液泵,自动进样器,柱温箱和在线脱气机(日本岛津公司);KQ5200E型超声清洗器(昆山市超声仪器有限公司);甲醇、乙腈(色谱纯,德国Merck公司);甲酸(色谱纯,美国Sigma-Aldrich公司); 蒸馏水(娃哈哈集团);三色片提取物由作者自制,现样品存放于复旦大学附属中山医院药剂科(SSP2018);补体、溶血素(自制);毛蕊异黄酮(批号:ST088101),雷公藤甲素(批号:ST020501),雷公藤内酯酮(批号:ST049901),丹参酮II A(ST014601)、黄芪甲苷(ST001601)(纯度≥ 98%,均购自上海斯丹德生物技术有限公司)。
2. 方法
2.1 三色片醇提物及各极性部位的制备
雷公藤、黄芪和丹参三味药材按1∶1∶1配伍,其中,黄芪和丹参加6倍量的水浸泡2 h后,煎煮2次,第一次1.5 h,第二次加水4倍量煎煮1 h,煎液滤过,合并滤液并浓缩至相对密度为1.10~1.20(70 ℃),加入2倍量的乙醇,静置沉淀24 h,取上清液备用。雷公藤分别加4倍量的乙醇加热回流2次,每次1.5 h,合并提取液,滤过,加入上述备用药液,混匀,回收乙醇至无醇味,浓缩后即得三色片醇提物,经现有的质量标准检验为制备三色片制剂合格的提取物。精密称取三色片醇提物2.0 g,置于100 ml萃取瓶中,加25 ml蒸馏水溶解后,用等量的石油醚、乙酸乙酯和正丁醇进行萃取,浓缩干燥后,放冷至室温,得到三色片醇提物的石油醚部位0.36 g,乙酸乙酯部位0.42 g,正丁醇部位0.56 g和水溶性部位。
2.2 经典途径的抗补体活性测定
取各极性部位样品2 mg溶于DMSO,采用BBS缓冲液稀释成不同浓度的样品,并加入临界浓度的补体(1∶80稀释的豚鼠血清),溶血素和2%绵羊红细胞(SRBC)。37 ℃水浴30 min,离心后取上清液在405 nm波长下测定吸光度(A)值。同时设置中药对照组(将等量的中药提取物加入BBS缓冲液中,用于测定中药本底A值)、补体组(取临界浓度的补体直接加入适量的BBS缓冲液、溶血素和2%SRBC,用于测定临界浓度补体所造成红细胞溶血的A值)和全溶血组(将2%SRBC加入水中使之全溶血,用于观察补体组是否达到或接近全溶血水平),并以肝素作为阳性对照组,计算溶血抑制率。以供试品浓度为横坐标(X),溶血抑制率为纵坐标(Y),计算CH50(经典途径50%抑制溶血所需供试品浓度)。溶血抑制率=1−(A中药−A中药对照)/A全溶血。
2.3 不同浓度的样品色谱与质谱条件
2.3.1 色谱条件
色谱柱为ACQUITY UPLC BEH C18(2.1 mm×100 mm,1.7 μm);流动相0.1%甲酸和水溶液(A)−乙腈(B);梯度洗脱:0~9 min,10%~23% B;9~13 min,23% B;13~28 min,23%~40% B;28~32 min,40%~50% B;32~37 min,50%~100% B;37~42 min,100% B;42~42.1 min,10%B;42.1~50 min,10% B;流速为0.25 ml/min,柱温为35 ℃;进样量为2 μl。
2.3.2 质谱条件
在正/负离子模式,离子源选择电喷雾离子化源(ESI);使用m/z 50~1250扫描范围;碰撞能量35 eV,碰撞能量叠加(35±15)eV;喷雾电压5 500 V;雾化气温度550 ℃;去簇电压100 V;雾化气和辅助气均为50 psi;气帘气25 psi;数据采集时间50 min;采用母离子触发的子离子(TOF-MS-IDA-MS/MS)扫描方式;多重质量亏损和动态背景扣除为触发二级的条件,满足该条件进行二级扫描。
2.4 对照品溶液的制备
精密称取毛蕊异黄酮、雷公藤甲素、雷公藤内酯酮、丹参酮Ⅱ A和黄芪甲苷对照品1.0 mg,加甲醇2 ml,溶解,摇匀,即得各对照品溶液。
2.5 供试品溶液的制备
取三色片醇提物的乙酸乙酯部位样品0.2 g,置于10 ml量瓶中,加入70%甲醇5 ml,超声处理(功率250 W,频率40 kHz)30 min,放冷至室温,70%甲醇定容至刻度,摇匀,滤过,取续滤液,即得供试品溶液。
2.6 三色片中化学成分数据库的建立
根据三色片中各药材化学成分研究文献,收集3种药材所含化合物成分的基本信息,包括化合物名称、分子式、精确分子量、准分子离子峰和碎片离子峰。通过精确分子量匹配,对照品的保留时间,二级谱所得到的离子碎片与文献报道进行比对,最终确定化合物的结构。
3. 结果与分析
3.1 三色片醇提物各极性部位的抗补体活性
分别对三色片醇提物的石油醚部位、乙酸乙酯部位和正丁醇部位进行经典途径的抗补体活性测定,以肝素为对照品,结果发现乙酸乙酯部位的抗补体活性最好,其抗补体活性略低于肝素钠,其次是正丁醇部位,结果见表1。
表 1 三色片提取物不同部位抗补体活性测定编号 研究对象 抗补体活性(CH50,μg/ml) 1 肝素 14.4±1.2 2 三色片-石油醚部位 − 3 三色片-乙酸乙酯部位 233.9±10.1 4 三色片-正丁醇部位 344.0±14.5 注:“—”表示该部位无抗补体活性。 3.2 三色片醇提物乙酸乙酯部位的UPLC-Q-TOF-MS分析
精密吸取对照品溶液和供试品溶液2 μl,采用“2.1”项下的色谱与质谱条件对样品进行分析,通过正、负离子全扫描,获得正、负离子模式下的总离子流图,见图1。
通过与对照品比对,分子离子峰质谱数据解析,与参考文献比对,共鉴定出三色片醇提物乙酸乙酯部位42个化合物,结果见表2。
表 2 三色片提取物中各成分主要碎片离子及谱峰归属化合物
编号tR/min 分子式 理论值(m/z) 模式 实测值(m/z) 误差(×10−6) 碎片离子(m/z) 化合物名称 参考文献 1 3.54 C7H6O3 139.039 0 [M+H]+ 139.039 4 3.0 121.028 7 原儿茶醛 [10] 2 4.76 C21H27N3O3 370.212 5 [M+H]+ 370.214 3 4.8 249.124 6,160.112 6,95.013 3,166.086 6,100.076 2,91.054 8 南蛇藤糠酰胺碱 [11] 3 6.63 C22H22O10 447.128 6 [M+H]+ 447.130 4 4.2 285.077 5,270.053 5,253.050 8,225.055 6,137.023 5 毛蕊异黄酮-7-O-β-D-葡萄糖苷 [12] 4 7.04 C23H29N3O2 380.233 3 [M+H]+ 380.235 1 4.8 176.106 9,160.112 6,105.033 8,100.076 5 苯代南蛇碱 [11] 5 9.41 C9H10O5 197.045 6 [M-H]− 197.044 7 −4.2 179.038 3,135.044 3 丹参素 [10] 6 9.42 C9H8O4 179.035 0 [M-H]− 179.034 2 4.7 135.044 8 咖啡酸 [13] 7 11.16 C16H12O4 431.133 7 [M+H]+ 431.136 3 6.1 269.082 6,253.050 3,225.055 5,213.091 7,197.060 2,136.014 6,118.041 7 芒柄花苷 [12] 8 12.71 C16H12O5 285.075 8 [M+H]+ 285.077 4 5.7 270.053 4,253.050 3,225.055 3,137.023 5 毛蕊异黄酮* [12] 9 12.76 C17H16O5 301.107 1 [M+H]+ 301.109 0 6.4 167.070 8,152.047 3,147.043 2,105.034 0,123.043 3 astrapterocarpan [12] 10 13.06 C20H24O6 361.164 6 [M+H]+ 361.166 5 5.5 269.154 3,227.108 3,185.096 9,157.101 7,129.070 3,91.054 9 雷公藤甲素* [14-15] 11 13.92 C17H14O6 315.086 3 [M+H]+ 315.088 1 5.6 300.064 7,243.065 5,167.034 2 熊竹素 [16] 12 18.21 C18H12O7 341.065 6 [M+H]+ 341.066 9 3.9 295.060 7,277.050 9,249.056 0 丹酚酸G [17] 13 19.76 C26H20O10 491.098 4 [M-H]− 491.097 0 −2.9 311.054 9,293.044 6,267.064 6,135.044 7 丹酚酸C [18] 14 21.46 C16H12O4 269.080 8 [M+H]+ 269.082 7 4.1 253.015 3,237.052 6,225.055 5,213.092 3,136.015 9,118.041 7,197.060 2 芒柄花素 [12] 15 22.57 C36H45NO17 764.276 0 [M+H]+ 764.278 3 2.9 746.276 1,686.246 3,644.235 1,206.081 7,188.070 9,178.086 5 aquifoliunine E-Ⅲ [14] 16 23.45 C20H22O6 359.148 9 [M+H]+ 359.150 7 4.9 267.138 0,225.019 5,183.079 9,128.061 8,91.054 3 雷公藤内酯酮* [19] 17 24.01 C38H47NO19 822.281 5 [M+H]+ 822.284 1 3.2 804.275 8,204.066 2,176.071 4 alatusinnine [20] 18 25.01 C39H45NO19 832.265 9 [M+H]+ 832.269 0 3.8 804.273 3,194.081 9,176.071 2 hypoglaunine E [11] 19 26.14 C41H68O14 829.458 0 [M+COOH]− 829.460 7 3.3 783.457 9,621.404 3,489.357 2 黄芪甲苷* [14] 20 28.19 C38H47NO18 806.286 6 [M+H]+ 806.290 3 3.8 788.279 5,686.247 0,206.082 1, 178.086 5 雷公藤定宁 E [20] 21 28.65 C39H45NO18 816.271 0 [M+H]+ 816.273 9 3.6 798.261 9,756.250 9,206.081 3,178.086 1,160.075 2 1-去乙酰基雷公藤吉碱 [11] 22 28.70 C43H70O15 871.468 6 [M+COOH]− 871.470 8 2.5 825.470 2,765.448 2,489.356 8 黄芪皂苷Ⅱ [13] 23 29.09 C41H47NO20 874.276 4 [M+H]+ 874.278 5 2.3 856.269 2,846.282 9,828.272 3,674.245 1,204.065 6,176.070 7 雷公藤春碱 [11] 24 29.72 C38H47NO18 806.286 6 [M+H]+ 806.291 2 3.8 788.280 4,686.247 4,206.082 4 peritassine A [20] 25 30.24 C43H70O15 871.468 6 [M+COOH]− 871.470 3 2.5 825.464 1,765.440 5 异黄芪皂苷Ⅱ
(异构体1)[16] 26 30.89 C19H16O4 309.112 1 [M+H]+ 309.114 2 6.7 281.667 0,263.106 0,235.076 7 丹参醛 [21] 27 31.58 C43H70O15 871.468 6 [M+COOH]− 871.470 8 2.5 825.470 2,765.448 0 异黄芪皂苷Ⅱ
(异构体2)[13] 28 32.12 C21H20O4 337.143 4 [M+H]+ 337.142 5 −2.7 309.686 6 丹参新醌丁 [10] 29 32.16 C43H49NO19 884.297 2 [M+H]+ 884.299 7 2.8 856.304 5,674.246 0,204.663 0,176.071 2 雷公藤定碱 [14] 30 32.96 C45H72O16 913.479 1 [M+COOH]− 913.482 4 3.5 867.481 7,825.469 8,807.464 3,765.450 6 黄芪皂苷Ⅰ [16] 31 32.99 C41H47NO19 858.281 5 [M+H]+ 858.285 4 4.6 840.275 7,798.263 8,746.269 1,738.243 5,686.248 0,206.082 5,178.087 1 雷公藤晋碱 [20] 32 33.04 C38H47NO18 806.286 6 [M+H]+ 806.289 7 3.8 788.278 3,686.244 4,206.082 1,728.257 0 卫矛碱 [20] 33 33.70 C45H72O16 913.479 1 [M+COOH]− 913.484 7 3.8 867.478 5,825.283 5,807.458 4,765.432 6 异黄芪皂苷Ⅰ
(异构体1)[16] 34 34.60 C45H72O16 913.479 1 [M+COOH]− 913.483 3 3.8 867.477 8,825.282 1,807.456 4,765.443 2 异黄芪皂苷Ⅰ
(异构体2)[16] 35 34.92 C46H49NO22 968.281 9 [M+H]+ 968.286 3 4.5 856.677 0,838.257 4,684.228 8,204.065 6,178.070 8 雷公藤素B [20] 36 35.01 C43H49NO18 868.302 2 [M+H]+ 868.304 6 2.7 868.364 0,850.295 8,746.268 9,686.247 6,206.082 4,178.087 1 雷公藤次碱 [20] 37 35.02 C41H47NO17 826.291 7 [M+H]+ 826.295 1 4.2 808.285 3,748.264 0,206.082 2,178.086 8 tripterygiumine Ⅰ [20] 38 35.49 C19H20O3 297.148 5 [M+H]+ 297.145 0 4.8 251.144 0,279.139 3,254.054 9,268.110 5,282.126 3 隐丹参酮 [10,17] 39 35.70 C20H28O2 299.201 7 [M-H]− 299.199 6 −6.7 283.168 2,213.090 8,201.916 0, 雷酚萜 [22] 40 35.86 C48H51NO18 930.317 9 [M+H]+ 930.321 3 3.7 912.308 7,310.111 0,206.081 8,188.071 2,178.086 5,105.033 6 ebenifoline E-Ⅱ [20] 41 36.81 C19H18O3 295.132 9 [M+H]+ 295.134 9 4.0 277.124 3,249.127 5,266.095 3,262.097 7,280.109 9 丹参酮Ⅱ A* [10,18,23] 42 37.49 C19H22O2 283.169 3 [M+H]+ 283.169 3 0 265.098 1,240.032 2,223.106 7,195.095 8,181.101 1 丹参新酮 [17,21,24] 注:*表示与对照品鉴定的化合物。 3.2.1 黄酮类化合物结构解析
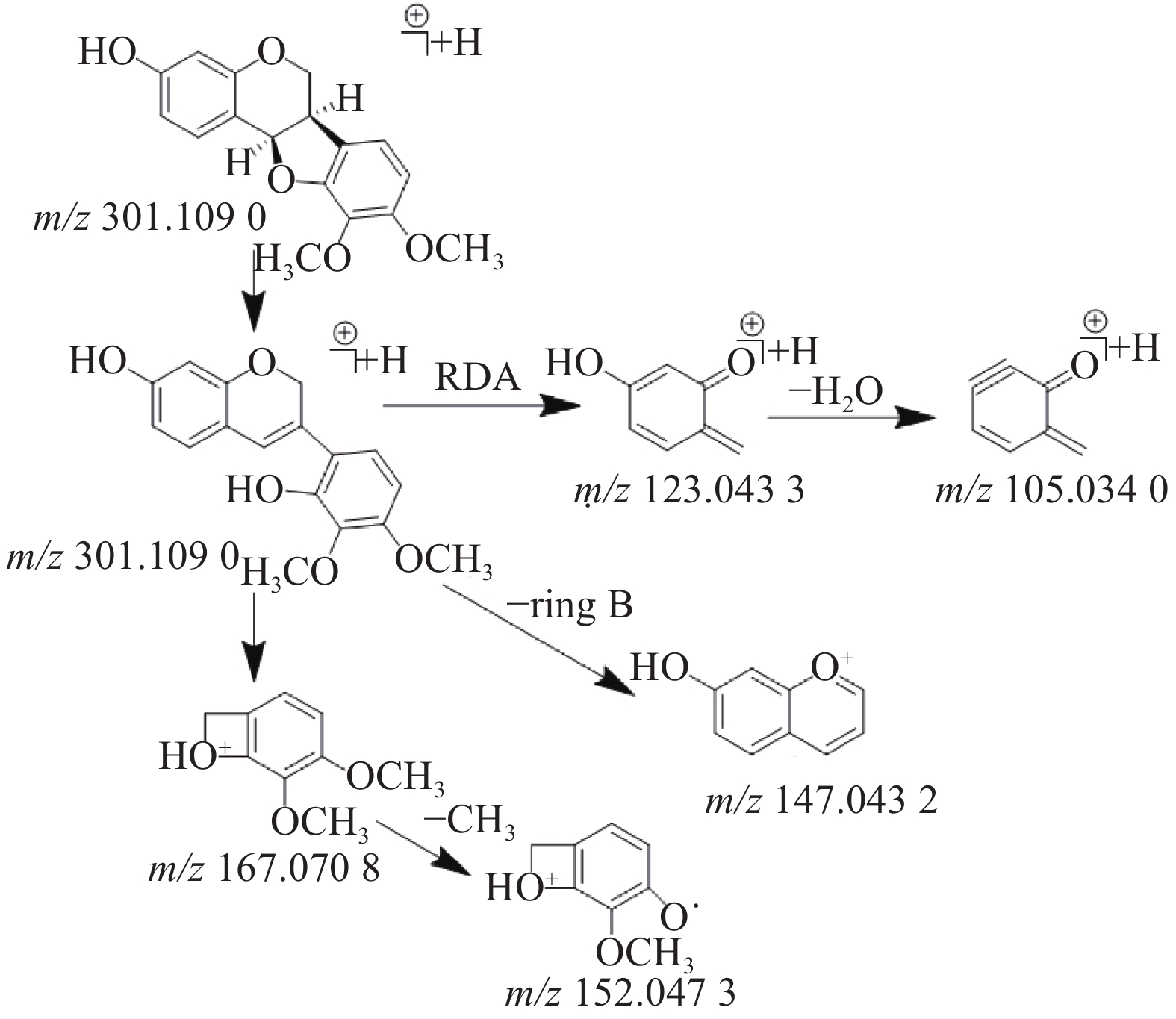
在乙酸乙酯部位中共鉴定出6个黄酮类化合物,其中4个黄酮苷元和2个黄酮苷,苷元为黄酮、异黄酮和紫檀烷,该类化合物在正离子模式下具有较好的响应。二级质谱中黄酮苷元易发生中性丢失,形成[M+H-H2O]+、[M+H-CO]+、[M+H-CH3]+等碎片离子,如在化合物8的二级质谱中可见m/z 270.053 4和m/z 253.050 3,则为m/z 285.077 4分别脱去-CH3和CH3OH形成的[M+H-CH3]+和[M+H-CH3OH]+碎片离子峰,m/z 225.055 3是m/z 253.050 3脱去1分子的CO形成的碎片离子峰,通过对照品的保留时间和参考文献[12]质谱数据比对确定化合物8为毛蕊异黄酮,m/z 137.023 5的碎片离子峰为异黄酮母核C环发生RDA裂解所产生。黄酮苷类易脱去糖基形成较强的分子离子峰,如化合物3(m/z 447.130 4)的二级质谱脱去糖基形成m/z 285.077 5的分子离子峰,并与化合物8(m/z 285.077 4)的二级质谱图非常相似,说明化合物3和化合物8在结构上是相似的,但化合物3的分子量多了162(C6H10O5),通过数据库比对和参考文献[12]推测化合物3则为毛蕊异黄酮-7-O-β-D-葡萄糖苷。化合物9(m/z 301.109 0)通过数据库比对发现两种候选化合物分别为astrapterocarpan和astraisoflavan,二级质谱中主要碎片离子峰为C环裂解产生的含A环和B环片段的碎片离子,其中,m/z 167.070 8为含B环的碎片离子峰且为基峰,进一步脱甲基形成m/z 152.047 3,m/z 123.043 3为含A环的碎片离子峰,进一步脱水形成m/z 105.034 0,m/z 147.043 2为母离子m/z 301.109 0脱去B环形成的碎片离子峰,根据m/z 167.070 8的碎片离子峰为基峰和含有m/z 147.043 2的碎片离子峰这两个特征,结合参考文献[12]的质谱数据,推测该化合物为astrapterocarpan,其相关裂解途径见图2。
3.2.2 三萜皂苷类化合物结构解析
在乙酸乙酯部位中鉴定出7个三萜皂苷类化合物,在负离子模式下均具有较好的响应,一级质谱中产生[M+COOH]−的准分子离子峰,二级质谱中产生较强的[M-H]-碎片离子峰和脱去糖基的较弱的分子离子峰。化合物19在负离子模式下产生的准分子离子峰为[M+COOH]−(m/z 829.458 0),二级质谱中产生m/z 783.457 9[M-H]−峰,脱去1分子六碳糖(C6H10O6)形成m/z 621.404 3的碎片离子峰,m/z 489.357 2则为m/z 621.404 3进一步脱去1分子五碳糖(C5H6O5)后形成的苷元碎片离子峰,推测其苷元为9,19-环阿尔廷烷,通过对照品的保留时间,参考文献[13]的离子碎片比对确定该化合物为黄芪甲苷。
3.2.3 生物碱类化合物结构解析
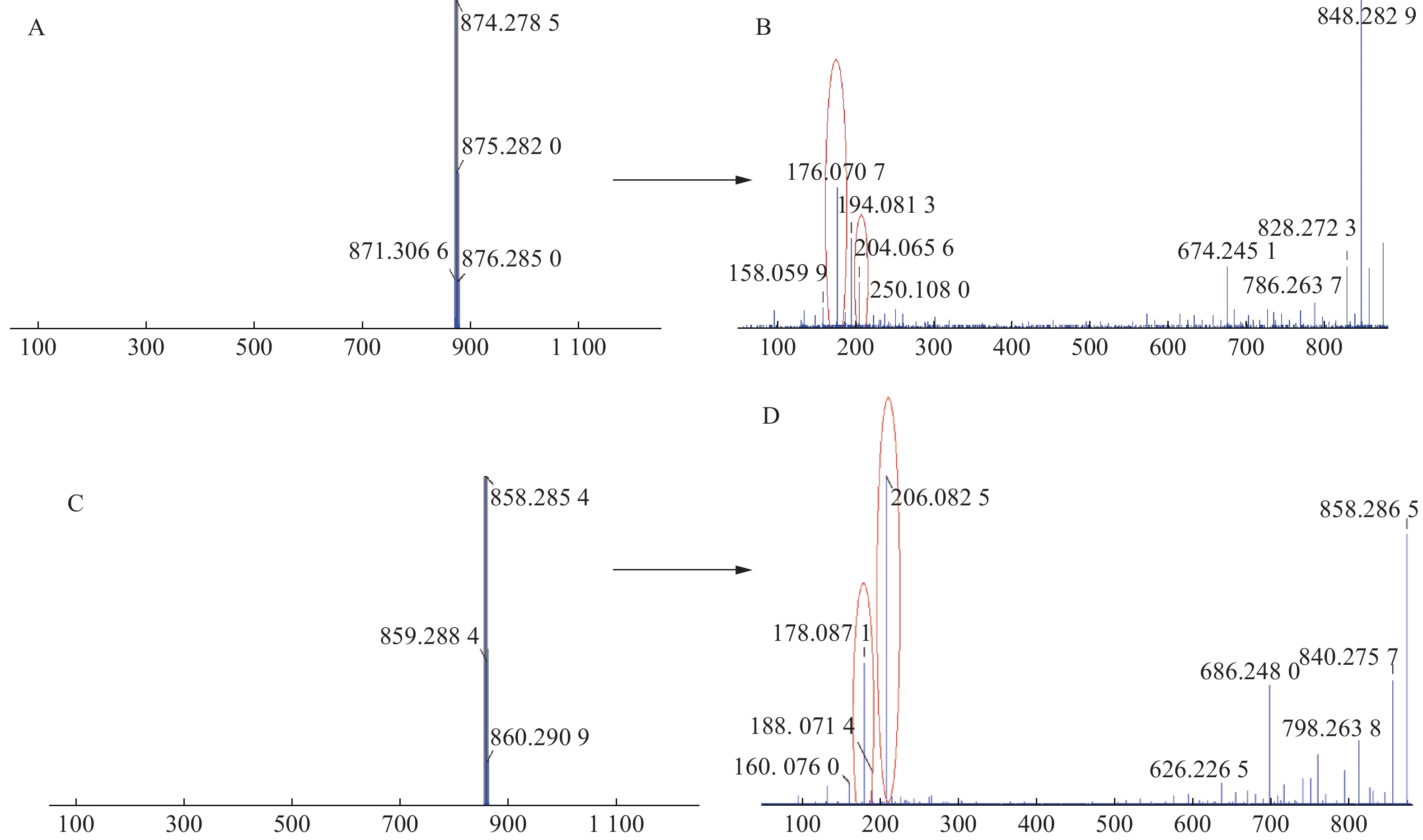
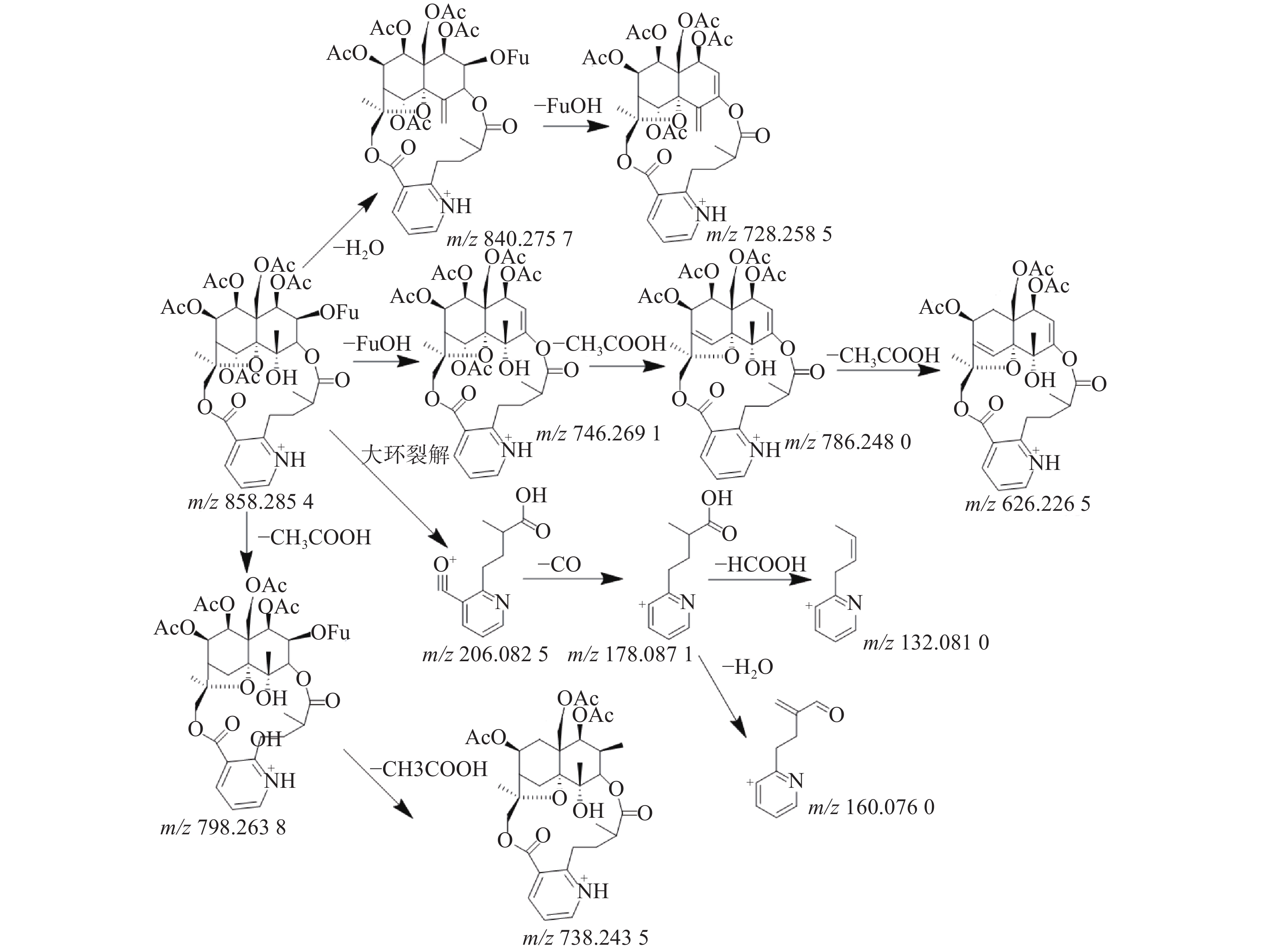
三色片提取物中共鉴定出16个生物碱类化合物,均来自雷公藤药材,在正离子模式下具有较好的响应,一级质谱中产生[M+H]+的准分子离子峰,二级质谱发现该类型的化合物容易脱去H2O、CO和CH3COOH等中性小分子而产生碎片离子峰,多数生物碱含有吡啶二羧酸部位的碎片离子峰。如化合物23在正离子模式下产生m/z 874.278 5的准分子离子峰,二级质谱中产生脱去1分子CO的m/z 846.282 9的基峰,脱去1分子H2O的m/z 856.2692的碎片离子峰和脱去1分子HCOOH的m/z 828.272 3的碎片离子峰,m/z 674.245 1峰为m/z 846.2829脱去C5H4O3侧链和CH3COOH形成的碎片离子峰,m/z 204.065 6峰为大环开裂产生的吡啶二羧酸部分脱水产生的碎片离子,该离子进一步脱羧形成m/z 176.070 7的碎片离子,通过数据库和参考文献[11]质谱数据的比对,推测化合物23为雷公藤春碱。化合物31在正离子模式下产生m/z 858.285 4的准分子离子峰,二级质谱中产生脱去1分子H2O的m/z 840.275 7的碎片离子峰,准分子离子峰脱去1分子CH3COOH形成较强的m/z 798.263 8峰,在进一步脱去1分子CH3COOH形成738.243 5峰,准分子离子峰m/z 858.285 4脱去FuOH(C5H4O3)侧链形成的m/z 746.269 1的碎片离子峰,再进一步脱去1分子CH3COOH,形成m/z 686.248 0的碎片离子,m/z 206.082 5峰为大环开裂产生的吡啶二羧酸部分脱水产生的碎片离子,该离子进一步脱羧形成m/z 178.087 1的碎片离子,通过数据库和参考文献[20]质谱数据的比对,推测化合物31为雷公藤晋碱。雷公藤晋碱中吡啶二羧酸部分较雷公藤春碱中少一个羟基,故其易产生m/z 206.0825的碎片离子峰,并通过脱羧产生m/z 178.087 1峰。两种化合物的质谱图见图3。以雷公藤晋碱为例,解析此类化合物的裂解规律,见图4。因此得出吡啶二羧酸部分含有羟基的生物碱会产生m/z 204系列的特征碎片离子峰,不含羟基的生物碱则产生m/z 206系列的特征碎片离子峰。
3.2.4 萜类化合物结构解析
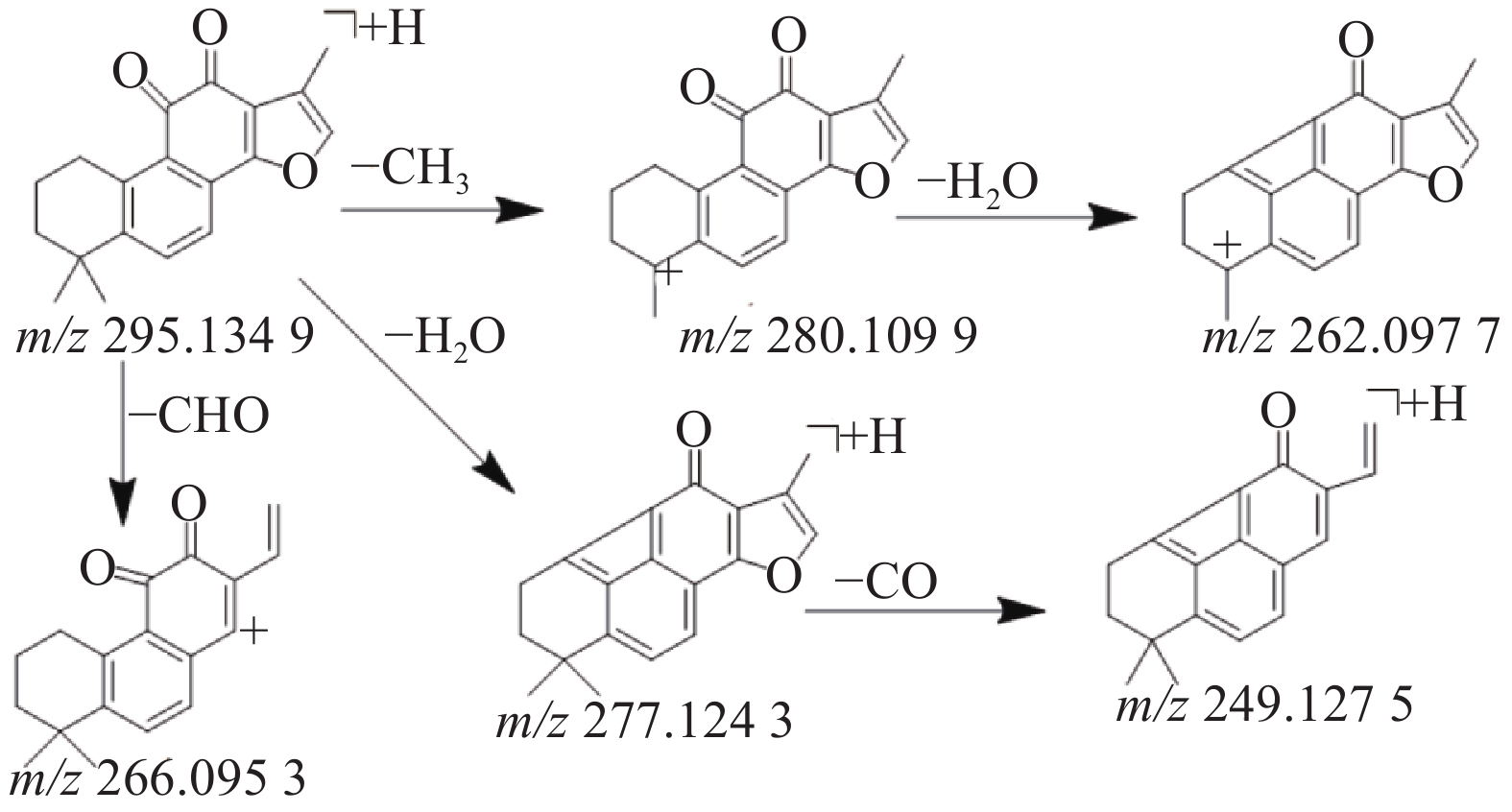
本研究共鉴定出8种萜类化合物,其中源于丹参药材中的5种萜类成分,丹参中的萜类化合物因其结构中主要含有羟基,羰基等取代基,所以质谱碰撞中主要丢失H2O,CO和-CH3等中性分子,产生一系列的碎片离子峰。化合物41在正离子模式下产生m/z 295.134 9的[M+H]+准分子离子峰,二级质谱中产生脱去1分子甲基形成的m/z 280.1099的碎片离子峰,在此基础上有丢失1分子水形成m/z 262.097 7峰,准分子离子峰脱去1分子H2O或脱去1个-CHO形成m/z 277.124 3峰或m/z 266.095 3峰,m/z 249.127 5峰是m/z 277.124 3脱去1分子H2O形成的碎片峰,通过对照品的保留时间和参考文献[10,18,23]数据比对,鉴定该化合物为丹参酮Ⅱ A,其质谱裂解规律见图5。
来源于雷公藤药材中的3种二萜类成分,该类化合物的二级质谱中出现一系列的脱水、脱CO和异丙基等碎片离子峰。化合物11在正离子模式下产生m/z 361.166 5的准分子离子峰,脱去2分子H2O和2分子CO形成m/z 269.154 3的碎片离子峰,m/z 227.108 3为m/z 269.154 3脱去1分子CH2CHCH3形成的碎片离子,其进一步脱1分子H2O和HCHO形成m/z 185.096 9的碎片离子,通过对照品比对和参考文献[14-15]的质谱数据,确定化合物10为雷公藤甲素。化合物18在正离子模式下产生m/z 359.148 9的准分子离子峰,脱去2分子H2O和2分子CO形成m/z 267.138 0的碎片离子峰,m/z 225.019 5为m/z 267.138 0脱去1分子CH2CHCH3形成的碎片离子,其进一步脱1分子H2O和HCHO形成m/z 183.0799的碎片离子,通过对照品比对和参考文献[19]的质谱数据,确定化合物16为雷公藤内酯酮。化合物39在负离子模式下产生m/z 299.199 6 的准分子离子峰,二级质谱中产生m/z 283.168 2的碎片离子, 提示为丢失1个-CH3后形成双键产生的碎片离子峰,A环发生RDA裂解产生m/z 213.090 8的碎片离子峰,通过数据库比对和参考文献[22]的质谱数据,推测化合物39为雷酚萜。
3.2.5 酚酸类化合物结构解析
在正负离子模式下共鉴定出乙酸乙酯部位中5种酚酸类成分,均来自于丹参药材,参考文献[18]报道的丹参中酚酸类成分的裂解规律发现,酚酸类化合物主要含有羰基、羧基和羟基,所以在质谱碰撞中易丢失CO、H2O和CO2的中性碎片;丹参素和咖啡酸作为基本母核而其他的水溶性酚酸类化合物大多数为这两者的聚合或缩合产物,主要为缩酚酸类的成分,在质谱碰撞中易丢失[M-H-180]−和[M-H-198]−中性碎片;含有羧基的单体化合物在负离子模式下会产生135[C8H7O2]−和179[C9H7O4]−的特征性碎片。化合物5中,在负离子模式下产生m/z 197.044 7的[M-H]−准分子离子峰,二级质谱进一步产生丢失1分子H2O和1分子CO2,形成的m/z 179.038 3和m/z 135.044 3的碎片离子峰,推测出结构中含有羧基,结合其精确分子量和参考文献[10]质谱数据,推测该化合物为丹参素。化合物12中,负离子模式下产生m/z 491.097 0的[M-H]−准分子离子峰,二级质谱中产生m/z 311.054 9和m/z 293.044 6的碎片离子峰,分别为[M-H-180]−和[M-H-198]−, m/z 267.064 6峰为m/z 311.054 9脱去1分子CO2所产生,根据m/z 135.044 7峰推测结构中含有羧基,结合其精确分子量和参考文献[18]质谱数据的比较,推测该化合物为丹酚酸C。化合物1中,正离子模式下给出m/z 139.039 4的[M+H]+准分子离子峰,脱去1分子H2O形成m/z 121.028 7的碎片离子峰,通过数据库比对和参考文献[10],推测该化合物1为原儿茶醛。化合物13中,在正离子模式下产生m/z 341.066 9的[M+H]+准分子离子峰,脱去1分子CO2形成m/z 295.060 7的碎片离子峰,m/z 277.050 9和m/z 249.056 0的碎片离子峰是m/z 295.060 7峰分别脱去1分子H2O和1分子CO2形成的,通过数据库比对和参考文献[17]质谱数据,推测该化合物为丹酚酸G。
4. 讨论
4.1 色谱与质谱条件考察
本实验流动相考察了乙腈-水系统和甲醇-水系统,结果乙腈-水系统中化合物的分离度较好,加入甲酸可以改善峰形,有助于化合物的离子化,提高质谱的响应,最终选择乙腈-0.1%甲酸水系统作为本次研究的流动相。
4.2 化学成分的定性分析
据以往文献中三色片各化学成分的研究报道,收集各药材的主要化学成分的精确分子量,碎片离子峰等信息,建立相应的化学成分数据库。通过数据库比对,对照品保留时间及参考文献中质谱数据鉴定三色片醇提物乙酸乙酯部位的化学成分。本研究共鉴定出42个化合物,其中5个是通过对照品鉴定得出,对无对照品的化合物,通过质谱的裂解特征及参考文献进行结构表征,对同分异构体应结合其在液相色谱中化合物的保留时间及质谱行为,综合对其定性鉴别。
三色片醇提物的乙酸乙酯部位具有较强的抗补体活性,本研究采用UPLC-Q-TOF-MS法对其中的化学成分进行结构表征,结果发现该部位主要含有生物碱类,萜类,黄酮和酚酸类等化学成分。其中以来源于雷公藤药材中极性中等的生物碱类成分含量较多,这与三色片提取物的制备工艺有关,三色片中雷公藤药材采用乙醇加热回流提取的方式,而黄芪和丹参药材采用水提取醇沉淀的方式。此外,先前的研究发现广藿香中的黄酮和萜类化合物对旁路途径的补体激活具有明显的抑制作用,紫花地丁中的生物碱类成分对旁路途径也有抑制作用(AP50=0.22~0.50 g/L), 牡丹皮和毛七公的抗补体活性成分研究中发现酚羟基决定抗补体活性的存在与否,没食子酰基可改善抗补体活性,甲基则对抗补体活性不利[25-27]。通过本次研究对三色片醇提物的乙酸乙酯部位的化学成分进行了初步表征,为阐明三色片的药效物质基础提供参考依据。研究的不足之处在于,仍有部分化学成分尚未定性鉴定,含量较高的单体成分未进行体外抗补体活性的测定,未来将通过中药化学的方法获得含量较高的单体成分,并进行结构鉴定和抗补体活性测定。
-
表 1 SGLT-2抑制剂的心血管安全性研究
试验项目 EMPA-REG OUTCOME CANVAS DECLARE-TIMI 58 干预措施 恩格列净(10~25 mg/d) 卡格列净(100~300 mg/d) 达格列净(10 mg/d) 样本量(例,T/C①) 4687/2333 5795/4347 8582/ 8578 平均年龄(年) 63.1 63.3 64.0 中位随访期(年) 3.1 2.4 4.2 合并ASCVD(例,%) 7020(100%) 6656(65.6%) 6974(40.6%) 主要事件(HR,95%CI) 0.86(0.74~0.99) 0.86(0.75~0.97) 0.93(0.84~1.03) 心肌梗死 0.87(0.70~1.09) 0.85(0.69~1.05) NA 缺血性卒中 1.18(0.89~1.56) 0.90(0.71~1.15) 1.01(0.84~1.21) 心血管死亡 0.62(0.49~0.77) 0.87(0.72~1.06) 0.98(0.82~1.17) 全因死亡 0.68(0.57~0.82) 0.87(0.74~1.01) 0.93(0.82~1.04) 心力衰竭住院 0.65(0.50~0.85) 0.67(0.52~0.87) 0.73(0.61~0.88) 注:T/C:试验组/对照组;ASCVD,动脉粥样硬化性心血管疾病;NA,无数据。 表 2 GLP-1受体激动剂的心血管安全性研究
试验项目 EXSCEL SUSTAIN-6 LEADER ELIXA 干预措施 艾塞那肽(2 mg/w) 索马鲁肽(0.5~1.0 mg/w) 利拉鲁肽(0.6~1.8 mg/d) 利司那肽(10~20 μg/d) 样本量(例,T/C①) 7256/7396 1648/1649 4668/4672 3034/3034 中位随访期(年) 3.2 2.1 3.5 2.1 合并ASCVD(例,%) 10782(73.1%) 2735(83%) 6764(72.4%) 6068(100%) 主要终点(HR,95%CI) 0.91 (0.83~1.00) 0.74(0.58~0.95) 0.87 (0.78~0.97) 1.02 (0.89~1.17) 心肌梗死 0.97 (0.85~1.10) 0.74 (0.51~1.08) 0.86 (0.73~1.00) 1.03 (0.87~1.22) 缺血性卒中 0.85 (0.70~1.03) 0.61 (0.38~0.99) 0.86 (0.71~1.06) 1.11 (0.47~2.62) 心血管死亡 0.88 (0.76~1.02) 0.98 (0.65~1.48) 0.78 (0.66~0.93) 0.98 (0.78~1.22) 全因死亡 0.86 (0.77~0.97) 1.05 (0.74~1.50) 0.85 (0.74~0.97) 0.94 (0.78~1.13) 心力衰竭住院 0.94 (0.78~1.13) 1.11 (0.77~1.61) 0.87 (0.73~1.05) 0.96 (0.75~1.23) 注:T/C:试验组/对照组。 -
[1] IDF DIABETES ATLAS, 9TH EDITION. Brussels; 2019. International Diabetes Federation. [2] 中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2017年版)[J]. 中华糖尿病杂志, 2018, 10(1):44-67. [3] GOLDFINE A B. Assessing the cardiovascular safety of diabetes therapies[J]. N Engl J Med,2008,359(11):1092-1095. doi: 10.1056/NEJMp0805758 [4] BAKRIS G L, FONSECA V A, SHARMA K, et al. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications[J]. Kidney Int,2009,75(12):1272-1277. doi: 10.1038/ki.2009.87 [5] ZINMAN B, WANNER C, LACHIN J M, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes[J]. N Engl J Med,2015,373(22):2117-2128. doi: 10.1056/NEJMoa1504720 [6] NEAL B, PERKOVIC V, MAHAFFEY K W, et al. Canagli- flozin and cardiovascular and renal events in type 2 diabetes[J]. N Engl J Med,2017,377(7):644-657. doi: 10.1056/NEJMoa1611925 [7] WIVIOTT S D, RAZ I, BONACA M P, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes[J]. N Engl J Med,2019,380(4):347-357. doi: 10.1056/NEJMoa1812389 [8] ZELNIKER T A, WIVIOTT S D, RAZ I, et al. SGLT2 inhibi- tors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials[J]. Lancet,2019,393(10166):31-39. doi: 10.1016/S0140-6736(18)32590-X [9] ARNOTT C, LI Q, KANG A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis[J]. J Am Heart Assoc,2020,9(3):e014908. [10] TOYAMA T, NEUEN B L, JUN M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis[J]. Diabetes Obes Metab,2019,21(5):1237-1250. doi: 10.1111/dom.13648 [11] KOSIBOROD M, CAVENDER M A, FU A Z, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors)[J]. Circulation,2017,136(3):249-259. doi: 10.1161/CIRCULATIONAHA.117.029190 [12] KOSIBOROD M, LAM C S P, KOHSAKA S, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study[J]. J Am Coll Cardiol,2018,71(23):2628-2639. doi: 10.1016/j.jacc.2018.03.009 [13] RYAN P B, BUSE J B, SCHUEMIE M J, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D)[J]. Diabetes Obes Metab,2018,20(11):2585-2597. doi: 10.1111/dom.13424 [14] PATORNO E, PAWAR A, FRANKLIN J, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care: a first analysis from the empagliflozin comparative effec- tiveness and safety (EMPRISE) Study[J]. Circulation,2019,139(25):2822-2830. doi: 10.1161/CIRCULATIONAHA.118.039177 [15] MAYO K E, MILLER L J, BATAILLE D, et al. International union of pharmacology. XXXV. The glucagon receptor family[J]. Pharmacol Rev,2003,55(1):167-194. doi: 10.1124/pr.55.1.6 [16] BAN K, NOYAN-ASHRAF M H, HOEFER J, et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and independent pathways[J]. Circulation,2008,117(18):2340-2350. doi: 10.1161/CIRCULATIONAHA.107.739938 [17] MARSO S P, DANIELS G H, BROWN-FRANDSEN K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes[J]. N Engl J Med,2016,375(4):311-322. doi: 10.1056/NEJMoa1603827 [18] MARSO S P, BAIN S C, CONSOLI A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes[J]. N Engl J Med,2016,375:1834-1844. doi: 10.1056/NEJMoa1607141 [19] HOLMAN R R, BETHEL M A, MENTZ R J, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes[J]. N Engl J Med,2017,377(13):1228-1239. doi: 10.1056/NEJMoa1612917 [20] PFEFFER M A, CLAGGETT B, DIAZ R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome[J]. N Engl J Med,2015,373(23):2247-2257. doi: 10.1056/NEJMoa1509225 [21] BETHEL M A, PATEL R A, MERRILL P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis[J]. Lancet Diabetes Endo,2018,6(2):105-113. doi: 10.1016/S2213-8587(17)30412-6 [22] KRISTENSEN S L, RORTH R, JHUND P S, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor ago- nists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials[J]. Lancet Diabetes Endo,2019,7(10):776-785. doi: 10.1016/S2213-8587(19)30249-9 [23] MIRANI M, FAVACCHIO G, SERONE E, et al. Liraglutide and cardiovascular outcomes in a real world type 2 diabetes cohort[J]. Pharmacol Res,2018,137:270-279. doi: 10.1016/j.phrs.2018.09.003 [24] TOULIS K A, HANIF W, SARAVANAN P, et al. All-cause mortality in patients with diabetes under glucagon-like peptide-1 agonists: a population-based, open cohort study[J]. Diabetes Metab,2017,43(3):211-216. doi: 10.1016/j.diabet.2017.02.003 [25] AMERICAN DIABETES ASSOCIATION. Standards of medi- cal care in diabetes-2020[J]. Diabetes Care,2020,43(Suppl 1):S1-S212. [26] TÄGER T, ATAR D, AGEWALL S, et al. Comparative effi- cacy of sodium-glucose cotransporter-2 inhibitors (SGLT2i) for cardiovascular outcomes in type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials[J]. Heart Fail Rev,2020. doi: 10.1007/s10741-020-09954-8 [27] YAMI M S A, ALFAYEZ O M, ALSHEIKH R, et al. Update in cardiovascular safety of glucagon like peptide-1 receptor ago- nists in patients with type 2 diabetes. a mixed treatment compari- son meta-analysis of randomised controlled trials[J]. Heart Lung Circ,2018,27(11):1301-1309. doi: 10.1016/j.hlc.2018.03.018 [28] FEI Y, TSOI M F, CHEUNG B M Y. Cardiovascular outcomes in trials of new antidiabetic drug classes: a network meta-analysis[J]. Cardiovasc Diabetol,2019,18(1):112. doi: 10.1186/s12933-019-0916-z [29] ZHENG S L, RODDICK A J, AGHAR-JAFFAR R, et al. Association between use of sodium-glucose cotransporter 2 inhibi- tors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis[J]. JAMA,2018,319(15):1580-1591. doi: 10.1001/jama.2018.3024 [30] ADVANCE COLLABORATIVE GROUP, PATEL A, MAC- MAHON S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes[J]. N Engl J Med,2008,358(24):2560-2572. doi: 10.1056/NEJMoa0802987 期刊类型引用(11)
1. 周超,尚丹丹,杨雯萱,代龙,姚景春. 荆防颗粒联合复方黄柏液涂剂对宫颈HPV感染的临床研究. 长春中医药大学学报. 2025(01): 55-59 .  百度学术
百度学术2. 孟珈同,邱智东,李军鸽,王永春,唐秋竹. 荆防败毒散关键信息考证. 中成药. 2024(04): 1262-1271 .  百度学术
百度学术3. 吕婧,高燕,赵渤年,姚景春,梁红宝. 荆防颗粒增强免疫作用机制研究. 中草药. 2024(16): 5541-5550 .  百度学术
百度学术4. 唐勇琛,张洪平,樊玲凤,杨玉竹,张亚洲. 心脉舒一号口服液治疗心脏病分子机制网络药理学研究. 中国药业. 2024(17): 66-73 .  百度学术
百度学术5. 尉雅洁,刘明飞,孙成宏,王伟,肖贺,程国良,陈颖. 基于网络药理学和动物实验探究荆防颗粒对高尿酸血症的治疗作用及机制. 中草药. 2023(03): 808-816 .  百度学术
百度学术6. 张永康,孙成宏,王西双,姚景春,张贵民. 基于网络药理学和实验验证探讨荆防颗粒对自身免疫性肝炎小鼠的治疗作用及作用机制. 中草药. 2023(05): 1461-1470 .  百度学术
百度学术7. 尉雅洁,刘明飞,周诗喆,项海鑫,孙成宏,陈颖. 荆防颗粒对氧嗪酸钾诱导小鼠高尿酸肾病的防治作用机制探讨. 山东医药. 2023(15): 1-5 .  百度学术
百度学术8. 鲍柏屹,孙美玲,陈祥,曹兆流,唐书炳,李歆. 基于网络药理学和分子对接研究地奥心血康治疗心脏神经官能症的相关机制. 中国医药导报. 2023(17): 9-13 .  百度学术
百度学术9. 阚雪纯,何润东,葛佳颖,吴俊,苗登顺. 基于网络药理学的续断抗骨质疏松分子作用机制研究. 南京医科大学学报(自然科学版). 2022(01): 35-40 .  百度学术
百度学术10. 姚世霞,刘东升,牛钰婷,朱旭江,刘志浩,朱琳. 荆防颗粒质量评价. 中成药. 2022(10): 3130-3136 .  百度学术
百度学术11. 徐庆仪,郁冬冬,黄烨炜. 临床治疗新型冠状病毒肺炎中药复方所使用高频数中药的网络药理学探讨. 海峡药学. 2021(10): 69-74 .  百度学术
百度学术其他类型引用(4)
-





 下载:
下载:
 下载:
下载: 

