-
肝细胞癌(HCC)是亚洲第五大常见癌症,也是癌症死亡的常见原因,其中中国占亚洲病例的62.4%[1]。HCC晚期治疗方法则有经肝动脉化疗栓塞术(TACE)、广谱络氨酸酶抑制剂(TKI)、免疫疗法和抗血管生成疗法。上述治疗策略延长了患者的生存期,但存在广谱毒副作用,长期应用分子靶向治疗还会出现严重耐药等问题[2]。中药治疗作为HCC的另一种疗法,在HCC中有独特的治疗作用。其能缓解癌症患者的临床症状、改善生活质量、提高免疫功能、预防复发和转移、延缓肿瘤进展和延长生存期[3-5]。因此,探究中药抗肝细胞癌的物质基础和作用机制越来越受到关注。
通关藤又称乌骨藤、通关散、通光藤等,在我国主要分布在云南和贵州两省,其最初记载于明代的《滇南本草》,药用历史悠久。通关藤富含多种生物活性成分,如甾体苷、多糖、有机酸等,其中C21甾体苷被认为是主要的抗肿瘤物质。大量临床研究发现通关藤在肝细胞癌和其它肿瘤中有特异性治疗作用[6]。在Huh7和HepG2细胞系中,通关藤皂苷H(TEH)通过下调PI3K/AKT/mTOR信号通路诱导自噬和凋亡,从而增加肿瘤细胞的敏感性[7]。在肝细胞癌患者来源的肿瘤异种移植物模型中,通关藤提取物通过下调血管生成标志物的表达、阻断血管生成因子的分泌,抑制肿瘤中血管网络的形成,从而延缓HCC的生长[8]。上述研究仅基于单个药物-单个靶点-单个通路的思路进行研究设计,未充分考虑中药成分的复杂性。中药发挥药效是一个多成分-多靶点-多通路的协同作用。因此,本研究拟利用网络药理学,寻找通关藤的多成分-多靶点-多通路的关系,构建药物成分-靶点网络图,从多个角度探讨通关藤治疗肝细胞癌的物质基础和作用机制,结合生物信息学方法,挖掘肝细胞癌基因芯片数据,寻找通关藤治疗HCC的潜在靶点,然后通过分子对接技术验证并展示通关藤活性成分与关键靶点间的相互作用,明确通关藤治疗肝细胞癌的物质基础和作用机制,以期为通关藤在HCC临床应用中提供理论参考。
-
检索《中国期刊全文数据库》(中国知网)和PubMed数据库,收集通关藤的成分。将获得的化合物输入到PubChem数据库中,获得Smiles结构式;对于未在PubChem数据库中获得Smiles结构式的化合物,进一步采用ChemDraw软件获得。通过Swiss ADME获得化合物的具体信息。依据“类药五原则”筛选出有效活性成分。利用Swiss Target Prediction预测化合物的作用靶点,并通过Uniprot数据库转化为标准的基因名称。
-
从GEO数据库以“hepatocellular carcinoma”和“Homo sapiens”为关键词,筛选得GSE147888系列数据。该系列包括12份肿瘤样本和12份邻近非肿瘤肝脏组织,采用R语言进行差异分析,收集两组之间差异基因表达的微阵列信息。微阵列数据以调整后P<0.05和│log2(FC)│≥1为标准筛选表达有明显差异的基因。采用R语言绘制差异基因热图和火山图。
在Genecards数据库和OMIM数据库中以“hepatocellular carcinoma”为关键词,搜索与肝细胞癌相关疾病靶点,合并、去除重复靶点。
-
利用Venny 2.1(https://bioinfogp.cnb.csic.es/tools/venny/)将活性成分靶点、GEO数据库筛选的靶基因和疾病数据库筛选的靶点取交集,得到共同靶点基因,并绘制韦恩图。将获得的共同靶点基因导入在线分析网站String,构建蛋白质-蛋白质相互作用(PPI)网络。设置条件为中等置信度>0.4。利用Cytoscape v3.8.0软件中的“Network analyze”功能进行网络拓扑分析。将药物活性成分与共同靶基因构建网络。
-
为了进一步探索通关藤活性成分对HCC的潜在作用机制和共同靶基因的功能,使用R语言中的Bioconductor包和Cluster Profiler包[9]对上一步获得的靶基因进行GO和KEGG信号通路分析。使用GOplot包对结果进行可视化。GO分析包括生物学过程(BP)、细胞组成(CC)、分子功能(MF)三方面。
-
在GEPIA数据库生存分析模块中分析HCC患者的总生存率与关键基因表达水平的相关性;使用人类蛋白质图谱(HPA)数据库分析关键基因的蛋白表达水平。
-
对通关藤活性成分和潜在靶点进行分子对接验证,探索它们之间的相互作用。首先从PubChem数据库下载通关藤活性成分的2D结构(SDF格式),利用OpenBabel 2.4.1转化为mol格式。然后从PDB数据库下载受体蛋白的3D结构(PDB格式)。PyMOL软件用于执行蛋白质的脱水和磷酸盐去除等操作。最后,利用PyMOL去掉受体蛋白配体,并用Auto Dock Tools软件对受体蛋白和配体分子进行去水、加氢、加电荷操作,保存为pdbqt格式,然后借助Auto Dock Vina软件进行分子对接。PyMOL将对接结果进行可视化。结合能<0表示配体和受体可以自发结合。选择结合能≤ −5.0 kcal/mol作为筛选依据,评价生物信息分析和预测的可靠性[10]。
-
通过文献检索[11-18],共收集到50种活性成分(表1)和588个靶基因。其中通关藤苷元甲未查询到靶基因。
表 1 通关藤活性成分信息
编号 化合物名称 Simles结构式 1 2,4-Decadienal(2,4-癸二烯醛) CCCCC/C=C/C=C/C=O 2 2,4-Dihydroxybenzoic Acid(2,4 -二羟基苯甲酸) C1=CC(=C(C=C1O)O)C(=O)O 3 2,6-Dihydroxybenzoic Acid(2,6 -二羟基苯甲酸) C1=CC(=C(C(=C1)O)C(=O)O)O 4 3,4-Dihydroxybenzoic Acid(3,4 -二羟基苯甲酸) C1=CC(=C(C=C1C(=O)O)O)O 5 Betulinic Acid(白桦脂酸) CC(=C)C1CCC2(C1C3CCC4C5(CCC(C(C5CCC4(C3(CC2)C)C)(C)C)O)C)C(=O)O 6 Caffeic Acid(咖啡酸) C1=CC(=C(C=C1/C=C/C(=O)O)O)O 7 Chlorogenic Acid(绿原酸) C1C(C(C(CC1(C(=O)O)O)OC(=O)C=CC2=CC(=C(C=C2)O)O)O)O 8 Cissogenin(西索苷元) CC(C1CCC2(C1(C(C(C3C2CC=C4C3(CCC(C4)O)C)O)O)C)O)O 9 Conduritol(环己烯四醇) C1=CC(C(C(C1O)O)O)O 10 D(+)-Digitoxose(D (+)-洋地黄毒糖) CC(C(C(CC=O)O)O)O 11 Daucosterol(胡萝卜甾醇) CCC(CCC(C)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)OC5C(C(C(C(O5)CO)O)O)O)C)C)C(C)C 12 D-Cymarose(加拿大麻糖) CC(C(C(CC=O)OC)O)O 13 Dihydrosarcostin(二氢肉珊瑚苷元) CC(C1(CCC2(C1(C(CC3C2(CCC4C3(CCC(C4)O)C)O)O)C)O)O)O 14 Dresgenin(苦绳甙元) CC(C1(CCC2(C1(C(CC3C2(CCC4C3(CCC(C4)O)C)O)OC(=O)C5=CC=CC=C5)C)O)O)O 15 Drevogenin A(南山藤皂苷元A) CC(C)CC(=O)OC1C(C2C(CC=C3C2(CCC(C3)O)C)C4(C1(C(CC4)C(=O)C)C)O)OC(=O)C 16 Drevogenin Q(南山藤皂苷元Q) CC(C)CC(=O)OC1C2C(CC=C3C2(CCC(C3)O)C)C4(CCC(C4(C1OC(=O)C)C)C(=O)C)O 17 D-Thevetose(D-黄花夹竹桃糖) CC(C(C(C(C=O)O)OC)O)O 18 Galactitol(半乳糖醇) C(C(C(C(C(CO)O)O)O)O)O 19 Gallic Acid(没食子酸) C1=C(C=C(C(=C1O)O)O)C(=O)O 20 Kaempferol(山奈酚) C1=CC(=CC=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O 21 Kaempferol-4'-Methylether(山柰酚-4-甲醚) COC1=CC=C(C=C1)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O 22 Lupeol(羽扇豆醇) CC(=C)C1CCC2(C1C3CCC4C5(CCC(C(C5CCC4(C3(CC2)C)C)(C)C)OC(=O)C6=
CC=CC=C6)C)C23 Marsectobiose(直立牛奶菜双糖) CC1C(C(C(C(O1)OC(C(C)O)C(CC=O)OC)O)OC)O 24 Marstenacigenin A(二氢肉珊瑚苷元的衍生物) CC(C1(CCC2(C1(C(CC3C2(CCC4C3(CCC(C4)O)C)O)C(=O)C=CC5=CC=CC=C5)C)O)O)O 25 Methyl Shikimate(莽草酸甲酯) COC(=O)C1=CC(C(C(C1)O)O)O 26 Nerolidol(橙花叔醇) CC(=CCCC(=CCCC(C)(C=C)O)C)C 27 Oleandrose(齐墩果糖) CC(C(C(CC=O)OC)O)O 28 P-Hydroxycinnamic Acid(对羟基肉桂酸) C1=CC(=CC=C1C=CC(=O)O)O 29 Propionic Acid(丙酸) CCC(=O)O 30 Protocatechualdehyde(中原儿茶醛) C1=CC(=C(C=C1C=O)O)O 31 Scopoletin(东莨菪素) COC1=C(C=C2C(=C1)C=CC(=O)O2)O 32 Scutellarein-4-Methylether(灯盏乙素-4-甲醚) COC1=CC=C(C=C1)C2=CC(=O)C3=C(O2)C=C(C(=C3O)O)O 33 Shikimic Acid(莽草酸) C1C(C(C(C=C1C(=O)O)O)O)O 34 Sinapic Acid(芥子酸) COC1=CC(=CC(=C1O)OC)C=CC(=O)O 35 Stigmasterol(豆甾醇) CCC(C=CC(C)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C)C(C)C 36 Succinic Acid(琥珀酸) C(CC(=O)O)C(=O)O 37 Syringic Acid(丁香酸) COC1=CC(=CC(=C1O)OC)C(=O)O 38 Taraxasterol(蒲公英甾醇) CC1C2C3CCC4C5(CCC(C(C5CCC4(C3(CCC2(CCC1=C)C)C)C)(C)C)O)C 39 Tenacigenin B(通光散苷元B) CC(=O)C1CCC23C1(C(C(C4C2(O3)CCC5C4(CCC(C5)O)C)O)O)C 40 Tenacigenin D(通光散苷元D) CCC(C)C(=O)OC1C2C3(CCC(=O)CC3CCC24C5(O4)CCC(C5(C1OC(=O)C)C)C(=O)C)C 41 Tenacissoside A(通关藤皂苷A) CC=C(C)C(=O)OC1C2C3(CCC(CC3CCC24C5(O4)CCC(C5(C1OC(=O)C)C)C(=O)C)OC6CC(C(C(O6)C)OC7C(C(C(C(O7)C)OC8C(C(C(C(O8)CO)O)O)O)OC)O)OC)C 42 Tenacissoside B(通关藤皂苷B) CC=C(C)C(=O)OC1C2C3(CCC(CC3CCC24C5(O4)CCC(C5(C1OC(=O)C(=CC)C)C)C(=O)C)OC6CC(C(C(O6)C)OC7C(C(C(C(O7)C)OC8C(C(C(C(O8)CO)O)O)O)OC)O)OC)C 43 Tenacissoside G(通关藤皂苷G) CC=C(C)C(=O)OC1C2C3(CCC(CC3CCC24C5(O4)CCC(C5(C1OC(=O)C)C)C(=O)C)OC6CC(C(C(O6)C)OC7C(C(C(C(O7)C)O)OC)O)OC)C 44 Tenacissoside H(通关藤皂苷H) CCC(C)C(=O)OC1C2C3(CCC(CC3CCC24C5(O4)CCC(C5(C1OC(=O)C)C)C(=O)C)OC6CC(C(C(O6)C)OC7C(C(C(C(O7)C)O)OC)O)OC)C 45 Tenacissoside I(通关藤皂苷I) CC1C(C(C(C(O1)OC2C(OC(CC2OC)OC3CCC4(C(C3)CCC56C4C(C(C7(C5(O6)CCC7C(=O)C)C)OC(=O)C)OC(=O)C8=CC=CC=C8)C)C)O)OC)O 46 Tenasogenin(通关苷元) CC(C1CCC2(C1(C(C(C3C2CC=C4C3(CCC(C4)O)C)OC(=O)C=C(C)C)O)C)O)O 47 Vanillic Acid(香草酸) COC1=C(C=CC(=C1)C(=O)O)O 48 β-Amyrin(β-香树脂醇) CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)O)C)C)C2C1)C)C)C 49 β-Sitosterol(β-谷甾醇) CCC(CCC(C)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C)C(C)C 50 17β-Tenacissoside B(17β-通光藤皂苷B) O[C@H]1CC[C@]2(C(C1)CC[C@@]13C2[C@H](O)[C@@H](O)[C@]2([C@]3(O1)CC[C@@H]2C(=O)C)C)C -
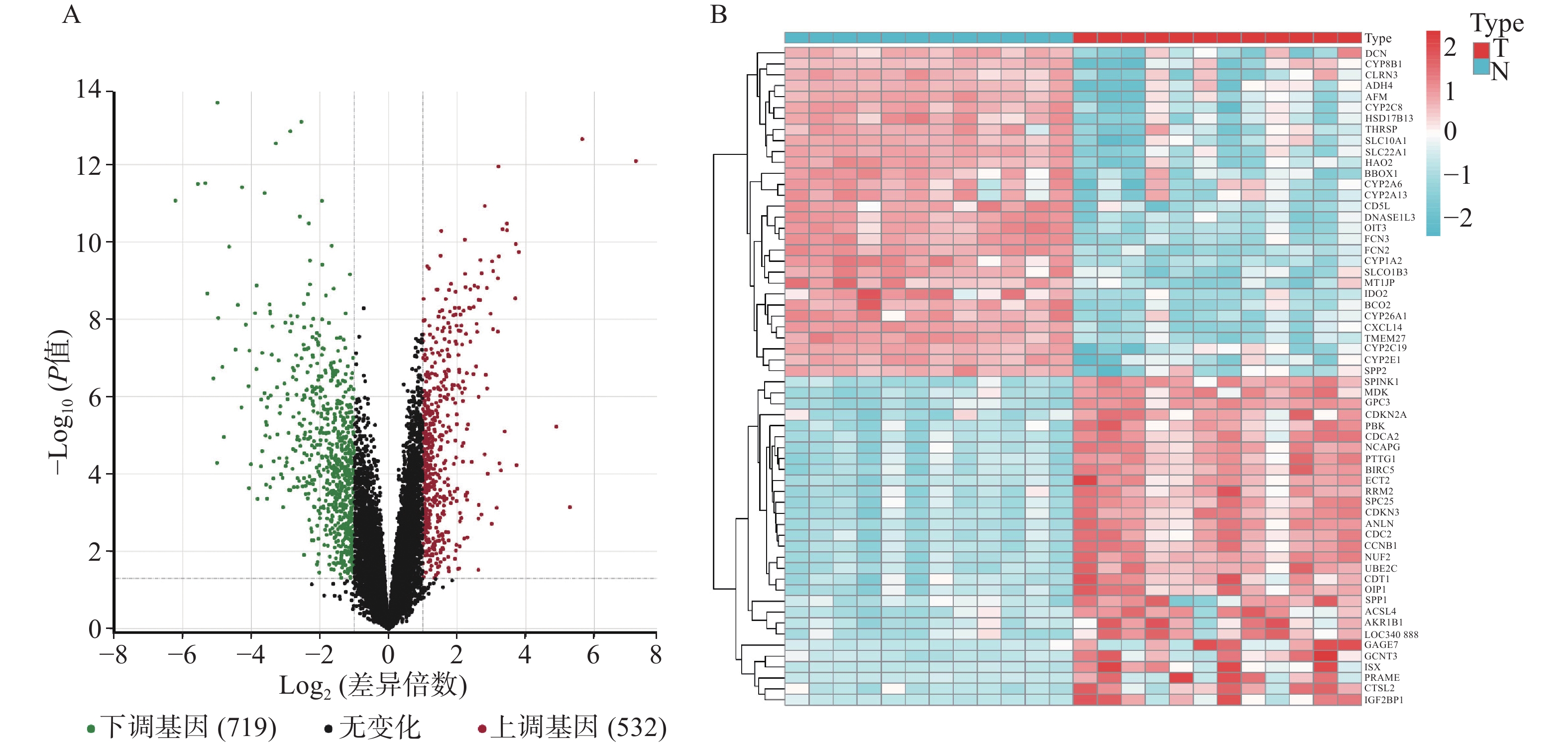
GSE147888包括12个正常人肝组织样本和12个肝细胞癌组织样本,对样本进行差异基因表达分析。结果显示(见图1),共有1251个差异基因,其中上调基因532个,下调基因719个。从Genecards和OMIM数据库中获得靶基因783个。
-
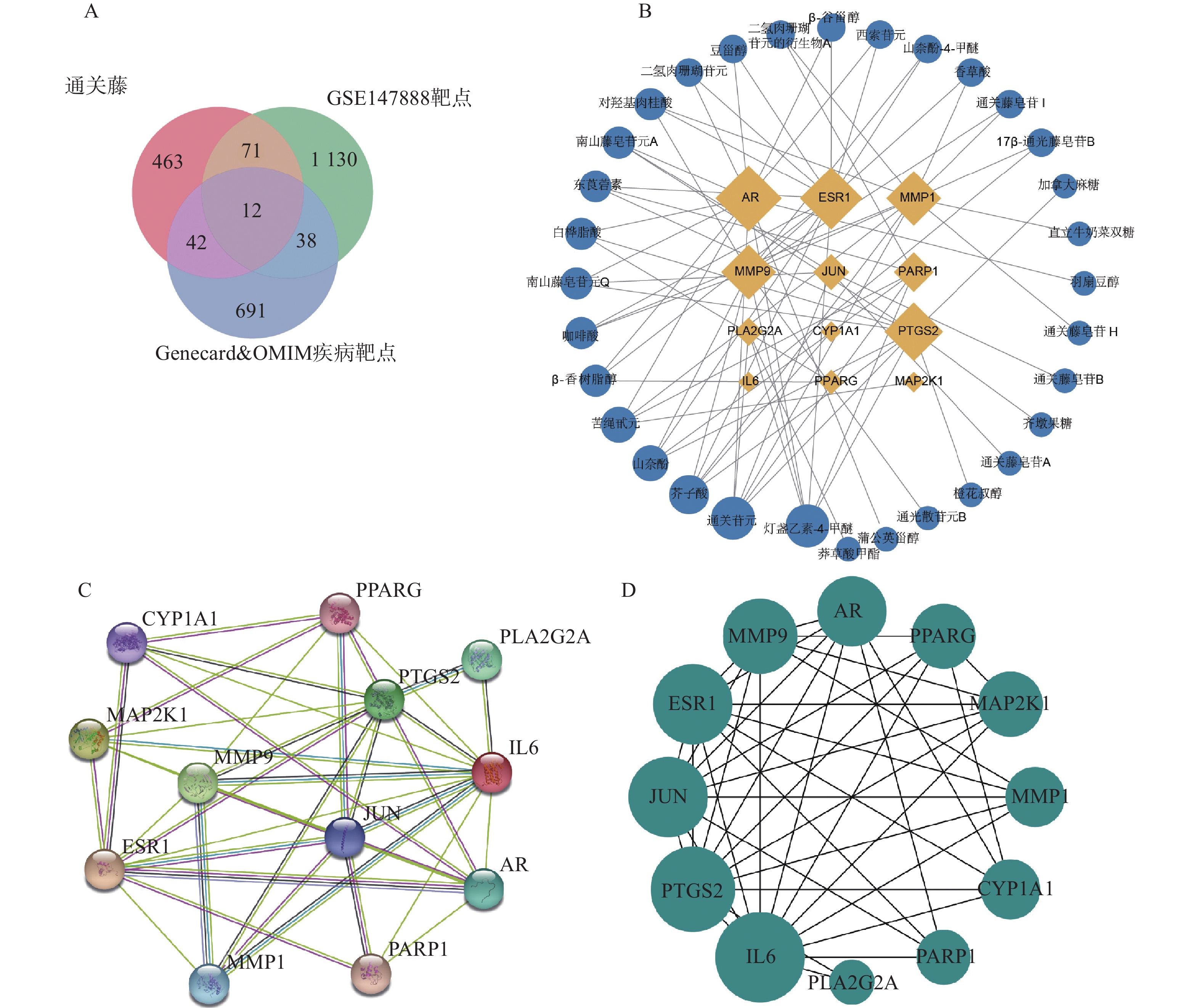
将活性成分靶点、GEO数据库筛选的靶基因和疾病数据库筛选的靶点取交集(见图2A),得到共同靶点基因12个,分别是ESR1、CYP1A1、PARP1、PTGS2、AR、JUN、MAP2K1、IL6、MMP1、MMP9、PPARG、PLA2G2A。将共同靶点基因与其对应的活性成分构建“成分-靶点网络图”。如图2B所示,网络图含44个节点、75条边,节点包含12个靶点基因和32个化合物。以度值对化合物进行排序,发现化合物野黄芩素四甲醚、通关苷元、芥子酸、苦绳苷元、山奈酚排名靠前。将交集基因构建PPI网络(见图2C和图2D),发现基因度值排名靠前。提示这些化合物和基因可能是通关藤治疗肝细胞癌的关键成分和关键靶点。
-
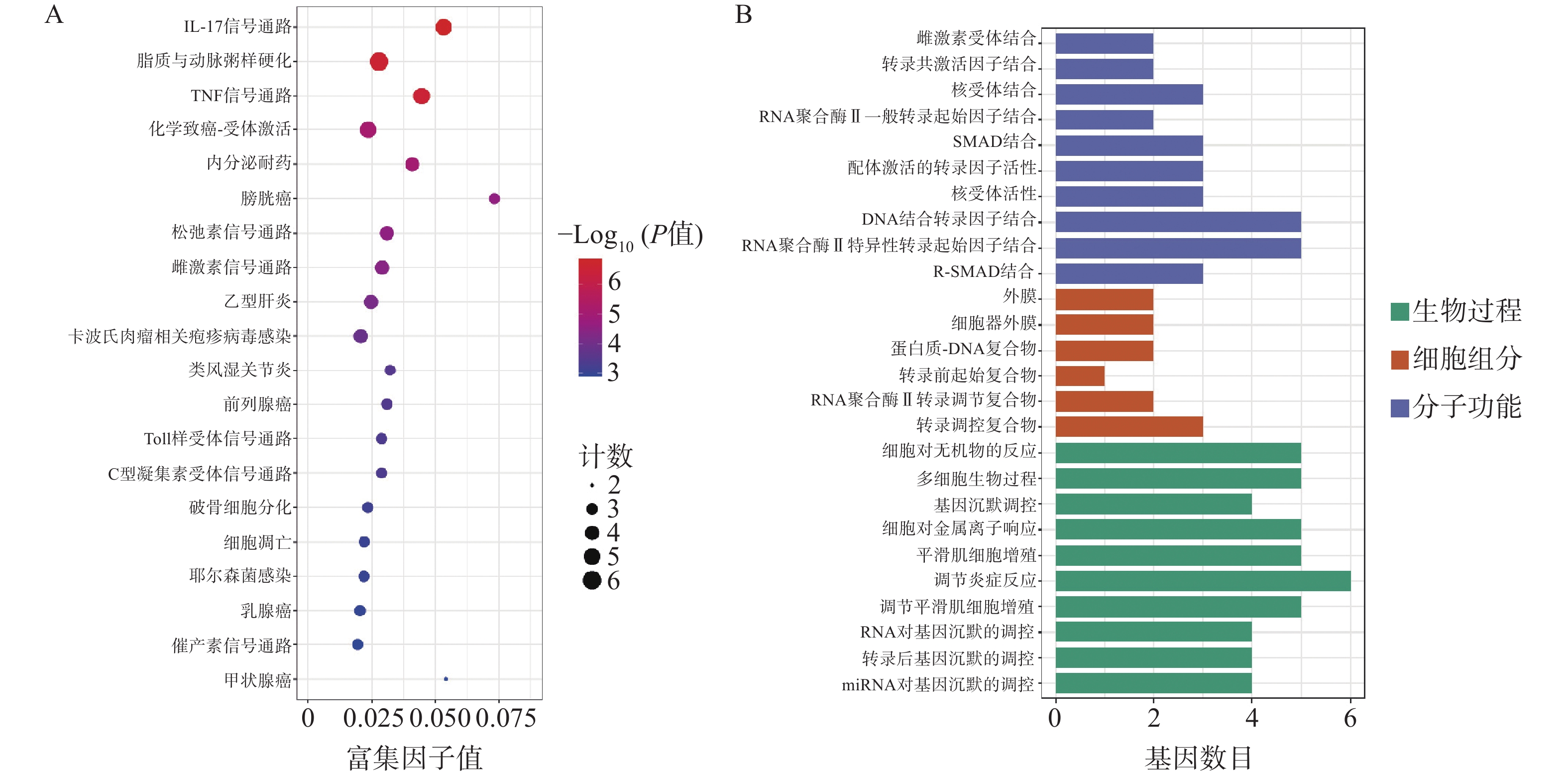
为了进一步探索通关藤活性成分对肝细胞癌的潜在作用机制和共同靶基因的功能,对共同靶基因进行GO/KEGG分析。GO分析获得条目BP 505个、CC 6个、MF 59个。图3A所示,生物过程主要涉及基因沉默调节、炎症反应调节、平滑肌细胞增殖,细胞组成涉及转录调节复合物、RNA聚合酶II转录调节复合物、转录前起始复合物、蛋白质-DNA复合物、细胞器外膜、外膜;分子功能涉及外膜、R-SMAD结合、RNA聚合酶II特异性DNA结合转录因子结合等。KEGG分析得61条通路(图3B),其中排名前列与肝细胞癌相关通路有IL-17信号通路、脂质和动脉粥样硬化、TNF信号通路等。
-
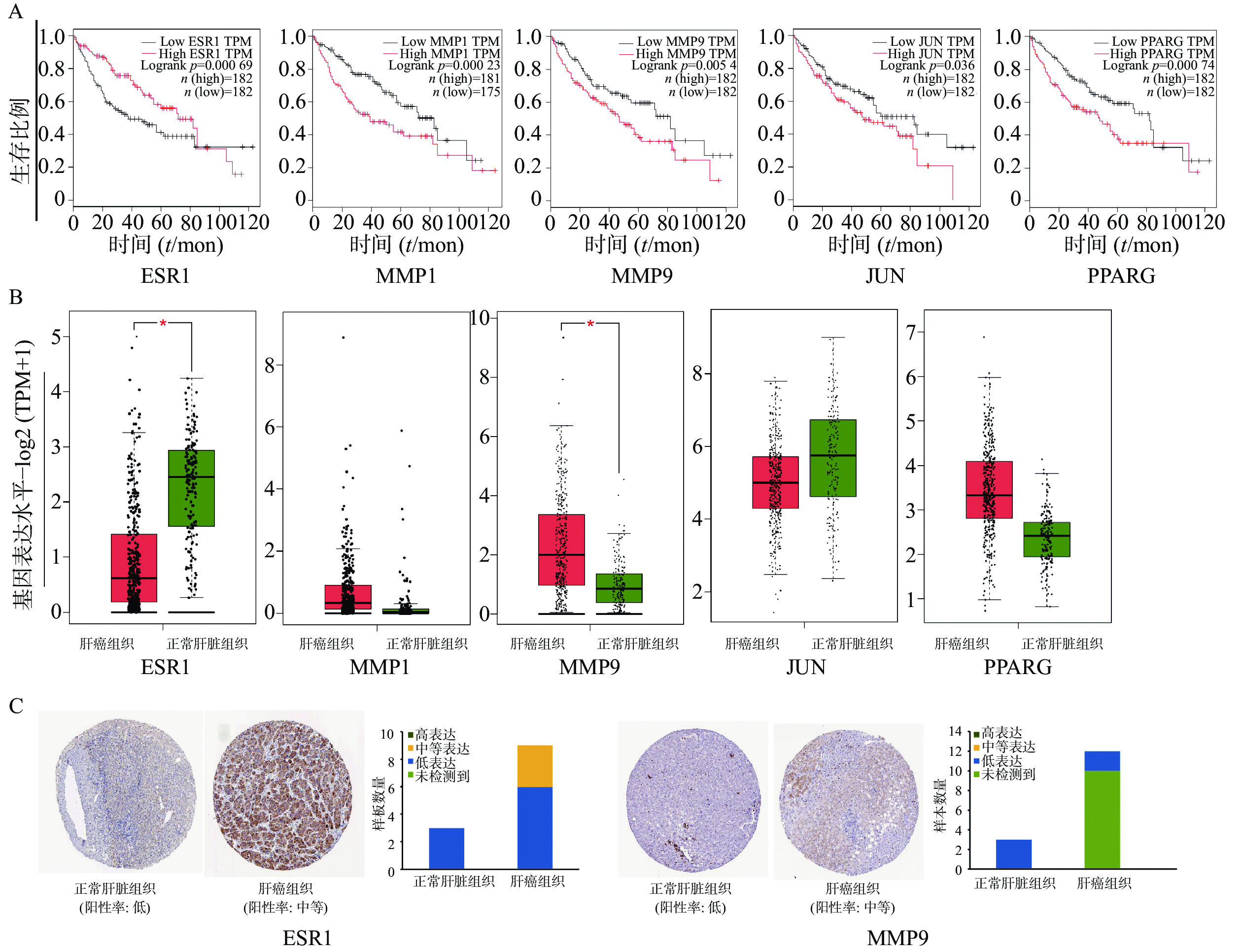
为证明关键基因与HCC之间的关系,通过GEPIA在线数据库中分析了共同靶基因与HCC患者的生存曲线。在12个靶基因中,ESR1、MMP1、MMP9、JUN、PPARG高表达的HCC患者的总生存期较差,与低表达患者相比,生存时间有明显差异(图4A)。ESR1和MMP9在肝细胞癌组织中mRNA水平表达存在显著差异(图4B)。HPA数据库中免疫组织化学结果显示(图4C),ESR1和MMP9在正常肝脏组织不表达,在肝癌组织中分别呈现中度表达和低表达状态。未在数据库中查询到MMP1、JUN、PPARG 在正常肝脏组织和肝癌组织中的表达。
-
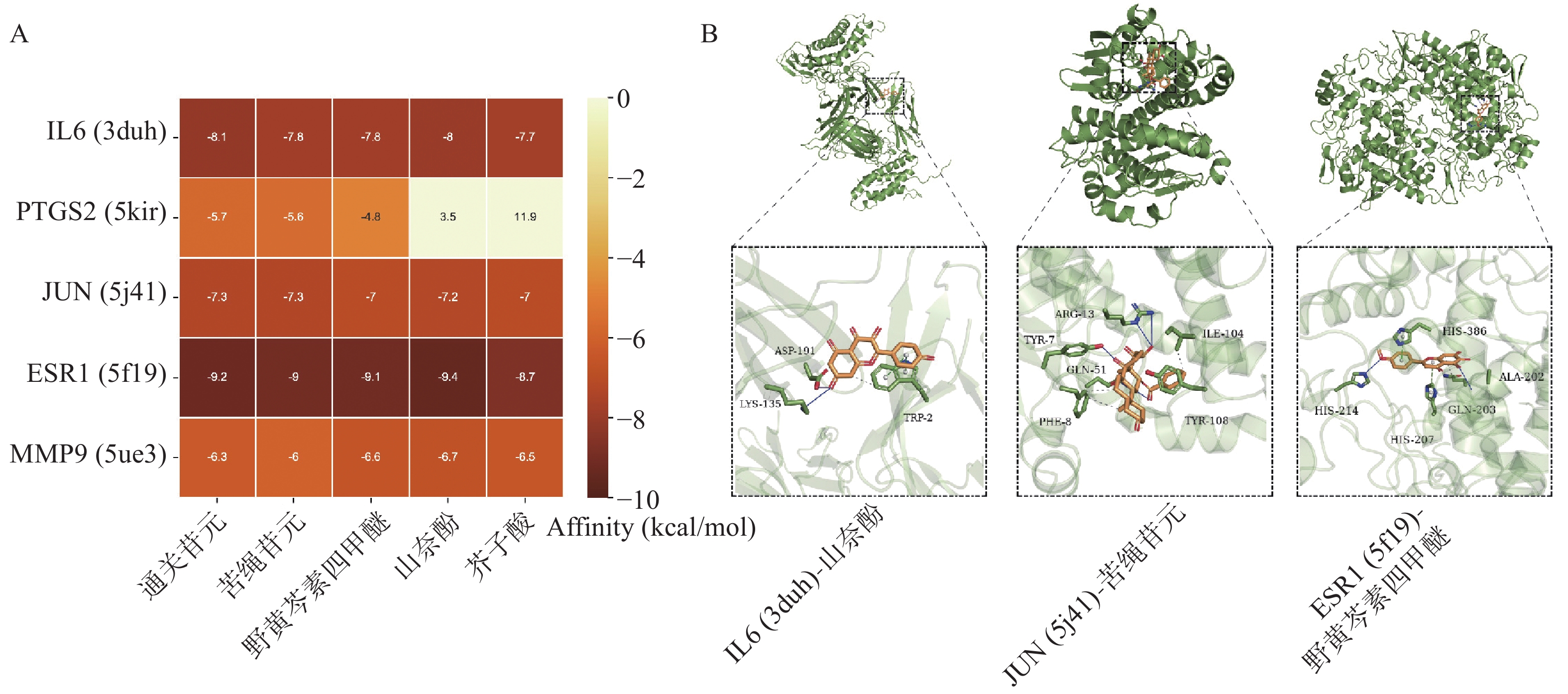
根据“2.3”项中结果,将度值排名靠前化合物野黄芩素四甲醚(Scutellarein-4-Methylether)、通关苷元(Tenasogenin)、芥子酸(Sinapic Acid)、苦绳苷元(Dresgenin)、山奈酚(Kaempferol)分别与基因IL6、PTGS2、JUN、ESR1、MMP9进行分子对接。对接结果如图5A所示,除PTGS2与野黄芩素四甲醚、芥子酸的结合自由能大于−5 kcal/mol,其余化合物与蛋白质受体结合能均低于−5 kcal/mol,有良好的结合活性。部分分子对接可视化结果如图5B所示。
-
本研究通过查询文献收集通关藤药物成分,依据“类药五原则”筛选出50个有效活性成分。由药物成分-靶点图可知,通关藤治疗HCC的关键成分有野黄芩素四甲醚、通关苷元、芥子酸、苦绳苷元、山奈酚等。芥子酸是一种小分子酚酸化合物,其与顺铂联用可以抑制肝癌细胞的增殖转移、诱导肝癌细胞凋亡[19]。山奈酚是一种多酚类中性化合物,具有较高的细胞毒性,在肿瘤治疗中有广阔的应用前景。其可以抑制肝癌细胞活性[20],改善索拉菲尼的化疗疗效[21]。目前关于野黄芩素四甲醚、通关苷元和苦绳苷元的研究分别在分离提取和心脏保护方面,尚无其在HCC中抗肿瘤活性的相关报道,需进一步研究[18,22]。
采用Swiss Target Prediction预测通关藤各成分的作用靶点;同时筛选Genecards、GEO数据库中HCC疾病基因,与GSE147888肝细胞癌芯片数据取交集,得出通关藤与HCC共有靶基因12个。如ESR1,是雌激素受体α(ERα)的编码基因,能够介导雌激素信号在HCC进展中的作用。肝脏是性激素的靶器官,其细胞表达ERα和雄激素受体,性激素特异性基因差异表达是肝癌发病的主要原因之一。流行病学研究发现HCC在女性中发病率明显低于男性,多项研究证实雌激素信号在HCC中具有保护作用[23-24]。ERα可通过STAT3信号通路、脂质代谢相关通路和非编码RNA多种途径抑制HCC的进展[25];而抑制ESR1基因的表达可促进HCC细胞的增殖、迁移和侵袭[26-27]。人肝癌组织中ERα表达缺失或减弱,表明雌激素信号在HCC中的保护作用。IL-6是一种多功能炎性细胞因子,在肝癌患者中表达增加,与肝癌的发生及预后密切相关[28]。肝癌中肝前体细胞通过分泌IL-6促进自身增殖,进一步促进肿瘤的恶性进展[29]。IL-6单克隆抗体阻断IL-6相关信号通路是治疗HCC的潜在药物[30]。在急性肝损伤模型中,芥子酸可改善肝脏中IL-6、TNF-α、ROS等炎症指标,对肝脏组织病理学变化起到保护作用[31]。PTGS2是前列腺素G/H合酶(又称诱导型环氧合酶2,COX-2)的编码基因,在肝癌发生过程中能够促进机体慢性炎症的形成。COX-2在肝癌患者中表达增高,且与患者总体生存率降低有关。COX-2抑制剂可有效抑制肝癌发生和进展[32-33]。在LPS诱导的斑马鱼炎症反应模型中,通关藤皂苷H通过NF-κB和P38通路调节COX-2、TNF-α、IL-8等炎症因子,发挥抗炎作用[34]。且本研究分子对接结果显示,通关藤关键成分与ESR1、IL-6、PTGS2均有较高的分子对接能,提示通关藤可能是通过交集靶基因发挥抗HCC作用。
为研究通关藤治疗HCC的作用机制,对交集靶基因进行GO和KEGG分析。GO分析发现,这些基因通过调节基因沉默、炎症反应、平滑肌细胞增殖等参与通关藤抗HCC作用。KEGG分析显示通关藤治疗HCC可能与IL-17、脂质和动脉粥样硬化、TNF通路相关。IL-17是由CD4+辅助性T细胞(Th17)分泌的炎症细胞因子,可通过上调MMP2、MMP9、VEGF的表达,促进HCC的侵袭和转移[35-36]。在非酒精性脂肪肝病(NASH)向HCC转变模型中,成纤维细胞生长因子21(FGF21)通过抑制肝细胞中TLR4、IL-17A信号通路,预防NASH向HCC的转变[37]。miR-383可能通过STAT3通路靶向IL-17发挥抗肿瘤作用,使得miR-383/IL-17轴成为HCC临床诊断和治疗的潜在靶点[38]。IL-17还可通过调节胆固醇合成影响HCC进展[39];胆固醇的过度累积可对肝细胞产生脂毒性作用,导致肝细胞代偿性增殖,释放促瘤细胞因子IL-6、TNF-α,促进HCC进展。LI等[40]采用多组学联合分析,在H22小鼠肝癌模型中确定了通关藤活性成分通关藤苷G可通过靶向HIF1α减少VEGF蛋白合成,从而抑制肝脏肿瘤中血管生成。PAN等[8]研究发现,在MHCC97H和HepG2细胞及肝癌患者来源的肿瘤异种移植小鼠模型中,通关藤提取物均可下调血管生成相关标志物(VEGF、VEGFA、PDGFRB、VWF)的表达,提示其有效成分通过抑制血管生成阻断供血,最终导致肿瘤组织坏死,抑制肝癌进展。另外,TNF信号通路在HCC发生发展中也有重要作用。术后接受索拉菲尼辅助用药治疗的HCC患者,TNFα高表达与不良预后相关[41]。高表达的lncRNA SPRY4-IT1可通过TNF信号通路促进HCC进展和转移[42]。在高脂饮食合并二乙基亚硝胺诱导的小鼠肝癌模型中,敲除TNFR1,可抑制HCC发生[43]。研究表明通关藤苷H可通过调节TNFα等炎症细胞因子水平,发挥抗炎作用[34]。
通过GEPIA 在线数据库分析交集靶基因与HCC患者生存率的关系,发现5个基因ESR1、MMP1、MMP9、JUN、PPARG的低表达与HCC生存率升高有关,这与GSE147888芯片分析中交集基因表达情况一致。Proteinatlas数据库免疫组化结果显示:与正常肝脏组织相比,HCC癌组织中ESR1、MMP9基因表达上调,进一步证实了其与HCC的相关性。MMP9(基质金属蛋白酶9),可通过调节生长板血管生成和干预内皮干细胞募集在肿瘤血管生成中发挥重要作用[44]。MMP9在HCC的过度表达通过增加淋巴结侵袭和促进转移导致更高的TNM分期,也导致分化差和总体预后差[45]。LIU等[46]研究表明,通关藤的4种C21甾体糖苷可下调MMP9的表达,削弱其降解细胞外间质成分和Ⅳ型胶原蛋白,抑制A549肿瘤细胞的迁移和侵袭。
综上所述,本研究通过生物信息学、网络药理学和分子对接技术对通关藤治疗HCC的药物成分、潜在靶点及作用机制进行了初步探讨,发现通关藤治疗HCC关键基因12个,药物成分50种。其中关键靶基因IL6、PTGS2、JUN、ESR1、MMP9可能有重要作用,涉及基因沉默调节、炎症反应调节等过程,参与R-SMAD结合、RNA聚合酶II特异性DNA结合转录因子结合等分子功能;可能通过IL-17信号通路、TNF信号通路等通路,抑制HCC的进展。本研究体现了中草药通关藤治疗HCC的多成分、多靶点、多通路的特点,为通关藤在HCC的临床应用中提供科学依据。
Exploring the mechanism of Marsdenia tenacissima in the treatment of hepatocellular carcinoma based on network pharmacology
-
摘要:
目的 通过生物信息学、网络药理学和分子对接技术研究通关藤治疗肝细胞癌(HCC)的作用机制。 方法 通过文献检索和ADME平台筛选通关藤活性成分并利用Swiss Target Prediction预测化合物的作用靶点;从GEO数据库获得HCC数据芯片GSE147888并筛选表达差异显著基因;通过Genecards和OMIM数据库获得HCC疾病相关靶点;Venny在线对上述靶点取交集。利用Cytoscape软件和String数据库构建药物成分-靶点网络图和PPI网络图;利用R软件进行GO功能富集分析和KEGG通路富集分析;利用GEPIA数据库对靶基因进行生存分析,筛选在HCC生存率中显著差异表达的基因,并用Proteinatlas数据库分析基因在HCC组织中免疫组化表达情况;将排名前5的靶蛋白与对应药物活性成分进行分子对接验证。 结果 共筛选通关藤活性成分50个,药物与疾病交集靶基因12个。通关藤治疗HCC的重要成分有野黄芩素四甲醚、通关苷元、芥子酸、苦绳苷元、山奈酚等,关键基因有JUN、MMP9、PTGS2等。GO、KEGG分析结果显示,关键靶点主要涉及基因沉默调节、炎症反应调节等过程,主要富集在IL-17、TNF等信号通路。生存分析显示ESR1、MMP1、MMP9、JUN、PPARG在高低风险组之间有显著差异。免疫组化结果显示ESR1和MMP9在正常组织和肝癌组织中差异表达。分子对接结果验证了药物活性成分与靶蛋白可稳定结合。 结论 研究体现了中草药通关藤治疗HCC的多成分、多靶点、多通路的特点,为通关藤在HCC的临床应用中提供科学依据。 Abstract:Objective To investigate the material basis and antitumor mechanism of Marsdenia tenacissima (MT) on hepatocellular carcinoma (HCC) by bioinformatics, network pharmacology and molecular docking technology. Methods Active ingredients of MT were collected by literature search and screened by Swiss ADME website, which targets were predicted by Swiss Target Prediction. The chip data of HCC (GSE147888) were downloaded from the NCBI Gene Expression Omnibus (GEO) database. Differentially expressed genes were screened by R software. HCC-related targets were collected from the Genecards and OMIM databases. The Venny online tool was used to obtain the intersection of the herbal medicine targets and the disease targets. Subsequently, drug-target network and protein–protein interaction (PPI) network were constructed by Cytoscape software and String platform. GO enrichment analysis and KEGG pathway analysis were performed to analysis the functions and pathways enriched by key genes. The expression of key genes in HCC and its effect on survival were analyzed by the GEPIA database. The Human Protein Atlas (HPA) was used to analyze the immunohistochemical expression of key genes in HCC. Finally, molecular docking was carried out to investigate interactions between the top five targets and their related active compounds. Results A total of 50 active components were screened and 12 common targets were identified related to MT and HCC. Scutellarein-4-Methylether, Tenasogenin, Sinapic Acid, Dresgenin and Kaempferol were considered as the critical components. JUN, MMP9 and PTGS2 were recognized as key therapeutic targets. The GO analyses demonstrated that key targets mainly involved in the process of gene silencing and inflammatory response. KEGG analysis suggested that key targets were enriched in TNF signaling pathway and IL-17 signaling pathway. Survival analysis by the GEPIA showed significant differences in the expression of ESR1, MMP1, MMP9, JUN, and PPARG between high and low risk groups. Immunohistochemical results showed that ESR1 and MMP9 were differentially expressed in normal and hepatocellular carcinoma tissues. The molecular docking results verified that the drug active ingredient could be stably bound to the target protein. Conclusion This study reflected the multi-component, multi-target and multi-pathway characteristics of the MT in the treatment of HCC, which could provide a scientific basis for the clinical application of MT in HCC. -
表 1 通关藤活性成分信息
编号 化合物名称 Simles结构式 1 2,4-Decadienal(2,4-癸二烯醛) CCCCC/C=C/C=C/C=O 2 2,4-Dihydroxybenzoic Acid(2,4 -二羟基苯甲酸) C1=CC(=C(C=C1O)O)C(=O)O 3 2,6-Dihydroxybenzoic Acid(2,6 -二羟基苯甲酸) C1=CC(=C(C(=C1)O)C(=O)O)O 4 3,4-Dihydroxybenzoic Acid(3,4 -二羟基苯甲酸) C1=CC(=C(C=C1C(=O)O)O)O 5 Betulinic Acid(白桦脂酸) CC(=C)C1CCC2(C1C3CCC4C5(CCC(C(C5CCC4(C3(CC2)C)C)(C)C)O)C)C(=O)O 6 Caffeic Acid(咖啡酸) C1=CC(=C(C=C1/C=C/C(=O)O)O)O 7 Chlorogenic Acid(绿原酸) C1C(C(C(CC1(C(=O)O)O)OC(=O)C=CC2=CC(=C(C=C2)O)O)O)O 8 Cissogenin(西索苷元) CC(C1CCC2(C1(C(C(C3C2CC=C4C3(CCC(C4)O)C)O)O)C)O)O 9 Conduritol(环己烯四醇) C1=CC(C(C(C1O)O)O)O 10 D(+)-Digitoxose(D (+)-洋地黄毒糖) CC(C(C(CC=O)O)O)O 11 Daucosterol(胡萝卜甾醇) CCC(CCC(C)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)OC5C(C(C(C(O5)CO)O)O)O)C)C)C(C)C 12 D-Cymarose(加拿大麻糖) CC(C(C(CC=O)OC)O)O 13 Dihydrosarcostin(二氢肉珊瑚苷元) CC(C1(CCC2(C1(C(CC3C2(CCC4C3(CCC(C4)O)C)O)O)C)O)O)O 14 Dresgenin(苦绳甙元) CC(C1(CCC2(C1(C(CC3C2(CCC4C3(CCC(C4)O)C)O)OC(=O)C5=CC=CC=C5)C)O)O)O 15 Drevogenin A(南山藤皂苷元A) CC(C)CC(=O)OC1C(C2C(CC=C3C2(CCC(C3)O)C)C4(C1(C(CC4)C(=O)C)C)O)OC(=O)C 16 Drevogenin Q(南山藤皂苷元Q) CC(C)CC(=O)OC1C2C(CC=C3C2(CCC(C3)O)C)C4(CCC(C4(C1OC(=O)C)C)C(=O)C)O 17 D-Thevetose(D-黄花夹竹桃糖) CC(C(C(C(C=O)O)OC)O)O 18 Galactitol(半乳糖醇) C(C(C(C(C(CO)O)O)O)O)O 19 Gallic Acid(没食子酸) C1=C(C=C(C(=C1O)O)O)C(=O)O 20 Kaempferol(山奈酚) C1=CC(=CC=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O 21 Kaempferol-4'-Methylether(山柰酚-4-甲醚) COC1=CC=C(C=C1)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O 22 Lupeol(羽扇豆醇) CC(=C)C1CCC2(C1C3CCC4C5(CCC(C(C5CCC4(C3(CC2)C)C)(C)C)OC(=O)C6=
CC=CC=C6)C)C23 Marsectobiose(直立牛奶菜双糖) CC1C(C(C(C(O1)OC(C(C)O)C(CC=O)OC)O)OC)O 24 Marstenacigenin A(二氢肉珊瑚苷元的衍生物) CC(C1(CCC2(C1(C(CC3C2(CCC4C3(CCC(C4)O)C)O)C(=O)C=CC5=CC=CC=C5)C)O)O)O 25 Methyl Shikimate(莽草酸甲酯) COC(=O)C1=CC(C(C(C1)O)O)O 26 Nerolidol(橙花叔醇) CC(=CCCC(=CCCC(C)(C=C)O)C)C 27 Oleandrose(齐墩果糖) CC(C(C(CC=O)OC)O)O 28 P-Hydroxycinnamic Acid(对羟基肉桂酸) C1=CC(=CC=C1C=CC(=O)O)O 29 Propionic Acid(丙酸) CCC(=O)O 30 Protocatechualdehyde(中原儿茶醛) C1=CC(=C(C=C1C=O)O)O 31 Scopoletin(东莨菪素) COC1=C(C=C2C(=C1)C=CC(=O)O2)O 32 Scutellarein-4-Methylether(灯盏乙素-4-甲醚) COC1=CC=C(C=C1)C2=CC(=O)C3=C(O2)C=C(C(=C3O)O)O 33 Shikimic Acid(莽草酸) C1C(C(C(C=C1C(=O)O)O)O)O 34 Sinapic Acid(芥子酸) COC1=CC(=CC(=C1O)OC)C=CC(=O)O 35 Stigmasterol(豆甾醇) CCC(C=CC(C)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C)C(C)C 36 Succinic Acid(琥珀酸) C(CC(=O)O)C(=O)O 37 Syringic Acid(丁香酸) COC1=CC(=CC(=C1O)OC)C(=O)O 38 Taraxasterol(蒲公英甾醇) CC1C2C3CCC4C5(CCC(C(C5CCC4(C3(CCC2(CCC1=C)C)C)C)(C)C)O)C 39 Tenacigenin B(通光散苷元B) CC(=O)C1CCC23C1(C(C(C4C2(O3)CCC5C4(CCC(C5)O)C)O)O)C 40 Tenacigenin D(通光散苷元D) CCC(C)C(=O)OC1C2C3(CCC(=O)CC3CCC24C5(O4)CCC(C5(C1OC(=O)C)C)C(=O)C)C 41 Tenacissoside A(通关藤皂苷A) CC=C(C)C(=O)OC1C2C3(CCC(CC3CCC24C5(O4)CCC(C5(C1OC(=O)C)C)C(=O)C)OC6CC(C(C(O6)C)OC7C(C(C(C(O7)C)OC8C(C(C(C(O8)CO)O)O)O)OC)O)OC)C 42 Tenacissoside B(通关藤皂苷B) CC=C(C)C(=O)OC1C2C3(CCC(CC3CCC24C5(O4)CCC(C5(C1OC(=O)C(=CC)C)C)C(=O)C)OC6CC(C(C(O6)C)OC7C(C(C(C(O7)C)OC8C(C(C(C(O8)CO)O)O)O)OC)O)OC)C 43 Tenacissoside G(通关藤皂苷G) CC=C(C)C(=O)OC1C2C3(CCC(CC3CCC24C5(O4)CCC(C5(C1OC(=O)C)C)C(=O)C)OC6CC(C(C(O6)C)OC7C(C(C(C(O7)C)O)OC)O)OC)C 44 Tenacissoside H(通关藤皂苷H) CCC(C)C(=O)OC1C2C3(CCC(CC3CCC24C5(O4)CCC(C5(C1OC(=O)C)C)C(=O)C)OC6CC(C(C(O6)C)OC7C(C(C(C(O7)C)O)OC)O)OC)C 45 Tenacissoside I(通关藤皂苷I) CC1C(C(C(C(O1)OC2C(OC(CC2OC)OC3CCC4(C(C3)CCC56C4C(C(C7(C5(O6)CCC7C(=O)C)C)OC(=O)C)OC(=O)C8=CC=CC=C8)C)C)O)OC)O 46 Tenasogenin(通关苷元) CC(C1CCC2(C1(C(C(C3C2CC=C4C3(CCC(C4)O)C)OC(=O)C=C(C)C)O)C)O)O 47 Vanillic Acid(香草酸) COC1=C(C=CC(=C1)C(=O)O)O 48 β-Amyrin(β-香树脂醇) CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)O)C)C)C2C1)C)C)C 49 β-Sitosterol(β-谷甾醇) CCC(CCC(C)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C)C(C)C 50 17β-Tenacissoside B(17β-通光藤皂苷B) O[C@H]1CC[C@]2(C(C1)CC[C@@]13C2[C@H](O)[C@@H](O)[C@]2([C@]3(O1)CC[C@@H]2C(=O)C)C)C -
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3):209-249. doi: 10.3322/caac.21660 [2] JIA W T, WANG L N. Using traditional Chinese medicine to treat hepatocellular carcinoma by targeting tumor immunity[J]. Evid Based Complement Alternat Med, 2020, 2020:9843486. [3] WANG Y S, ZHANG Q F, CHEN Y C, et al. Antitumor effects of immunity-enhancing traditional chinese medicine[J]. Biomed Pharmacother, 2020, 121:109570. doi: 10.1016/j.biopha.2019.109570 [4] LIAO Y H, LIN C C, LAI H C, et al. Adjunctive traditional Chinese medicine therapy improves survival of liver cancer patients[J]. Liver Int, 2015, 35(12):2595-2602. doi: 10.1111/liv.12847 [5] ZHAI B T, ZHANG N N, HAN X M, et al. Molecular targets of β-elemene, a herbal extract used in traditional Chinese medicine, and its potential role in cancer therapy: a review[J]. Biomed Pharmacother, 2019, 114:108812. doi: 10.1016/j.biopha.2019.108812 [6] WANG X, YAN Y L, CHEN X, et al. The antitumor activities of Marsdenia tenacissima[J]. Front Oncol, 2018, 8:473. doi: 10.3389/fonc.2018.00473 [7] LIN J T, RUAN J Y, ZHU H, et al. Tenacissoside H induces autophagy and radiosensitivity of hepatocellular carcinoma cells by PI3K/Akt/mTOR signaling pathway[J]. Dose Response, 2021, 19(2): 15593258211011023. [8] PAN Y T, LIAO X Y, YANG L L, et al. Extract of Marsdenia tenacissima (roxb.) moon[Apocynaceae]suppresses hepatocellular carcinoma by inhibiting angiogenesis[J]. Front Pharmacol, 2022, 13:900128. doi: 10.3389/fphar.2022.900128 [9] BOBYN J D, GLASSMAN A H, GOTO H, et al. The effect of stem stiffness on femoral bone resorption after canine porous-coated total hip arthroplasty[J]. Clin Orthop Relat Res, 1990(261):196-213. [10] VEBER D F, JOHNSON S R, CHENG H Y, et al. Molecular properties that influence the oral bioavailability of drug candidates[J]. J Med Chem, 2002, 45(12):2615-2623. doi: 10.1021/jm020017n [11] WANG P L, YANG J, ZHU Z F, et al. Marsdenia tenacissima: a review of traditional uses, phytochemistry and pharmacology[J]. Am J Chin Med, 2018: 1-32. [12] LI S Y, PEI W H, ZHANG H. Simultaneous determination of eight bioactive components in Marsdenia tenacissima extract in rat plasma by LC-MS/MS and its application in a pharmacokinetic study[J]. Biomed Chromatogr, 2020, 34(11):e4946. doi: 10.1002/bmc.4946 [13] XIE B, LU Y Y, LUO Z H, et al. Tenacigenin B ester derivatives from Marsdenia tenacissima actively inhibited CYP3A4 and enhanced in vivo antitumor activity of paclitaxel[J]. J Ethnopharmacol, 2019, 235:309-319. doi: 10.1016/j.jep.2019.02.028 [14] XIA Z H, MAO S L, LAO A N, et al. Five new pregnane glycosides from the stems of Marsdenia tenacissima[J]. J Asian Nat Prod Res, 2011, 13(6):477-485. doi: 10.1080/10286020.2011.570263 [15] 康乐, 苗晋鑫, 方晓艳, 等. 基于网络药理学及分子对接的通关藤治疗非小细胞肺癌的现代机制[J]. 重庆理工大学学报(自然科学), 2021, 35(7):191-200. [16] 覃薇, 殷梓辛, 华维维, 等. 基于网络药理学和分子对接探讨通关藤治疗胃癌的作用机制[J]. 实用临床医药杂志, 2022, 26(1):1-7,17. [17] 肖雪峰, 张稳, 张新卓, 等. 药用植物通关藤研究进展[J]. 安徽农业科学, 2018, 46(9):19-21. doi: 10.13989/j.cnki.0517-6611.2018.09.006 [18] 张薇, 王泽锋, 王静, 等. 乌骨藤化学成分及其抗肿瘤活性[J]. 中成药, 2017, 39(2):334-338. [19] ZHAO J, LI H W, LI W Y, et al. Effects of sinapic acid combined with cisplatin on the apoptosis and autophagy of the hepatoma cells HepG2 and SMMC-7721[J]. Evid Based Complement Alternat Med, 2021, 2021:6095963. [20] ZHU G L, LIU X L, LI H J, et al. Kaempferol inhibits proliferation, migration, and invasion of liver cancer HepG2 cells by down-regulation of microRNA-21[J]. Int J Immunopathol Pharmacol, 2018, 32: 2058738418814341. [21] NAIR B, ANTO R J, M S, et al. Kaempferol-mediated sensitization enhances chemotherapeutic efficacy of sorafenib against hepatocellular carcinoma: an in silico and in vitro approach[J]. Adv Pharm Bull, 2020, 10(3):472-476. doi: 10.34172/apb.2020.058 [22] MATHEW A A, ASIRVATHAM R, TOMY D V. Cardioprotective effect of Marsdenia tenacissima and Sansevieria roxburghiana in doxorubicin-induced cardiotoxicity in rats in vivo: the role of dresgenin and lupeol[J]. Turk J Pharm Sci, 2021, 18(3):271-281. doi: 10.4274/tjps.galenos.2020.27880 [23] ZHANG Y Y, CHENG J X, ZHONG C, et al. ESR1 regulates the obesity- and metabolism-differential gene MMAA to inhibit the occurrence and development of hepatocellular carcinoma[J]. Front Oncol, 2022, 12:899969. doi: 10.3389/fonc.2022.899969 [24] O'BRIEN M H, PITOT H C, CHUNG S H, et al. Estrogen receptor-α suppresses liver carcinogenesis and establishes sex-specific gene expression[J]. Cancers, 2021, 13(10):2355. doi: 10.3390/cancers13102355 [25] MENG X Z, LIU X. Therapeutic value of estrogen receptor α in hepatocellular carcinoma based on molecular mechanisms[J]. J Clin Transl Hepatol, 2022, 10(1):140-146. doi: 10.14218/JCTH.2021.00224 [26] WANG L B, CUI M X, CHENG D M, et al. MiR-9-5p facilitates hepatocellular carcinoma cell proliferation, migration and invasion by targeting ESR1[J]. Mol Cell Biochem, 2021, 476(2):575-583. doi: 10.1007/s11010-020-03927-z [27] CHEN F, NI X Y, CHEN L X, et al. MiR-939-3p promotes epithelial-mesenchymal transition and may be used as a prognostic marker in hepatocellular carcinoma[J]. Oncol Lett, 2020, 19(4):2727-2732. [28] KAO J T, LAI H C, TSAI S M, et al. Rather than interleukin-27, interleukin-6 expresses positive correlation with liver severity in naïve hepatitis B infection patients[J]. Liver Int, 2012, 32(6):928-936. doi: 10.1111/j.1478-3231.2011.02742.x [29] HE G B, DHAR D, NAKAGAWA H, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling[J]. Cell, 2013, 155(2):384-396. doi: 10.1016/j.cell.2013.09.031 [30] KAMPAN N C, XIANG S D, MCNALLY O M, et al. Immunotherapeutic interleukin-6 or interleukin-6 receptor blockade in cancer: challenges and opportunities[J]. Curr Med Chem, 2018, 25(36):4785-4806. doi: 10.2174/0929867324666170712160621 [31] ROSTAMI A, BALUCHNEJADMOJARAD T, ROGHANI M. Sinapic acid ameliorates paracetamol-induced acute liver injury through targeting oxidative stress and inflammation[J]. Mol Biol Rep, 2022, 49(6):4179-4191. doi: 10.1007/s11033-022-07251-1 [32] QIU Z P, ZHANG C, ZHOU J X, et al. Celecoxib alleviates AKT/c-Met-triggered rapid hepatocarcinogenesis by suppressing a novel COX-2/AKT/FASN cascade[J]. Mol Carcinog, 2019, 58(1):31-41. doi: 10.1002/mc.22904 [33] HU J W, CHEN B, ZHANG J, et al. Novel combination of celecoxib and metformin improves the antitumor effect by inhibiting the growth of hepatocellular carcinoma[J]. J Cancer, 2020, 11(21):6437-6444. doi: 10.7150/jca.47532 [34] LI J J, ZHANG Y, HAN L W, et al. Tenacissoside H exerts an anti-inflammatory effect by regulating the nf-κb and p38 pathways in zebrafish[J]. Fish Shellfish Immunol, 2018, 83:205-212. doi: 10.1016/j.fsi.2018.09.032 [35] GU F M, LI Q L, GAO Q, et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma[J]. Mol Cancer, 2011, 10:150. doi: 10.1186/1476-4598-10-150 [36] LI J, LAU G K, CHEN L L, et al. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression[J]. PLoS One, 2011, 6(7):e21816. doi: 10.1371/journal.pone.0021816 [37] ZHENG Q Q, MARTIN R C, SHI X J, et al. Lack of FGF21 promotes NASH-HCC transition via hepatocyte-TLR4-IL-17A signaling[J]. Theranostics, 2020, 10(22):9923-9936. doi: 10.7150/thno.45988 [38] WANG J C, LU L B, LUO Z J, et al. MiR-383 inhibits cell growth and promotes cell apoptosis in hepatocellular carcinoma by targeting IL-17 via STAT3 signaling pathway[J]. Biomed Pharmacother, 2019, 120:109551. doi: 10.1016/j.biopha.2019.109551 [39] MA H Y, YAMAMOTO G, XU J, et al. IL-17 signaling in steatotic hepatocytes and macrophages promotes hepatocellular carcinoma in alcohol-related liver disease[J]. J Hepatol, 2020, 72(5):946-959. doi: 10.1016/j.jhep.2019.12.016 [40] LI S Y, PEI W H, YUAN W, et al. Multi-omics joint analysis reveals the mechanism of action of the traditional Chinese medicine Marsdenia tenacissima (Roxb.) Moon in the treatment of hepatocellular carcinoma[J]. J Ethnopharmacol, 2022, 293:115285. doi: 10.1016/j.jep.2022.115285 [41] TAN W L, LUO X, LI W D, et al. TNF-α is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma[J]. EBioMedicine, 2019, 40:446-456. doi: 10.1016/j.ebiom.2018.12.047 [42] MA W J, CHEN X, WU X L, et al. Long noncoding RNA SPRY4-IT1 promotes proliferation and metastasis of hepatocellular carcinoma via mediating TNF signaling pathway[J]. J Cell Physiol, 2020, 235(11):7849-7862. doi: 10.1002/jcp.29438 [43] PARK E J, LEE J H, YU G Y, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression[J]. Cell, 2010, 140(2):197-208. doi: 10.1016/j.cell.2009.12.052 [44] HEISSIG B, HATTORI K, DIAS S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand[J]. Cell, 2002, 109(5):625-637. doi: 10.1016/S0092-8674(02)00754-7 [45] SUN S J, WANG N, SUN Z W, et al. MiR-5692a promotes the invasion and metastasis of hepatocellular carcinoma via MMP9[J]. Eur Rev Med Pharmacol Sci, 2018, 22(15):4869-4878. [46] LIU P, XU D W, LI R T, et al. A combined phytochemistry and network pharmacology approach to reveal potential anti-NSCLC effective substances and mechanisms in Marsdenia tenacissima (roxb.) moon (stem)[J]. Front Pharmacol, 2021, 12:518406. doi: 10.3389/fphar.2021.518406 -





 下载:
下载: