-
河豚毒素(tetrodotoxin,TTX)是一种存在于河豚、蝾螈、斑足蟾等动物中的天然毒素,其选择性作用于电压门控钠离子通道(voltage-gated sodium channels,VGSCs),可强效阻滞神经、肌肉兴奋传导,导致神经和肌肉的麻痹,甚至死亡[1]。基于VGSCs在体内的广泛分布和作用,TTX的药用价值也备受关注,尤其在麻醉、镇痛、戒断等方面,一直是研究的热点[2-6]。TTX对多种疼痛尤其是炎性疼痛和神经病理性疼痛表现出优异的镇痛效果,临床试验也表明,TTX在治疗无法控制的中、重度癌症相关疼痛方面有良好的疗效,且不会产生耐药性和成瘾性,具有较好的应用前景[7-8]。然而,目前对TTX的急性镇痛研究较少,有报道认为TTX对急性疼痛的镇痛效应较弱[7,9]。但也有研究表明,TTX在小鼠甩尾实验、醋酸扭体实验中有显著镇痛效应。为明确TTX的急性镇痛效应,本研究拟通过4种急性疼痛模型,即扭体实验、福尔马林刺激实验、热板实验和甩尾实验,进一步评估TTX对急性疼痛的镇痛效果,为其安全、合理应用提供实验支持。
-
ICR小鼠,体重18~22 g,Wistar大鼠,雄性,体重150~180 g,购自北京华阜康生物科技股份有限公司,实验动物合格证号:SCXK(京)2019-0008。动物经适应性饲养4~7 d后开始进行实验。
-
TTX(批号:E2011088,上海阿拉丁试剂有限公司);盐酸吗啡(批号:20110601,青海制药厂有限公司);花生四烯酸检测试剂盒(批号:202101,江苏雨桐生物科技有限公司);冰醋酸(批号:C10309476,上海麦克林生化科技有限公司);甲醛溶液(福尔马林,批号:030430,北京化学试剂公司)。
-
DK-S28型电热恒温水浴锅(上海精宏实验设备有限公司);YLS-6B智能热板仪(济南益延科技发展有限公司);5424R型离心机(德国Eppendorf公司);Synergy HTX酶标仪(美国BioTeK公司)。
-
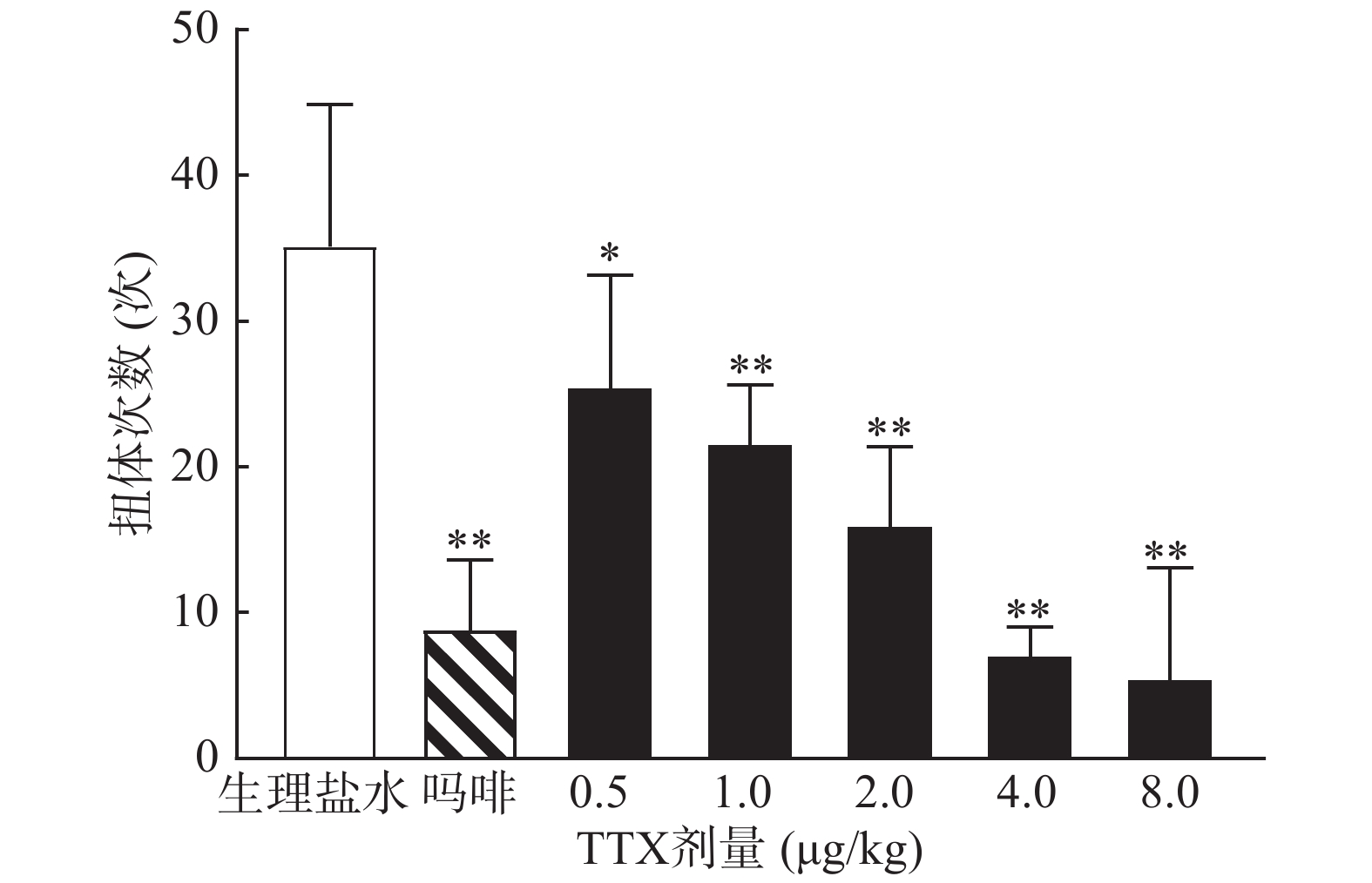
小鼠70只,雌雄各半,随机分为7组,每组10只,实验前禁食12 h,自由饮水。给药组分别肌内注射0.5、1、2、4、8 μg/kg的TTX或1 mg/kg吗啡,对照组肌内注射等体积的生理盐水,给药后40 min,小鼠腹腔注射0.6%的醋酸溶液(0.1 ml/10 g),记录15 min内的扭体次数,以小鼠出现腹部内凹、躯干与后肢伸张、臀部高起等行为为扭体反应阳性。计算各组疼痛抑制率,公式为:
$$\begin{array}{c} {\text{疼痛抑制率}}\left( \% \right) =\\ \displaystyle\frac{{{\text{生理盐水平均扭体数}} - {\text{给药平均扭体数}}}}{{\text{生理盐水平均扭体数}}} \times 100\% \end{array} $$ -
受试大鼠进行“疼痛反应累积分值”预筛实验,试验时,在大鼠左后肢足趾部皮下注射2.5%的福尔马林溶液50 μl后,分别观察1 ~10 min(Ⅰ相)和10 ~40 min(Ⅱ相)内大鼠的疼痛反应。表现为舔、咬、抖足为3分,提足为2分,轻触地面但不负重行走时跛行为1分,正常负重,行走自如为0分。记录各时间段出现上述各级反应的秒数乘以相应反应的分值,以乘积之和为疼痛反应累积分值,公式为:疼痛反应累积分值=跛行时间×1+提足时间×2+舔咬抖足时间×3。选择累积分值评分相近的动物进行实验。
动物恢复7 d,然后重新分组,每组10只,进行正式实验,给药组分别肌内注射0.5 ~8 μg/kg的TTX或1 mg/kg吗啡,对照组注射等体积生理盐水,给药40 min后,在大鼠右后肢足趾部皮下注射2.5%的福尔马林溶液50 μl,再次观察I相和II相疼痛反应,并计算各组的疼痛反应累积分值和疼痛抑制率。疼痛抑制率计算公式为:
$$\begin{array}{c} {\text{疼痛抑制率}}\left( \% \right) =\\ \displaystyle \frac{{{\text{生理盐水组疼痛反应均值}} - {\text{给药组疼痛反应均值}}}}{{\text{生理盐水组疼痛反应均值}}} \times 100\% \end{array}$$ -
受试动物均进行“基础痛阈”预筛实验,实验时,将小鼠尾下部垂直浸入(52±0.5)℃的恒温水浴中,浸入长度为3 cm左右,以尾回缩出水面的潜伏期为测痛指标,给药前间隔5 min测定2次,以其均值作为基础痛阈。选择基础痛阈相近(3~7 s)的动物作为合格动物进行实验。筛选后动物恢复24 h,重新分成7组,每组10只,进行正式实验。小鼠肌内注射TTX(0.5~8 μg/kg)、吗啡(1 mg/kg)或等体积生理盐水,给药后40 min,进行痛阈测定,间隔5 min测定2次,以其均值作为给药后痛阈。为防止尾部烫伤,若痛阈超过14 s则停止水浴,以14 s计算。疼痛抑制率计算公式为:
$$ {\text{疼痛抑制率}}\left( \% \right) = \frac{{{\text{给药后痛阈}} - {\text{基础痛阈}}}}{{14 - {\text{基础痛阈}}}} \times 100\% $$ -
受试小鼠为雌性,均进行“基础痛阈”预筛实验,实验时,将小鼠放在预热至(55±0.5)℃金属板上,恒温,以小鼠舔足反应或跳跃反应的潜伏期为痛阈指标。每只动物测定间隔5 min,测定2次,取其平均值作为基础痛阈值。选择基础痛阈相近(5~20 s)的小鼠进行正式试验。筛选后小鼠恢复24 h以上,重新分为7组,每组10只,进行正式实验。小鼠肌内注射TTX(0.5~8 μg/kg)、吗啡(1 mg/kg)或等体积生理盐水,给药40 min后,进行痛阈测定。间隔5 min测定2次,以其均值作为给药后痛阈值。为防止足部烫伤,若痛阈值超过40 s则停止测定,以40 s计算。疼痛抑制率计算公式为:
$$ {\text{疼痛抑制率}}\left( \% \right) = \frac{{{\text{给药后痛阈}} - {\text{基础痛阈}}}}{{40 - {\text{基础痛阈}}}} \times 100\% $$ -
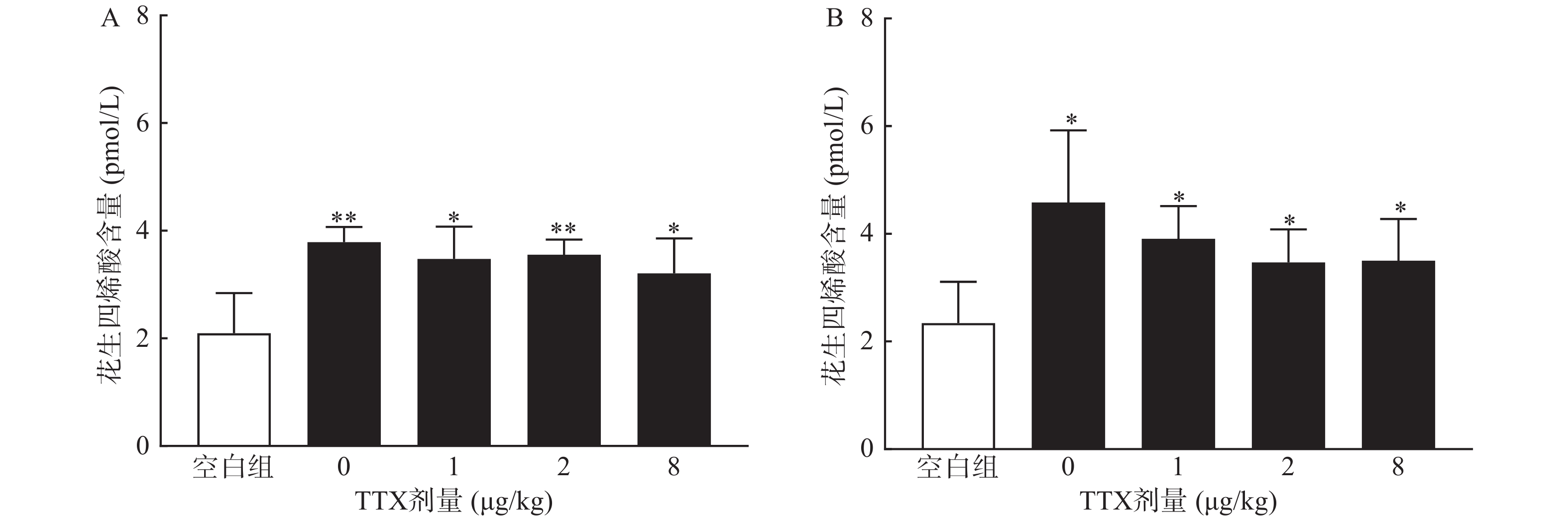
为进一步阐明TTX对醋酸扭体和福尔马林疼痛模型的镇痛机制,进行血清相关炎性介质的测定。将小鼠分为5组:空白对照组(只注射生理盐水),醋酸对照组(肌内注射生理盐水40 min后,腹腔注射0.6%的醋酸),TTX组(肌内分别注射1、2、8 μg/kg TTX 40 min后,腹腔注射0.6%的醋酸),每组10只,待测定镇痛效应后,小鼠眼眶取血;大鼠分组参照小鼠,注射生理盐水或TTX 40 min后足底注射福尔马林,待测定镇痛效应后,眼内眦取血;血液静置30 min后,3 000 r/min离心15 min,取上清液,用花生四烯酸Elisa试剂盒测定血清中花生四烯酸含量。
-
实验数据以(
$\bar x $ ±s)表示。用SPSS15.0统计分析软件进行统计学处理,组间差异采用单因素方差分析(ANOVA)和t检验,以P<0.05为差异有显著性。 -
0.6%的醋酸可诱导小鼠扭体反应,15 min内平均扭体次数为(35.1±9.8)次,如图1所示。1 mg/kg盐酸吗啡显著抑制醋酸诱导的扭体反应,0.5~8 μg/kg的TTX呈剂量依赖性地降低醋酸诱导的小鼠扭体次数,最高抑制率约为81.26%。TTX抑制醋酸诱导疼痛效应的半数效应剂量(ED50)为1.51 μg/kg,95%置信区间(CI)为1.16 ~1.93 μg/kg,表明TTX对醋酸诱导的小鼠扭体疼痛模型具有较好的镇痛效果。
-
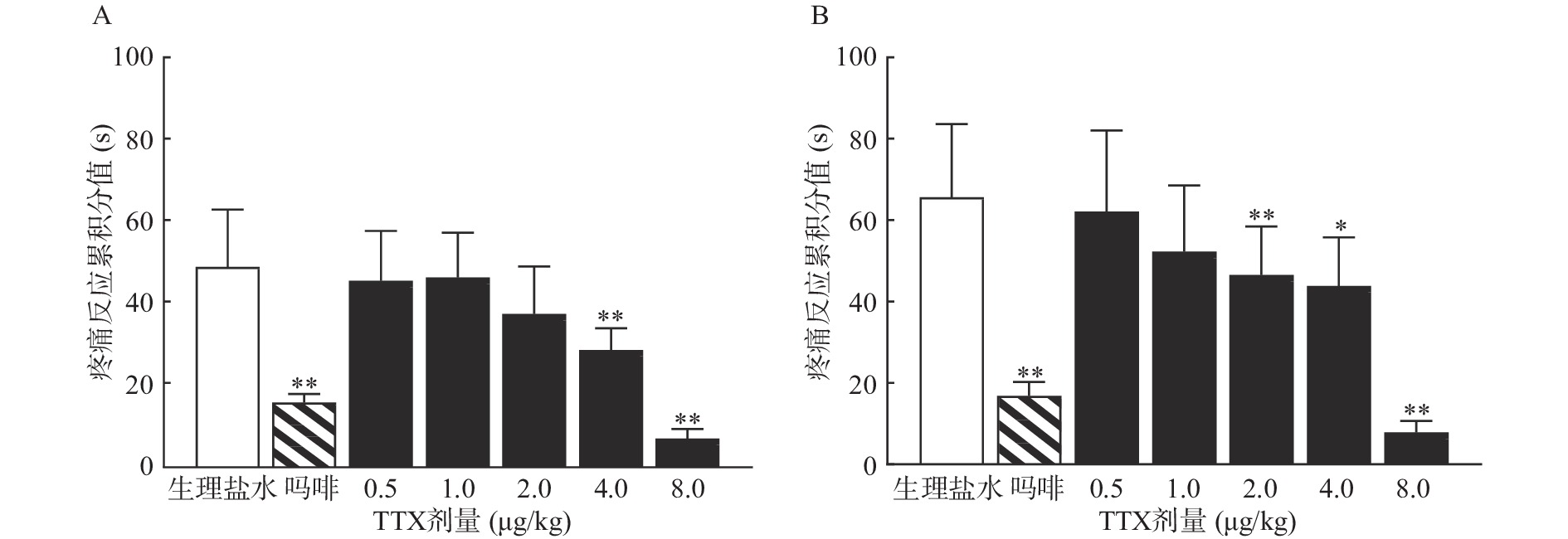
TTX 4 μg/kg和8 μg/kg剂量组对福尔马林致大鼠Ⅰ相疼痛反应累积分值与生理盐水组相比有显著差异(P<0.01),当TTX给药剂量为2~8 μg/kg 时,对福尔马林致大鼠Ⅱ相疼痛反应累积分值与阴性对照组相比有显著差异(P<0.05或P<0.01)(图2)。结果表明,吗啡及TTX对福尔马林致大鼠Ⅰ相、Ⅱ相疼痛反应均有明显的镇痛作用,TTX的最高疼痛抑制率分别为85.58%、88.05%。TTX抑制福尔马林致大鼠Ⅰ相、Ⅱ相疼痛反应的ED50值(95% CI)分别为4.12 μg/kg(3.22~5.25 μg/kg)、4.00 μg/kg(2.18 ~9.12 μg/kg)。
-
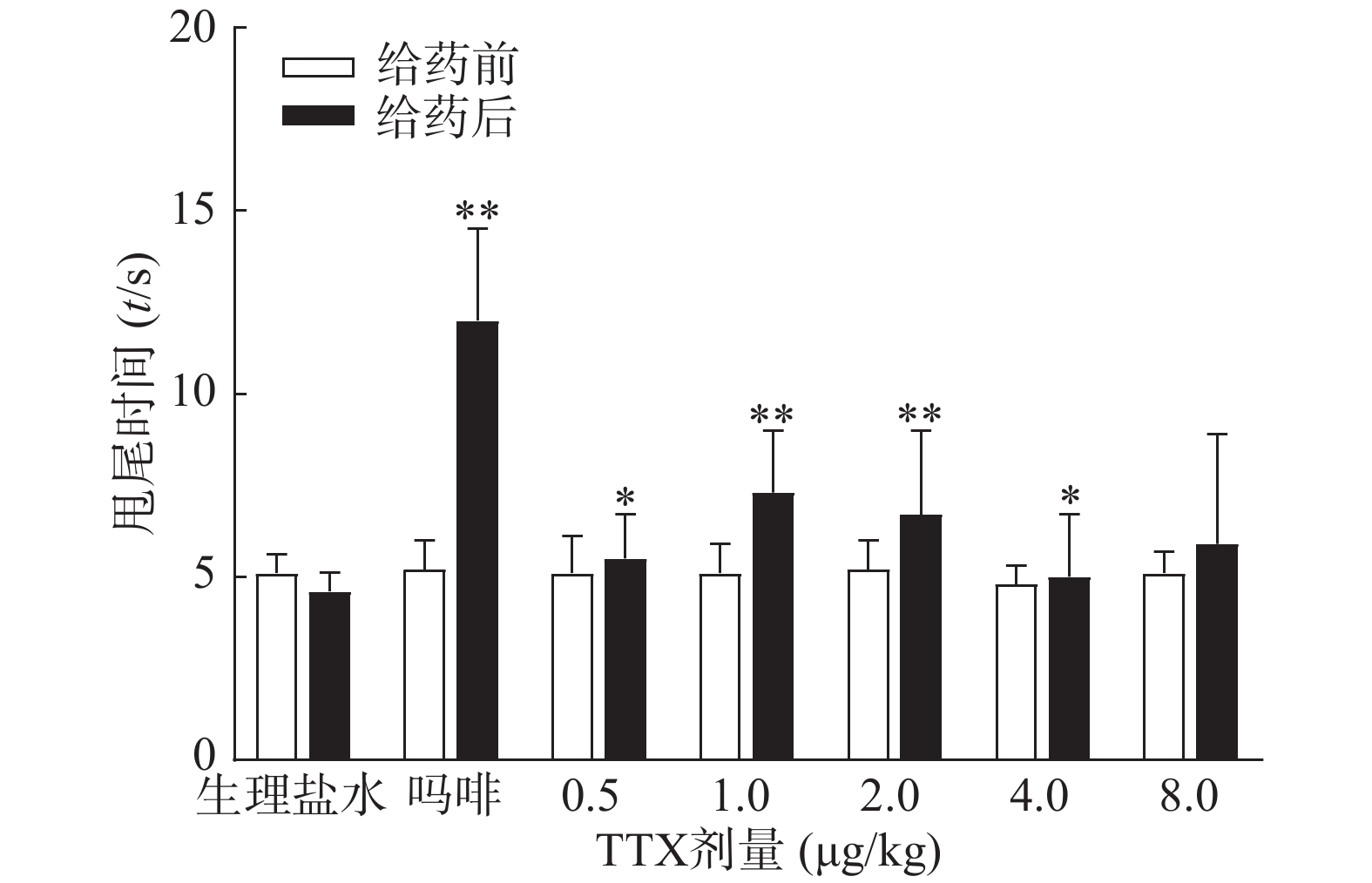
图3结果显示,1 mg/kg吗啡显著延长小鼠的甩尾时间,TTX在0.5~8 μg/kg的剂量范围,1 μg/kg镇痛效果达峰值,最高疼痛抑制率仅为25.0%,随着给药剂量增加,其镇痛效应并未提高,表明TTX对小鼠甩尾疼痛模型的镇痛效应较弱。
-
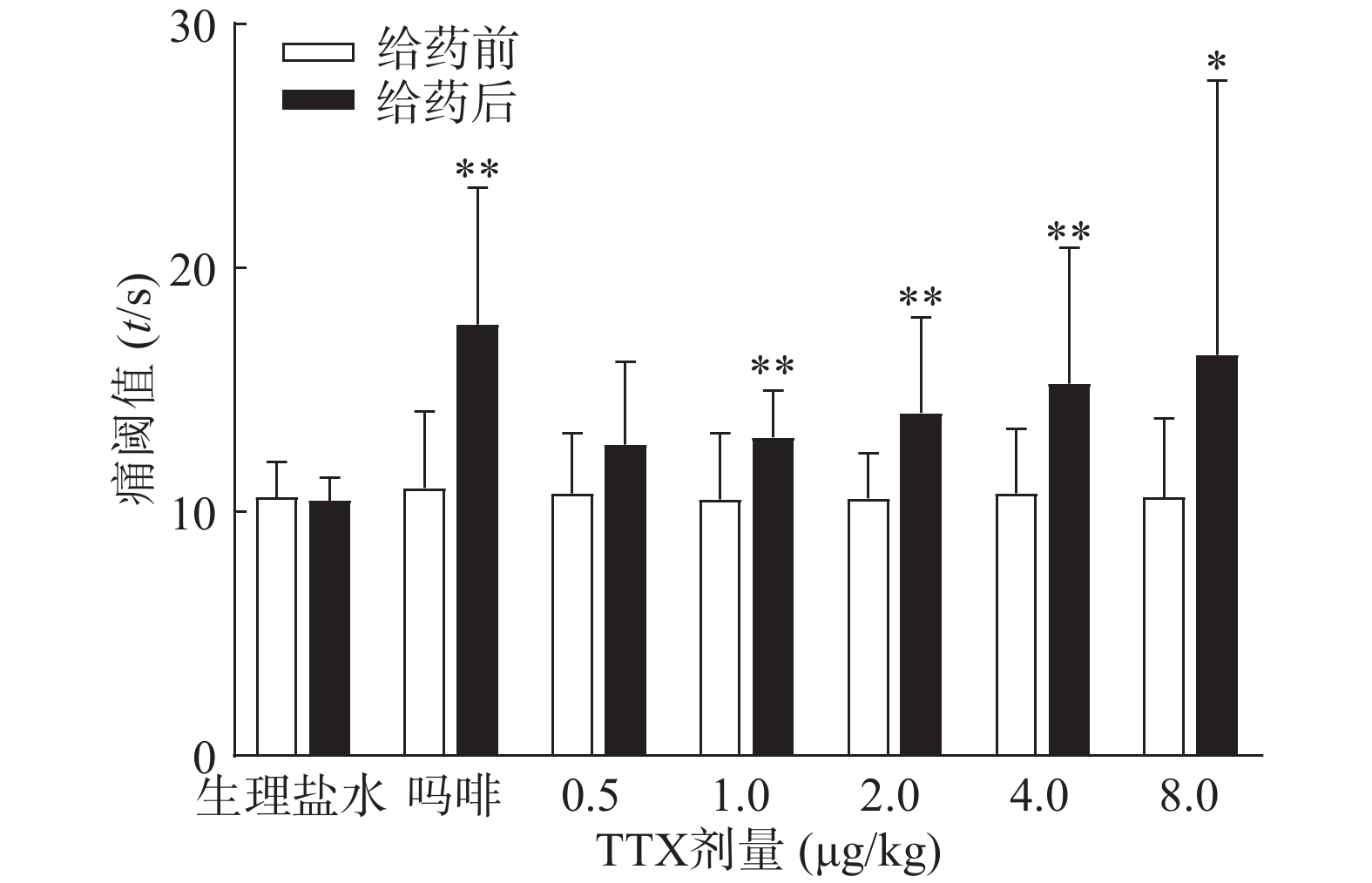
吗啡可显著延长小鼠热板时间(P<0.01),TTX在0.5 ~8 μg/kg的给药剂量范围,0.5 μg/kg剂量组与给药前相比无显著性差异,其余各剂量组可明显增加小鼠的痛阈值(P<0.05或P<0.01)(图4),但疼痛抑制率最高仅为19.79%,镇痛效应偏低,表明TTX对小鼠热板疼痛模型的镇痛效应较弱。
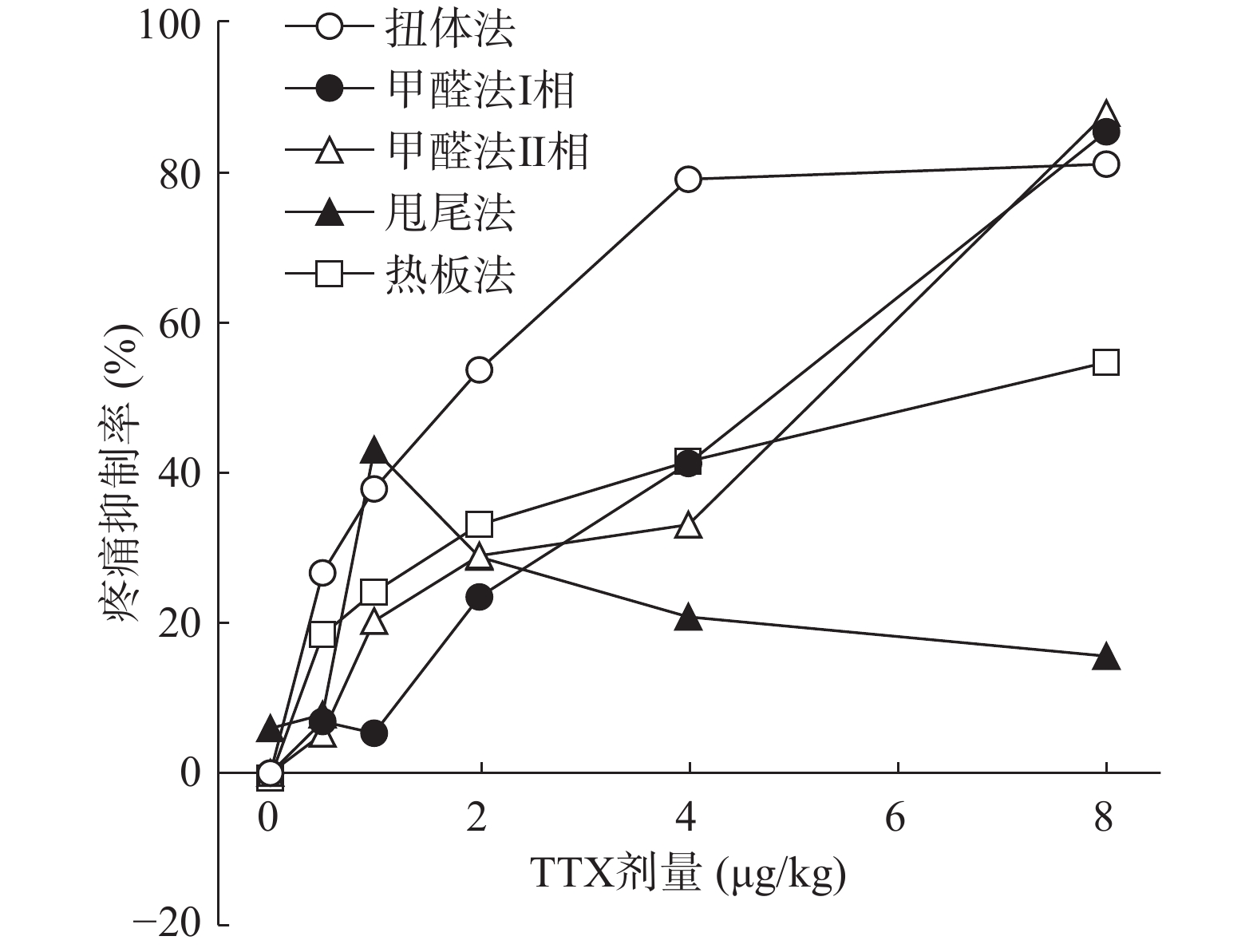
将4种模型计算得到的疼痛抑制率作图,如图5所示,TTX对动物扭体及福尔马林疼痛模型具有较好的镇痛效果,疼痛抑制率可达80%以上。在甩尾和热板模型上,TTX虽表现出一定的镇痛效果,但疼痛抑制率较低,整体镇痛效果偏弱。
-
对动物血清测定结果显示,与正常空白对照小鼠对比,腹腔注射0.6%的醋酸可显著提高血清中炎性介质花生四烯酸的水平(P<0.01),然而,TTX各剂量组血清花生四烯酸含量与醋酸对照组(TTX 0 μg/kg剂量组)比较,未有显著改变(P>0.05)。2.5%的福尔马林能显著升高大鼠血清花生四烯酸的含量(P<0.05),但TTX各剂量组未表现出对花生四烯酸的显著抑制作用(图6)。
-
目前,机体内已发现9种VGSCs亚型(Nav1.1~Nav1.9),根据对TTX的敏感性,又分为TTX敏感型钠通道和TTX非敏感型钠通道。其中Nav1.1、Nav1.2、Nav1.3、Nav1.4、Nav1.6和Nav1.7属于TTX敏感型钠通道,纳摩尔浓度的TTX即可抑制其电流。因此,TTX是已知毒性最大的神经毒素之一,其小鼠口服、皮下注射和腹腔注射的半数致死剂量分别为532、12.5和10.7 μg/kg,对人类的毒性剂量尚不明确[9]。本研究中应用的TTX剂量为0.5~8 μg/kg,各剂量组肌内注射后未观察到明显不良反应,且实验过程中及实验后14 d内无动物死亡。目前,报道的与疼痛相关的TTX敏感型钠通道有Nav1.1、Nav1.7、Nav1.3。近期的一项研究发现,激活脊根神经节Nav1.1通道会提升机械超敏小鼠的疼痛行为,且不会引起神经炎症[10]。Nav1.3通道参与外周和中枢神经系统对各种损伤的疼痛信号传导,应用Nav1.3反义核苷酸降低通道的表达,会减轻大鼠和小鼠的坐骨神经和脊髓损伤的疼痛反应[11-12]。Nav1.7通道在疼痛敏感性形成方面起重要作用,炎症反应如各种损伤、截肢或外科手术,导致Nav1.7的过度表达,Nav1.7基因敲除的小鼠对机械和热创伤性疼痛痛阈降低[2]。
TTX对多种疼痛尤其是炎症性疼痛和神经病理性疼痛的镇痛效应得到了广泛验证。低剂量TTX可显著降低脊神经结扎动物的疼痛行为,剂量依赖性地抑制角叉菜胶引起的机械性痛觉过敏、热痛觉过敏以及化疗药物诱导的神经病理性疼痛[7, 9]。近期研究也发现,TTX可有效抑制辣椒素等刺激诱发的内脏痛[13]。然而,TTX在急性疼痛治疗效果方面存在较大争议。本研究与其他研究结果都显示,TTX可有效抑制醋酸诱导的小鼠扭体次数[14-16]。Marcil等[16]的研究表明,TTX腹腔注射不能抑制福尔马林诱导的大鼠Ⅰ相疼痛反应,且只有高剂量TTX(6 μg/kg)显著抑制Ⅱ相疼痛反应。而徐英等[15]研究通过肌内注射TTX,可显著降低2.5%福尔马林注射后5 min内的疼痛反应。本研究结果也表明,TTX对福尔马林刺激引起的Ⅰ相和Ⅱ相疼痛均有较强的镇痛作用。
TTX对两种模型镇痛作用的机制可能与TTX的炎性疼痛抑制作用相关。小鼠扭体模型中,腹腔注射醋酸可刺激脏层和壁层腹膜,引起深部较大面积较长时间的炎性疼痛;足底皮下注射福尔马林引起的反应分为2个时相:0~10 min出现者为Ⅰ相(早期相),10 ~60 min出现的反应为Ⅱ相(迟发相)。Ⅰ相反应主要是刺激C纤维所致,Ⅱ相反应有炎症机制参与[17]。因此,为进一步明确TTX的镇痛效应与化学物质导致的炎性疼痛是否相关,本研究进行了血清花生四烯酸的测定,结果显示,醋酸和福尔马林均能显著提高动物血清中的花生四烯酸的水平,表明两者诱导的疼痛反应有炎性机制参与,但TTX并不能降低血清花生四烯酸水平,推测其可能是通过阻断炎性介质介导的疼痛反应产生镇痛效果,但不抑制炎性介质的产生或释放。
在急性物理性疼痛方面,虽然有研究显示TTX对热板和甩尾模型具有一定镇痛效应,但对两种模型的镇痛效应不一致,可能与足底和尾部的神经分布数量、类型差异相关[14]。然而,也有报道表明,皮下注射1~6 μg/kg TTX对热刺激、冷刺激及机械刺激导致的急性疼痛的抑制作用并不明显[18-20]。本研究发现,TTX通过肌内注射途径给药,对小鼠热板和甩尾疼痛模型镇痛效应较弱,TTX可能对热刺激引起的急性疼痛的镇痛作用较弱,但此结论还需要通过更多的给药途径和疼痛模型进一步验证和阐明。
本研究分别通过化学诱导和物理刺激方法,建立了4种急性疼痛模型并评估TTX的镇痛作用,结果表明TTX对醋酸和福尔马林诱导的化学诱导疼痛模型具有良好的镇痛效果,其作用可能与阻断化学物质诱导的炎性疼痛相关;而对热诱导(热板和热水)的物理刺激疼痛模型的镇痛效果较弱。本研究结果为TTX的安全、合理应用提供了进一步的实验支持。
Comparative study on analgesic effect of tetrodotoxin in four acute pain models
-
摘要:
目的 评估河豚毒素(tetrodotoxin,TTX)对4种急性疼痛模型的镇痛效果,为其合理应用提供实验支持。 方法 动物肌内注射1 mg/kg盐酸吗啡或不同剂量TTX,TTX剂量为0、0.5、1、2、4、8 μg/kg,给药后40 min,分别进行醋酸扭体实验、福尔马林刺激实验、热板实验和甩尾实验,记录动物疼痛反应或痛阈,计算疼痛抑制率;取动物血清,Elisa法测定花生四烯酸含量。 结果 盐酸吗啡对4种急性疼痛模型均有显著镇痛效应;TTX可减少醋酸诱导的小鼠扭体次数,降低福尔马林诱导的大鼠I相和II相疼痛反应,对两种疼痛模型的最高疼痛抑制率均达到80.00%以上;TTX在甩尾实验和热板实验中有一定的镇痛作用,最高疼痛抑制率分别为25.00%、19.79%。醋酸和福尔马林均能导致动物血清花生四烯酸升高,但是TTX对花生四烯酸无显著抑制作用。 结论 TTX对醋酸和福尔马林诱导的化学性刺激疼痛模型具有良好的镇痛效果,而对热诱导(热板和热水)的物理性刺激疼痛模型的镇痛效果较弱,TTX可能通过阻断炎性介质介导的疼痛反应产生镇痛效果。 Abstract:Objective To evaluate the analgesic effect of tetrodotoxin (TTX) in four types of acute pain models and provide experimental support for its rational application. Methods Mice or rats were intramuscularly pretreated with morphine (1 mg/kg) or TTX (0, 0.5, 1, 2, 4 and 8 μg/kg) 40 min before acetic acid writhing test, formalin stimulation test, hot plate test or tail flick test. Pain response or pain threshold were recorded, and inhibition rate was calculated during the tests. The arachidonic acid of serum was determined by Elisa. Results Significant analgesic effects were observed with morphine in all four acute pain models. TTX dose-dependently reduced the number of writhing induced by acetic acid and inhibited the pain response induced by formalin during phase I and phase II, with the highest inhibition rate of more than 80.00% in two pain models. TTX showed analgesic effect in tail flick test and hot plate test, with the highest inhibition rate of 25.00% and 19.79%, respectively. Both acetic acid and formalin increased arachidonic acid in animal serum, but TTX had no significant inhibitory effect on the releasing of arachidonic acid. Conclusion TTX showed significant analgesic effect in the chemical stimulation pain models induced by acetic acid and formalin, but limited analgesic effect was observed on the physical stimulation pain model induced by heat (hot plate and hot water). TTX may produce analgesic effect by blocking the inflammatory mediators mediating pain response. -
Key words:
- tetrodotoxin /
- morphine hydrochloride /
- analgesic effect /
- acute pain models
-
肝纤维化是由肝炎病毒、酒精摄入过量或代谢紊乱引起的急性/慢性肝损伤的一种病理伤口愈合反应,也是慢性肝病发病率和病死率高的主要原因[1, 2]。肝纤维化的特点是I 型胶原和纤维连接蛋白等细胞外基质(ECM)成分的过多聚集,形成纤维疤痕扭曲肝脏结构,最终造成肝脏器官功能损伤[2]。研究显示,肝星状细胞(HSCs)的过度激活是肝纤维化进程中的关键环节,也是肝纤维化防治研究的重要靶点[3, 4]。
全反式维甲酸(ATRA)是维生素A主要的生物活性形式,已是急性早幼粒细胞白血病的标准治疗方案[5]。近期研究证实,ATRA可逆转HSCs的活化,并对肝纤维化具有抑制作用,但其具体机制尚未完全阐明[6]。本文拟在细胞水平探索ATRA抑制HSCs增殖及活化的作用和机制,为ATRA的临床应用提供理论和实验基础。
1. 材料与方法
1.1 主要试剂
HSCs系LX-2细胞和培养基购自上海中乔新舟生物科技公司。胎牛血清(FBS)购自美国Gibco公司。血小板源性生长因子(PDGF-bb)、ATRA购自美国MedChemExpress公司。RNAiso试剂,逆转录和定量PCR试剂盒购自大连宝生物公司。PCR引物由上海生工生物工程公司合成。CCK-8、活性氧(ROS)、还原型谷胱甘肽(GSH)和丙二醛(MDA)等检测试剂盒购自上海碧云天生物科技公司。抗α-SMA、Collagen I、NRF2和LC3的抗体购自武汉三鹰生物科技公司;抗HO-1、ATF4、Beclin 1和GAPDH的抗兔购自武汉博士德生物科技公司。辣根过氧化物酶及FITC标记的二抗购自美国thermo Fisher Scientific公司。
1.2 HSCs的培养和诱导
HSCs系LX-2细胞解冻后,在DMEM培养基(含2%FBS),37 ℃,5%CO2条件下生长,每2 d更换一次培养基,细胞融合度达到85%以上时传代培养。以含PDGF-bb(10 ng/ml)的DMEM培养基作为诱导培养基;ATRA以5 μmol/L的浓度刺激。
1.3 细胞生长活力的检测
细胞生长活力通过CCK-8法检测。以每孔2×103个细胞的密度将LX-2细胞接种于96孔培养板,培养过夜。分别在常规培养基(含10%FBS)和PDGF-bb诱导培养基中培养,并添加ATRA(5 μmol/L)刺激。培养目标时间后,每孔加入10 μl的CCK-8溶液,继续培养1 h,轻轻拍动培养板充分混匀后,在酶标仪上测定450 nm的吸光度(A),并绘制细胞生长活力曲线。
1.4 蛋白表达的荧光检测
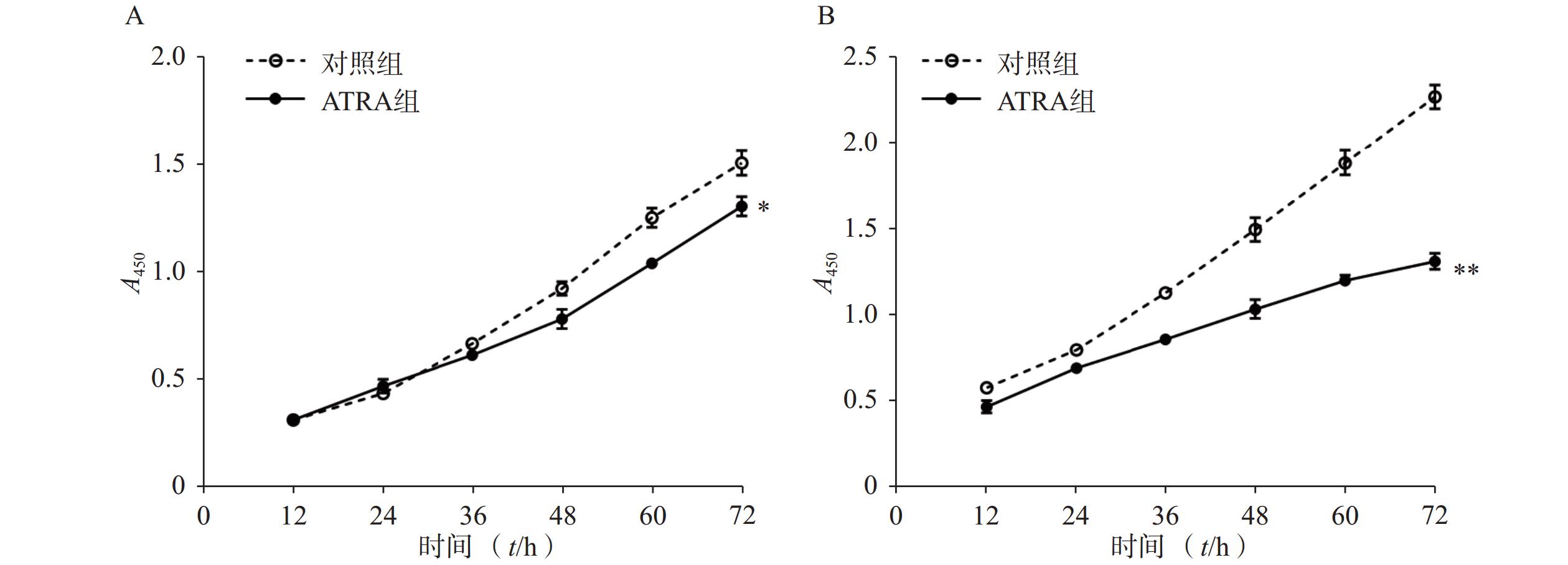
以每孔5×104个细胞的密度将LX-2细胞接种于24孔培养板(预置细胞爬片),培养过夜后以不同方式刺激培养48 h,以4%的多聚甲醛固定,并经Triton X-100(0.1%)透化10 min,随后经1%的BSA封闭1 h,加入一抗,4 ℃过夜孵育后,加入FITC标记的二抗,避光孵育1 h。加入DAPI染色1 min,以PBS-T缓冲液清洗后,用抗淬灭封片剂封片,于荧光倒置显微镜下观察并拍照。
1.5 蛋白表达的免疫印迹检测
以每孔2×105个细胞的密度将LX-2细胞接种于12孔培养板,培养过夜后以不同方式刺激培养48 h,利用RIPA试剂提取总蛋白。以20 μg总蛋白作为上样量,经SDS凝胶电泳分离后,转至甲醇预处理的PVDF膜。以5%脱脂牛奶常温封闭1 h,加入一抗,4 ℃过夜孵育后,加入辣根过氧化物酶标记二抗,常温孵育1 h。用TBS-T缓冲液清洗3次,经显色后在凝胶成像仪上观察并拍照记录。
1.6 基因表达的实时定量PCR检测
以每孔2×105个细胞的密度将LX-2细胞接种于12孔培养板,培养过夜后以不同方式刺激培养48 h,利用trizol试剂提取总RNA。以200 ng总RNA为模板,逆转录成互补DNA(cDNA),反应程序为37 ℃,10 min,85 ℃,5 s。以稀释后的cDNA为模板进行实时定量PCR反应,反应程序为95 ℃,15 s;56 ℃,20 s;72 ℃,20 s,共40个循环。以GAPDH基因作为内参,每个样品重复3次,经2−△△Ct法计算目的基因的相对表达水平。
1.7 细胞氧化应激的检测
细胞内ROS利用DCFH-DA荧光探针检测。以每孔1×104个细胞的密度将LX-2细胞接种于24孔培养板,培养过夜后以不同方式刺激培养48 h。去除培养液并加入100 μl含DCFH-DA(10 μmol/L)的无血清培养基,继续孵育20 min。用无血清培养基清洗3次后,在荧光显微镜下观察并拍照记录。
以每孔1×105个细胞的密度将LX-2细胞接种于6孔培养板,培养过夜后以不同方式刺激培养48 h。细胞内GSH和MDA水平根据试剂盒说明书进行检测,计算总蛋白中GSH和MDA的含量(nmol/mg)。
1.8 细胞自噬水平的检测
双荧光自噬流通过转染自噬双标腺病毒(pAd-mRFP-GFP-LC3)后检测。以每孔1×104个细胞的密度将LX-2细胞接种于24孔培养板,培养过夜。将腺病毒转染细胞后,以不同方式刺激培养48 h,即在荧光显微镜下分别观察红色及绿色荧光信号并拍照记录。自噬激活后,自噬体与溶酶体融合后绿色荧光发生淬灭,红色荧光增强。
1.9 统计学分析
所有数据均使用SPSS 26.0软件进行统计学分析,满足正态分布的计量数据以(
$ \bar x \pm s $ )表示。组间差异以独立样本t检验比较分析,以P<0.05说明差异具有统计学意义。2. 结果
2.1 ATRA对HSCs生长活力的影响
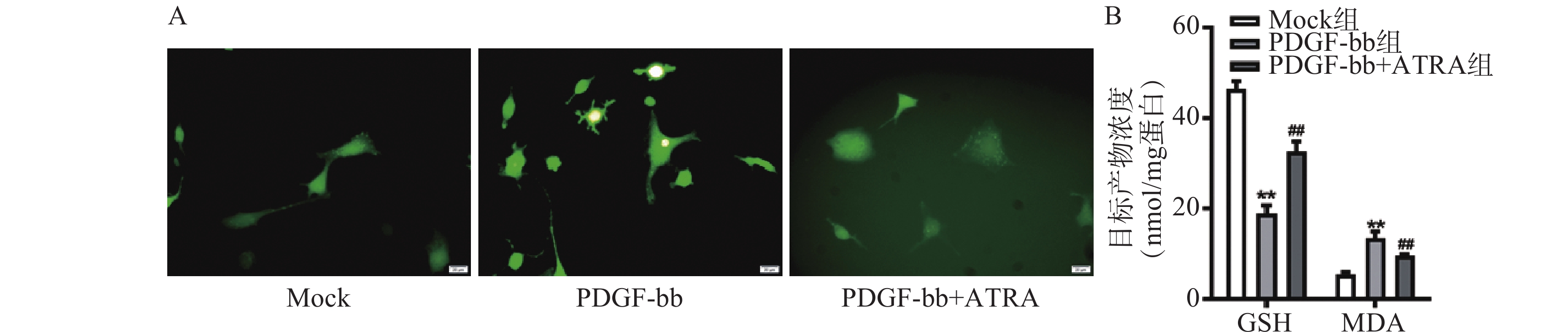
如图1A所示,常规培养条件下,5 μmol/L的ATRA处理48 h和72 h后的HSCs生长活力为对照组的(84.5±4.8)%和(86.7±3.0)%,具有一定的抑制作用。如图1B所示,在PDGF-bb诱导条件下,5 μmol/L的ATRA处理48 h和72 h后的HSCs生长活力为对照组的(52.4±3.0)%和(57.6±2.0)%,具有显著的抑制作用(P<0.01)。
2.2 ATRA对PDGF-bb诱导HSCs活化的影响
免疫荧光结果如图2A所示,与对照组相比,PDGF-bb刺激的HSCs中α-SMA的绿色荧光信号较强,而ATRA处理后,α-SMA荧光信号显著降低。蛋白质免疫印迹的结果如图2B所示,与对照组相比,PDGF-bb刺激的HSCs中α-SMA和Collagen I的蛋白表达明显增加,而ATRA处理后,α-SMA和Collagen I蛋白表达明显降低。
2.3 ATRA对HSCs氧化应激的影响
ROS的检测如图3A所示,PDGF-bb刺激后HSCs中的荧光强度明显强于对照组,而ATRA处理后荧光强度明显降低,提示ATRA抑制HSCs中ROS的生成。如图3B所示,PDGF-bb刺激后,HSCs中GSH的含量明显低于对照组(18.82±1.83 nmol/mg vs. 46.45±1.69 nmol/mg),MDA的含量则明显高于对照组(13.46±1.43 nmol/mg vs. 5.45±0.47 nmol/mg);ATRA处理后GSH的含量明显升高(32.60±2.23)nmol/mg,MDA的含量则明显降低(9.56±0.34)nmol/mg。
2.4 ATRA对HSCs中抗氧化基因表达的影响
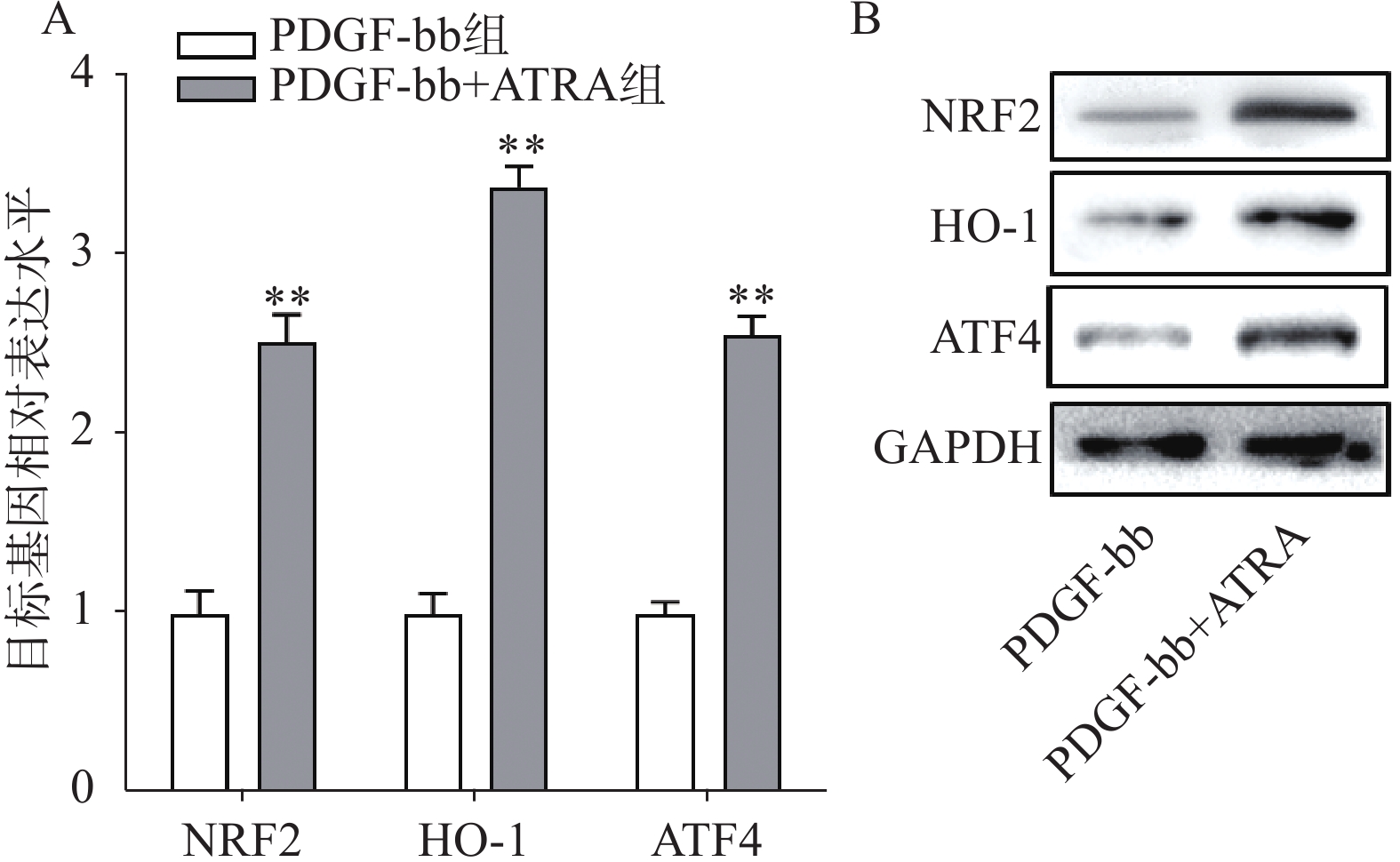
实时定量PCR的检测结果如图4A所示,ATRA处理后HSCs中抗氧化基因NRF2、HO-1和ATF4的表达明显增加(P<0.01),分别是PDGF-bb组的(2.53±0.15)倍、(3.34±0.12)倍和(2.58±0.10)倍。蛋白质免疫印迹的结果如图4B所示,NRF2、HO-1和ATF4的蛋白表达在ATRA处理后也明显增加。
2.5 ATRA对HSCs自噬活力的影响
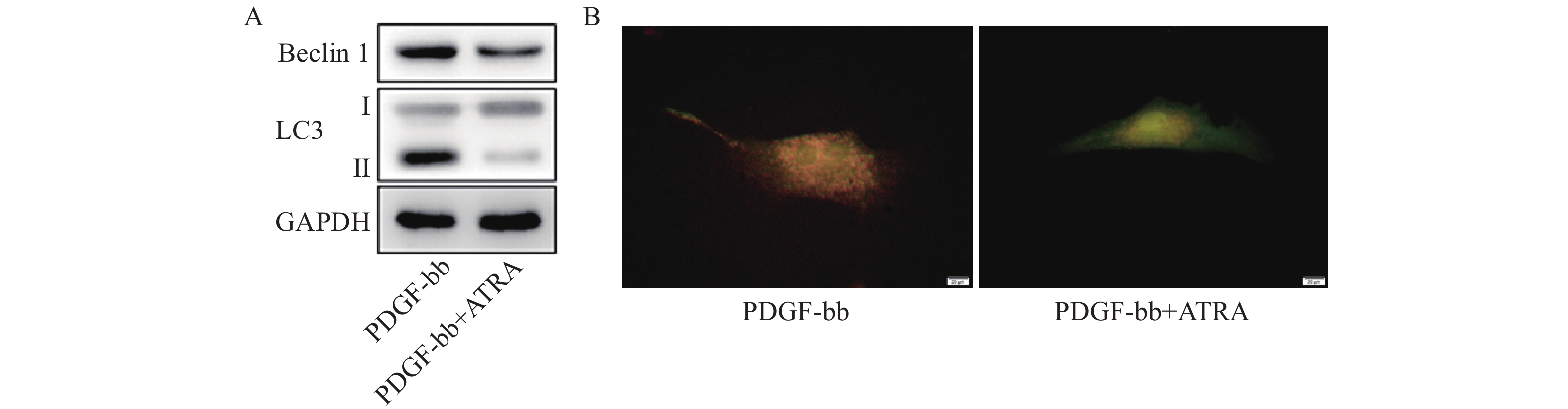
蛋白质免疫印迹的结果如图5A所示,ATRA处理后HSCs中自噬标志蛋白Beclin 1的表达和LC3 II/I均明显减少。双荧光自噬流的检测结果如图5B所示,ATRA处理后HSCs中红色荧光信号显著降低,绿色荧光信号明显增强,自噬流信号显著降低。
3. 讨论
目前,还没有特定的抗纤维化疗法来预防或逆转肝纤维化,现有的治疗方案旨在去除潜在的致病因子或紊乱,但也证明了肝纤维化的潜在可逆性[7]。研究证实,HSCs是主要的肝胶原生成细胞,并被认为是肝纤维化的主要效应细胞[8]。在健康肝脏中,HSCs位于窦周间隙,处于静止状态;当受到损伤或刺激时,HSCs会对各种促纤维化信号做出反应,如来自受损肝细胞的产物、来自Kupffer细胞的生长因子和细胞因子、重构的ECM以及肝外信号等[9]。HSCs的活化涉及多个进程,包括细胞死亡、衰老和恢复静止状态等。在此过程中,HSCs会失去其特有的细胞质脂滴,并转分化为肌成纤维样细胞,并合成ECM成分(如胶原纤维I型和III型),增殖、收缩和迁移等能力增强,并具有促炎作用。HSCs活化的作用机制和干预是肝纤维化防治研究的重要内容。
静息状态HSCs中富含的脂滴,其主要成分是甘油三酯和维生素A。脂滴脱落被认为是HSCs活化的形态学标志,但其生物学作用仍被广泛研究[10-12],作为维生素A的主要代谢产物,ATRA被发现广泛参与肝纤维化以及肝癌等的生物学过程[13, 14]。研究发现,ATRA能通过抑制硫氧还蛋白互用蛋白的表达,改善TGF-β诱导的HSCs的活化和维生素A缺乏诱导的肝纤维化[6]。目前的研究证实,ATRA 通过与 RAR(α、β、γ)或 PPARβ/δ 起作用,但这两组受体的生物效应在某些进程中却完全相反[15],提示ATRA抗肝纤维化的作用机制未完全阐明。还有研究发现,纤维化药物对HSCs的激活最终会导致这些细胞的衰老,尽管有助于纤维化的逆转,但也可能会导致肝癌的发生[16]。因此,ATRA作为抗肝纤维化潜在药物的应用潜能仍需深入探索和验证。
研究证实,氧化应激与肝纤维化之间存在密切关系,当肝脏受到氧自由基的攻击时,机体抗氧化防御系统的平衡就会被打破,从而导致氧化应激[17, 18]。MDA是脂质过氧化的主要代谢产物,过量时会严重破坏细胞膜结构,也被认为是肝脏中自由基的间接指标[19]。GSH是哺乳动物细胞中最主要的自由基清除剂,广泛分布于包括肝脏在内的多个器官。研究发现,GSH可保护肝细胞免受各种自由基的侵害,包括ROS、脂质氢过氧化物、异生物毒物和重金属[20]。因此,调节MDA和GSH水平有助于控制氧化应激,最终可能有助于抑制肝纤维化。有研究报道,抑制氧化应激显著降低MDA水平,提高GSH水平,对CCl4诱导的小鼠肝脏纤维化具有明显的保护作用[21]。本课题研究发现ATRA处理促进了HSCs中抗氧化基因的表达,造成细胞中GSH的增加和MDA的降低,减少细胞内ROS的水平,缓解了PDGF-bb诱导的HSCs氧化应激,研究结果提示,以氧化应激为靶点可能是肝纤维化防治的潜在策略。
自噬是真核细胞消除一次性或有潜在危险的细胞质物质的一种新陈代谢过程,可以消除有缺陷的蛋白质和细胞器、细胞内的病原体,防止异常蛋白质的积累,在许多疾病的病理过程中发挥着积极作用[22]。越来越多的证据表明,肝细胞和非实质性细胞(HSCs、Kupffer 细胞等)的自噬反应对肝脏的生理功能至关重要[23]。近年来,HSCs自噬成为肝纤维化研究领域的热点,其调控机制日益受到关注。研究显示,自噬水平的增加可加速HSCs中脂滴的降解,为HSCs的激活提供能量支持[24, 25]。降低HSCs自噬活性显著抑制其活化,降低小鼠的肝纤维化程度[26]。本课题的结果证实,ATRA能抵抗PDGF-bb诱导的HSCs自噬水平的增加,这可能是ATRA抑制HSCs活化的分子机制之一。
HSCs是目前研究药物代谢和毒理的重要工具,也是研究肝纤维化的绝佳模型[27]。HSCs具有高度的可塑性,在有害刺激下会改变其表型和代谢,并产生大量ECM成分来替代受伤细胞,生成纤维化疤痕[28]。在各种HSCs细胞系中,LX-2是目前广泛使用的细胞类型[29]。研究发现,LX-2细胞会根据培养基中FBS的浓度改变其代谢,从而诱导基因的差异表达。在低浓度FBS下,LX-2细胞呈静止样表型;在高浓度 FBS下,细胞呈肌成纤维细胞样表型,产生ECM成分[30]。本课题研究发现,PDGF-bb可诱导LX-2细胞向肌成纤维细胞样表型分化,表现为高增殖水平,且高表达α-SMA和Collagen I,而ATRA的处理显著降低细胞增殖能力并抑制α-SMA和Collagen I的表达,表明ATRA具有抵抗HSCs活化的潜在功能。
本课题在细胞水平探索了ATRA对HSCs激活的作用和潜在机制。ATRA促进抗氧化基因的表达,降低PDGF-bb诱导的HSCs氧化应激及自噬活力,抑制了HSCs的激活。研究结果证实了ATRA对肝纤维化防治的潜在应用。需要注意的是,自噬活性的激活存在“双刃剑”作用,ATRA对HSCs自噬活性的调节还需要进行剂量和分子机制等方面的深入研究。
-
[1] 赖晓慧, 肖义军. 河豚毒素的研究进展[J]. 福建畜牧兽医, 2019, 41(4):23-24, 29. doi: 10.3969/j.issn.1003-4331.2019.04.010 [2] MELNIKOVA D, KHOTIMCHENKO Y, MAGARLAMOV T. Addressing the issue of tetrodotoxin targeting[J]. Mar Drugs,2018,16(10):352. doi: 10.3390/md16100352 [3] 于翠萍, 王长都, 安建雄, 等. 河豚毒素可能的镇痛机制及镇痛作用[J]. 中国处方药, 2009, 7(3):82-84. [4] MA R S Y, KAYANI K, WHYTE-OSHODI D, et al. Voltage gated sodium channels as therapeutic targets for chronic pain[J]. J Pain Res,2019,12:2709-2722. doi: 10.2147/JPR.S207610 [5] ZHAO C, LIU A D, SANTAMARIA C M, et al. Polymer-tetrodotoxin conjugates to induce prolonged duration local anesthesia with minimal toxicity[J]. Nat Commun,2019,10(1):2566. doi: 10.1038/s41467-019-10296-9 [6] LIU Q, SANTAMARIA C M, WEI T, et al. Hollow silica nanoparticles penetrate the peripheral nerve and enhance the nerve blockade from tetrodotoxin[J]. Nano Lett,2018,18(1):32-37. doi: 10.1021/acs.nanolett.7b02461 [7] NIETO F R, COBOS E J, TEJADA M Á, et al. Tetrodotoxin (TTX) as a therapeutic agent for pain[J]. Mar Drugs,2012,10(12):281-305. doi: 10.3390/md10020281 [8] HAGEN N A, CANTIN L, CONSTANT J, et al. Tetrodotoxin for moderate to severe cancer-related pain: a multicentre, randomized, double-blind, placebo-controlled, parallel-design trial[J]. Pain Res Manag,2017,2017:7212713. [9] LAGO J, RODRÍGUEZ L P, BLANCO L, et al. Tetrodotoxin, an extremely potent marine neurotoxin: distribution, toxicity, origin and therapeutical uses[J]. Mar Drugs,2015,13(10):6384-6406. doi: 10.3390/md13106384 [10] OSTEEN J D, HERZIG V, GILCHRIST J, et al. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain[J]. Nature,2016,534(7608):494-499. doi: 10.1038/nature17976 [11] HAINS B C, SAAB C Y, KLEIN J P, et al. Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury[J]. J Neurosci,2004,24(20):4832-4839. doi: 10.1523/JNEUROSCI.0300-04.2004 [12] HAINS B C, KLEIN J P, SAAB C Y, et al. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury[J]. J Neurosci,2003,23(26):8881-8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003 [13] GONZÁLEZ-CANO R, TEJADA M Á, ARTACHO-CORDÓN A, et al. Effects of tetrodotoxin in mouse models of visceral pain[J]. Mar Drugs,2017,15(6):E188. doi: 10.3390/md15060188 [14] 于丽华, 周力, 谢克勤. 河豚毒素对小鼠镇痛作用的实验研究[J]. 山东医科大学学报, 1999, 37(2):120-121, 126. [15] 徐英, 耿兴超, 韩继生, 等. 河豚毒素单用及与吗啡合用对大鼠福尔马林致痛的影响[J]. 中国海洋药物, 2003, 22(2):39-41, 56. doi: 10.3969/j.issn.1002-3461.2003.02.011 [16] MARCIL J, WALCZAK J S, GUINDON J, et al. Antinociceptive effects of tetrodotoxin (TTX) in rodents[J]. Br J Anaesth,2006,96(6):761-768. doi: 10.1093/bja/ael096 [17] 徐叔云, 卞如濂, 陈修. 药理实验方法学[M]. 3版. 北京: 人民卫生出版社, 2002. [18] KAYSER V, VIGUIER F, IOANNIDI M, et al. Differential anti-neuropathic pain effects of tetrodotoxin in sciatic nerve- versus infraorbital nerve-ligated rats - Behavioral, pharmacological and immunohistochemical investigations[J]. Neuropharmacology,2010,58(2):474-487. doi: 10.1016/j.neuropharm.2009.09.003 [19] ENTRENA J M, COBOS E J, NIETO F R, et al. Sigma-1 receptors are essential for capsaicin-induced mechanical hypersensitivity: studies with selective Sigma-1 ligands and Sigma-1 knockout mice[J]. Pain,2009,143(3):252-261. doi: 10.1016/j.pain.2009.03.011 [20] NIETO F R, ENTRENA J M, CENDÁN C M, et al. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice[J]. Pain,2008,137(3):520-531. doi: 10.1016/j.pain.2007.10.012 期刊类型引用(4)
1. 王冲,朱攀科,王雪玲. 宫颈癌组织PCNA、Derlin-1表达与术前分期及术后复发转移的关系. 医学理论与实践. 2024(08): 1378-1381 .  百度学术
百度学术2. 郑晓骏,张蕾,张晓君,来保勇. 中医药治疗乳腺癌的机制和临床研究进展. 中医药信息. 2024(06): 76-81 .  百度学术
百度学术3. 郑洋,杨智荟,李灿婷,张璟钊,黄麟翔,赵铁建,王佳慧,段雪琳. 莪术醇对肝星状细胞内质网应激和凋亡的作用研究. 中国临床药理学杂志. 2023(10): 1431-1435 .  百度学术
百度学术4. 刘芙蓉,顾宇,张树明. 黑龙江省不同产区五味子的木脂素及有机酸的含量测定及对比分析研究. 黑龙江中医药. 2023(06): 363-367 .  百度学术
百度学术其他类型引用(2)
-





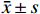
 下载:
下载:

 下载:
下载: