-
随着经济的发展和人民生活水平的提高,糖尿病的患病率仍在逐年上升,血管并发症是导致患者残疾和死亡的主要原因,并给社会和经济的发展带来了沉重负担。内皮祖细胞(endothelial progenitor cells, EPCs)在各种因素刺激下,从骨髓动员到外周血,参与损伤内皮的修复,在血管新生中具有重要作用[1-3]。但是高血糖会导致EPCs数量减少及功能受损[4-5]。研究表明,雌激素降低是心血管疾病发病的危险因素。因此,本实验通过研究雌激素对糖尿病大鼠EPCs功能的改善作用并探讨可能的作用机制,为探讨糖尿病血管并发症提供理论依据。
-
SPF 级 Wistar大鼠,雄性,体重(180±10)g(上海斯莱克实验动物有限公司)。实验期间,保持动物房室温在22 ℃左右,相对湿度70%左右,早8点至晚8点自动照明。动物自由进食,自由饮水,所有实验动物均符合实验动物伦理学要求。
-
雌激素(Abcam公司);链脲佐菌素(Sigma aldrich公司);FITC标记的荆豆凝集素I(FITC-UAE-I)(美国Sigma公司);Dil 标记的乙酰化低密度脂蛋白(Dil-ac-LDL)(Molecular Probe公司);甲醛溶液(国药集团化学试剂有限公司);基质胶(matrigel)(Thermo Fisher公司);EGM-2 培养基(LON-ZA 公司);CCK-8试剂盒(日本Dojindo公司);NO检测试剂盒(美国Abcam公司)。
-
Wistar雄性大鼠,10~12周,适应性地喂养1周后,连续7 d空腹腹腔注射新鲜配制的链脲佐菌素(streptozotocin, STZ)55 mg/(kg·d),对照组大鼠腹腔注射等体积枸橼酸钠缓冲液。7 d后测空腹血糖(禁食12 h),将血糖值为13.5~25 mmol/L的大鼠作为糖尿病大鼠进行实验。注射STZ后,每周称体重,观察体重变化。大鼠给药、饲养过程中勤换垫料,勤补水。
-
大鼠麻醉后处死,将大鼠整体置于75%乙醇浸泡10 min。取出大鼠,使用吸水纸吸干动物身上水分后转移至超净工作台,剥离胫骨,使用无菌剪刀减去骨头两端,使用1ml PBS通过注射器冲洗骨髓并将冲洗液转移至15 ml无菌离心管,冲洗过程重复操作3次。用密度梯度离心法获取单核细胞,将单核细胞重悬于培养基EGM-2并调整细胞浓度至1×106个/ml,接种于预先包被好纤维连接蛋白的细胞培养皿,置于细胞培养箱37 ℃和5%CO2条件下培养。培养3 d后洗去未贴壁细胞,以后每3 d换培养液培养至7 d。PBS洗去未贴壁细胞,贴壁细胞供实验用。
-
培养7 d的EPCs用0.25%胰蛋白酶消化后收集于15 ml离心管中,将细胞用PBS 1000r/min离心5 min,清洗3次。用PBS 100 μl重悬细胞,每管加入CD34和CD133抗体各2 μl,4 ℃避光孵育30 min后用PBS清洗离心,将细胞重悬于300 μl PBS中避光保存待上机检测;同时将消化的细胞采用DiI-Ac-LDL 和FITC-UAE-I双染,倒置荧光显微镜观察染色结果,双染色阳性细胞为正在分化的EPCs。
-
实验分3组:①对照组;②糖尿病组:M199培养基;③雌激素组:含雌激素10 nmol/L的M199培养基。在37 ℃,5% CO2培养箱中孵育24 h 后进行实验。
-
3组EPCs用0.25%胰酶消化,将细胞重悬于EBM-2 培养基中,调整细胞密度为5×105个/ml,并取100 μl接种于Transwell 上室,在下室中加入600 μl含50 ng/ml 血管内皮生长因子的EGM-2 培养基。37 ℃、5% CO2孵育24 h 后,取出上室,用棉球轻轻擦拭上室底部膜内上表面的细胞,下室上表面用甲醛室温固定20 min,于37 ℃用0.1%结晶紫染色30 min,用PBS多次冲洗晾干后在160倍光学显微镜下观察并拍照,随机选取5个视野计算迁移细胞数并计算平均值。
-
在冰上将48 孔板预冷,然后加入150 μl的基质胶Matrigel,放置于37 ℃孵育30 min。3组EPCs用0.25%胰酶消化,将5×104个细胞接种于Matrigel上,在37 ℃、5% CO2培养箱中孵育24 h 后,于160倍光学显微镜下观察并拍照,随机选取5个视野计算形成小管的数目并计算平均值。
-
3组EPCs用0.25%胰酶消化,经PBS清洗2次,制成细胞悬液,采用NO检测试剂盒测定EPCs中NO的水平。按照说明书的操作方法,完成后置于酶标仪在540 nm波长处测定吸光度(A)。
-
3组EPCs用0.25%胰酶消化,将100 μl细胞悬液接种于96孔培养板中并置于37 ℃、5% CO2培养箱培养24 h。每孔加入CCK-8溶液10 μl培养4 h,置于酶标仪于450 nm处测A值。
-
收集3组EPCs并提取蛋白,应用BCA法进行蛋白质定量。蛋白样本经煮沸变性、SDS-PAGE电泳后加入兔抗人锰超氧化物歧化酶(MnSOD)和肌动蛋白(β-actin)多克隆抗体(1∶1500稀释),蛋白质湿法转移至硝基纤维素膜,羊抗兔二抗(1∶2000稀释)室温孵育2 h,用ECL化学发光法显色,Image J凝胶成像分析系统扫描成像,测定目标条带的A值,以β-actin为内参比较同一条带与β-actin的灰度值进行分析。
-
收集3组EPCs上清液,按照说明书步骤进行操作。取各组细胞上清液各100 μl加入酶标板中37 ℃孵育90 min,然后加入100 μl生物素标记抗体工作液37 ℃孵育60 min。洗涤3次,加入100 μl酶结合物工作液在37 ℃孵育30 min后,洗涤5次。加入90 μl底物溶液37 ℃孵育15 min,最后加入终止液50 μl后,立即在450 nm波长处测量A值。
-
实验数据以(
$ \overline{\text{x}}\text{±}\text{s} $ )表示,采用GraghPad Prism软件进行统计处理,采用单因素方差分析(One-way ANOVA)检验差异的显著性。以P<0.05为差异有统计学意义。 -
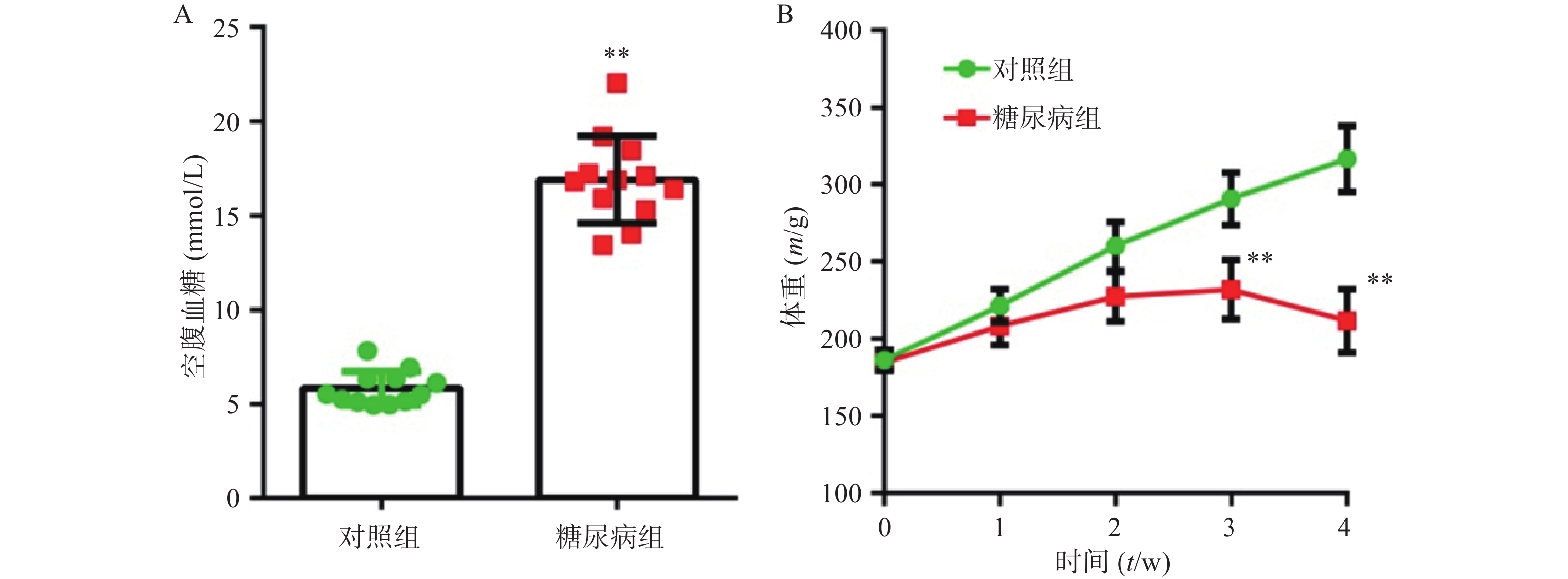
正常组大鼠,精神状态良好,动作自如,反应灵敏,毛发平伏有光泽。而糖尿病大鼠体重变轻,精神萎靡,反应迟钝,毛杂乱无光泽,动作迟缓,弓背捲体,尿量显著增加。在建模4周后,糖尿病模型组空腹血糖值(23.33±3.61)mmol/L明显高于对照组(16.91±2.30)mmol/L,组间差异有统计学意义(P<0.01) (图1A) 。对照组动物体重随时间增加而增加,模型组在前3周随时间缓慢增加,但最后1周出现体重下降趋势。在第3、4周,两组动物体重有显著差异(P<0.01) (图1B) 。
-
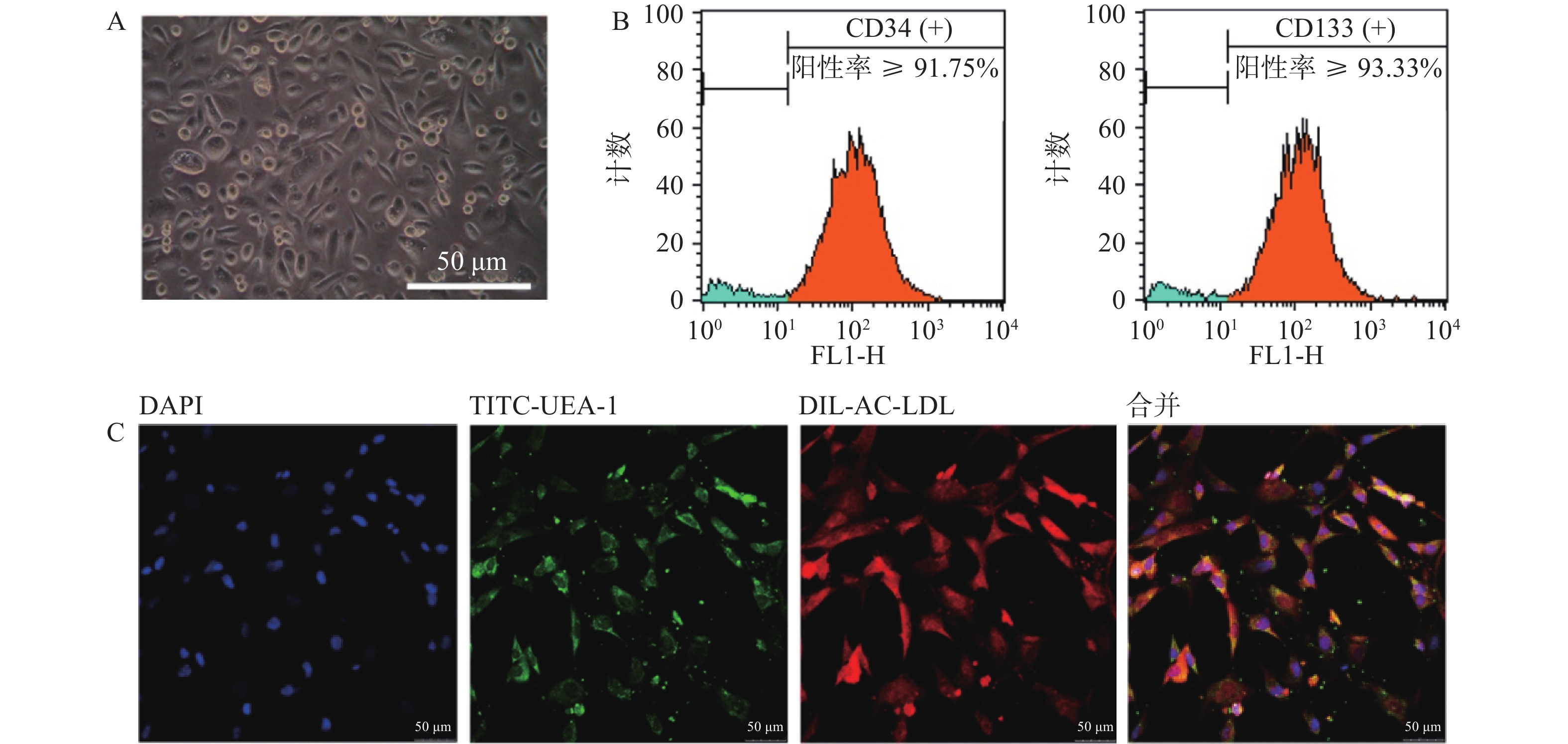
以EGM-2培养基定向诱导培养5 d后,镜下观察可见贴壁细胞形态由圆形逐渐向梭形转化(图2A)。流式细胞仪检测结果显示,CD34与CD133双阳性细胞占比不小于总细胞数的85.63%(图2B)。采用Dil-Ac-LDL和TITC-UEA-1双染细胞,显示红绿荧光双阳性细胞占视野中细胞的绝大多数(图2C)。上述结果表明,我们成功地制备了大鼠骨髓来源EPCs细胞,且细胞具有较高纯度。
-
与对照组比较,糖尿病大鼠EPCs的对数期增殖活性明显降低(P<0.01),而雌激素孵育后其增殖活性明显改善(P<0.01)(图3A)。与对照组比较,糖尿病大鼠EPCs的小管形成功能明显下降(P<0.01),而雌激素能够明显改善其小管形成功能(P<0.01)(图3B)。细胞迁移实验表明,与对照组比较,糖尿病大鼠EPCs细胞迁移能力明显受损(P<0.01),而雌激素孵育能够明显改善受损的细胞迁移能力(P<0.01)(图3C)。
-
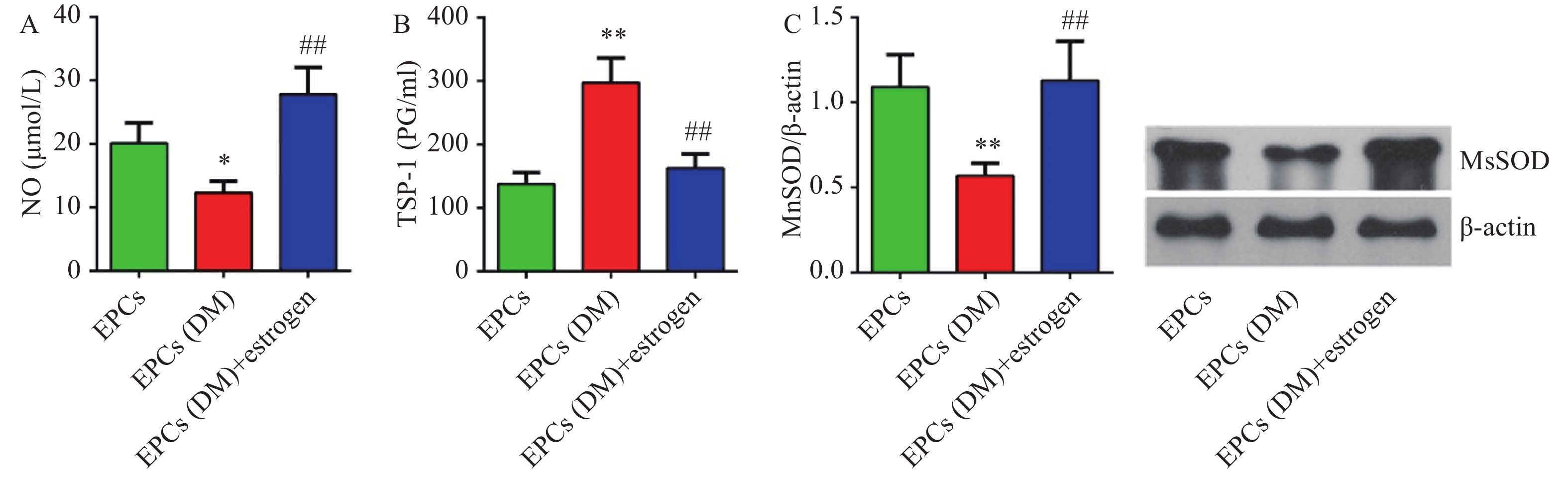
糖尿病大鼠EPCs细胞中NO含量较对照组明显降低(P<0.05),而雌激素孵育能够明显上调NO的含量(P<0.01)(图4A)。
-
糖尿病组大鼠EPCs细胞上清液中TSP-1含量明显高于对照组(P<0.01),而给与雌激素孵育后细胞上清液中TSP-1含量明显降低(P<0.05,图4C)。Western blotting检测显示,糖尿病组大鼠EPCs中MnSOD蛋白表达较对照组明显降低(P<0.01),而雌激素孵育能够明显上调细胞中MnSOD蛋白表达(P<0.01)(图4D)。
-
EPCs主要来源于骨髓,在血管新生中具有重要作用。1997年Asahara等[1]首次将其命名为内皮祖细胞,而后其在心血管方面的研究越来越多。EPCs与心血管疾病存在密不可分的联系。研究表明,EPCs的数量和功能与高血压、糖尿病、血脂等呈负相关[8-11]。此外,EPCs在脑缺血、阿尔茨海默症治疗中表现出较好的疗效[12-13]。故改善EPCs功能及增加其数量可作为改善糖尿病血管并发症的新靶点。并将能表达CD34、CD133 等干细胞特征或血管内皮生长因子受体2 (VEGFR2)等细胞标志物定义为EPCs[14]。用密度梯度离心法获取骨髓单核细胞,经培养并采用流式细胞术和荧光显微镜法对细胞进行鉴定,结果表明本实验成功制备大鼠骨髓来源EPCs细胞,且细胞具有较高纯度。
流行病学提示雌激素为心脑血管疾病的保护因素。绝经后女性卒中发生风险明显高于绝经前女性[15]。美国的调查数据显示,年龄在18~39岁的成年男性发生高血压的风险高于同龄女性,但是在年龄大于60岁成人中男性风险低于女性[16]。不同性别发生高血压及靶器官损伤的差异与雌激素相关[17]。此外,杨莹莹[18]等的研究表明雌激素对急性期高血压脑出血患者的EPCs功能有改善作用且与雌激素浓度呈正相关。但是雌激素对糖尿病EPCs功能改善作用及相关机制的研究较少。
本实验采用腹腔注射链脲佐菌素制作大鼠糖尿病模型,提取其骨髓来源的EPCs进行实验,结果发现糖尿病大鼠骨髓EPC的增殖能力较正常EPCs明显下降,而雌激素体外孵育能明显促进其增殖活力;除增殖活力受到抑制外,糖尿病大鼠EPCs的迁移能力和小管形成功能也明显受损,而在体外给予雌激素10 nmol/L孵育后细胞功能得到明显改善。说明雌激素能促进糖尿病大鼠EPCs的增殖并改善其功能。
研究表明氧化应激可导致血管内皮损伤。MnSOD是一种存在于线粒体并能清除机体新陈代谢中产生的过多的氧自由基(O2·−)等有害物质的一种酶[19,]。MnSOD在一定程度上能抵抗高糖所增加线粒体中的活性氧(ROS)细胞的伤害[20]。本实验采用Western blotting检测EPCs细胞中MnSOD蛋白表达,糖尿病组大鼠EPCs细胞中MnSOD蛋白表达明显低于对照组,而给与雌激素体外孵育后能明显上调细胞中MnSOD蛋白表达,说明雌激素通过上调EPCs中MnSOD水平而改善细胞功能。
一氧化氮(NO)与血管健康密切相关。NO通过促进EPCs向内皮细胞分化进而修复受伤的内皮,而抑制内源性NO的合成对EPCs的迁移能力有不良影响[21]。有文献报道,高血糖通过增加活性氧积聚、降低一氧化氮生物利用度及抑制内皮依赖性血管舒张功能,导致严重的血管内皮功能障碍[22-23]。凝血酶敏感蛋白-1(TSP-1)属细胞外基质糖蛋白,其过表达能抑制EPCs的功能[24],而NO含量降低能诱导TSP-1的表达[25]。本实验发现,糖尿病组大鼠EPCs中NO含量明显降低而细胞上清中TSP-1含量明显升高,雌激素处理能够逆转上述改变,说明雌激素通过增加EPC内NO水平并降低TSP-1含量进而改善糖尿病EPCs功能。
综上所述,本研究证实雌激素能改善受损的糖尿病大鼠EPCs功能并促进其增殖,作用机制可能与其降低细胞内的氧化应激及下调TSP-1的表达相关。本研究为将来进一步进行雌激素在糖尿病血管并发症方面的研究提供了深入的实验与理论依据。
Effect and mechanism of estrogen on EPCs function in diabetic rats
-
摘要:
目的 探索雌激素对糖尿病大鼠内皮祖细胞(EPCs)功能的影响及其可能的作用机制。 方法 取健康Wistar大鼠骨髓提取EPCs并采用流式细胞仪和荧光显微镜鉴定。大鼠给予链脲佐菌素诱导为糖尿病模型,提取正常大鼠和糖尿病大鼠骨髓EPCs并培养,糖尿病大鼠EPCs体外给予雌激素10 nmol/L孵育24 h。检测EPCs增殖和功能;测定EPCs中锰超氧化物歧化酶(MnSOD)水平和NO水平及上清液中凝血酶敏感蛋白-1(TSP-1)蛋白水平。 结果 与对照组比较,糖尿病EPCs的细胞增殖能力、迁移能力和小管形成功能受损(P<0.01),而雌激素体外干预后细胞增殖能力、迁移能力和小管形成功能均得到改善(P<0.01);糖尿病EPCs中MnSOD水平和NO水平明显下调,上清液中TSP-1蛋白水平升高(P<0.01);雌激素孵育能逆转上述改变(P<0.01)。 结论 雌激素能改善糖尿病大鼠EPCs迁移能力和小管形成功能,作用机制可能与其降低糖尿病EPCs内的氧化应激及下调TSP-1的表达相关。 Abstract:Objective To explore the effect and mechanism of estrogen on endothelial progenitor cells(EPCs)function in diabetic rats. Methods EPCs were isolated from bone marrow of rats and characterized by fluorescence microscopy and flow cytometry. Rat diabetic model was established via streptozotocin induction. The bone marrow was taken to culture EPCs. EPCs of diabetes were incubated with Estrogen 10 nmol/L for 24h. The functions and proliferation of EPCs in vitro were detected. The levels of MnSOD and NO in EPCs and TSP-1 in supernatant were assayed. Results Compared with control group, EPCs proliferation, adhesion and angiogenesis functions were impaired in diabetic rats. The level of MnSOD and NO in diabetic EPCs were significantly decreased, while TSP-1 protein level in the supernatant increased. The above changes can be reversed with estrogen incubation. Conclusion Estrogen improved the EPCs migration and tubule formation in diabetic rats. The mechanism may be related to the reduction of oxidative stress and downregulation of TSP-1 expression in diabetic EPCs. -
随着经济的发展和人民生活水平的提高,糖尿病的患病率仍在逐年上升,血管并发症是导致患者残疾和死亡的主要原因,并给社会和经济的发展带来了沉重负担。内皮祖细胞(endothelial progenitor cells, EPCs)在各种因素刺激下,从骨髓动员到外周血,参与损伤内皮的修复,在血管新生中具有重要作用[1-3]。但是高血糖会导致EPCs数量减少及功能受损[4-5]。研究表明,雌激素降低是心血管疾病发病的危险因素。因此,本实验通过研究雌激素对糖尿病大鼠EPCs功能的改善作用并探讨可能的作用机制,为探讨糖尿病血管并发症提供理论依据。
1. 材料
1.1 实验动物
SPF 级 Wistar大鼠,雄性,体重(180±10)g(上海斯莱克实验动物有限公司)。实验期间,保持动物房室温在22 ℃左右,相对湿度70%左右,早8点至晚8点自动照明。动物自由进食,自由饮水,所有实验动物均符合实验动物伦理学要求。
1.2 药物与主要试剂
雌激素(Abcam公司);链脲佐菌素(Sigma aldrich公司);FITC标记的荆豆凝集素I(FITC-UAE-I)(美国Sigma公司);Dil 标记的乙酰化低密度脂蛋白(Dil-ac-LDL)(Molecular Probe公司);甲醛溶液(国药集团化学试剂有限公司);基质胶(matrigel)(Thermo Fisher公司);EGM-2 培养基(LON-ZA 公司);CCK-8试剂盒(日本Dojindo公司);NO检测试剂盒(美国Abcam公司)。
2. 方法
2.1 动物模型的制备
Wistar雄性大鼠,10~12周,适应性地喂养1周后,连续7 d空腹腹腔注射新鲜配制的链脲佐菌素(streptozotocin, STZ)55 mg/(kg·d),对照组大鼠腹腔注射等体积枸橼酸钠缓冲液。7 d后测空腹血糖(禁食12 h),将血糖值为13.5~25 mmol/L的大鼠作为糖尿病大鼠进行实验。注射STZ后,每周称体重,观察体重变化。大鼠给药、饲养过程中勤换垫料,勤补水。
2.2 大鼠骨髓来源EPCs的分离培养
大鼠麻醉后处死,将大鼠整体置于75%乙醇浸泡10 min。取出大鼠,使用吸水纸吸干动物身上水分后转移至超净工作台,剥离胫骨,使用无菌剪刀减去骨头两端,使用1ml PBS通过注射器冲洗骨髓并将冲洗液转移至15 ml无菌离心管,冲洗过程重复操作3次。用密度梯度离心法获取单核细胞,将单核细胞重悬于培养基EGM-2并调整细胞浓度至1×106个/ml,接种于预先包被好纤维连接蛋白的细胞培养皿,置于细胞培养箱37 ℃和5%CO2条件下培养。培养3 d后洗去未贴壁细胞,以后每3 d换培养液培养至7 d。PBS洗去未贴壁细胞,贴壁细胞供实验用。
2.3 大鼠骨髓来源EPCs的鉴定
培养7 d的EPCs用0.25%胰蛋白酶消化后收集于15 ml离心管中,将细胞用PBS 1000r/min离心5 min,清洗3次。用PBS 100 μl重悬细胞,每管加入CD34和CD133抗体各2 μl,4 ℃避光孵育30 min后用PBS清洗离心,将细胞重悬于300 μl PBS中避光保存待上机检测;同时将消化的细胞采用DiI-Ac-LDL 和FITC-UAE-I双染,倒置荧光显微镜观察染色结果,双染色阳性细胞为正在分化的EPCs。
2.4 实验分组
实验分3组:①对照组;②糖尿病组:M199培养基;③雌激素组:含雌激素10 nmol/L的M199培养基。在37 ℃,5% CO2培养箱中孵育24 h 后进行实验。
2.5 大鼠骨髓来源EPCs功能测定[6-7]
2.5.1 细胞迁移实验
3组EPCs用0.25%胰酶消化,将细胞重悬于EBM-2 培养基中,调整细胞密度为5×105个/ml,并取100 μl接种于Transwell 上室,在下室中加入600 μl含50 ng/ml 血管内皮生长因子的EGM-2 培养基。37 ℃、5% CO2孵育24 h 后,取出上室,用棉球轻轻擦拭上室底部膜内上表面的细胞,下室上表面用甲醛室温固定20 min,于37 ℃用0.1%结晶紫染色30 min,用PBS多次冲洗晾干后在160倍光学显微镜下观察并拍照,随机选取5个视野计算迁移细胞数并计算平均值。
2.5.2 EPCs小管形成实验
在冰上将48 孔板预冷,然后加入150 μl的基质胶Matrigel,放置于37 ℃孵育30 min。3组EPCs用0.25%胰酶消化,将5×104个细胞接种于Matrigel上,在37 ℃、5% CO2培养箱中孵育24 h 后,于160倍光学显微镜下观察并拍照,随机选取5个视野计算形成小管的数目并计算平均值。
2.6 骨髓来源EPCs中NO水平的测定
3组EPCs用0.25%胰酶消化,经PBS清洗2次,制成细胞悬液,采用NO检测试剂盒测定EPCs中NO的水平。按照说明书的操作方法,完成后置于酶标仪在540 nm波长处测定吸光度(A)。
2.7 EPCs增殖能力的测定
3组EPCs用0.25%胰酶消化,将100 μl细胞悬液接种于96孔培养板中并置于37 ℃、5% CO2培养箱培养24 h。每孔加入CCK-8溶液10 μl培养4 h,置于酶标仪于450 nm处测A值。
2.8 Western blotting测定EPCs中MnSOD水平
收集3组EPCs并提取蛋白,应用BCA法进行蛋白质定量。蛋白样本经煮沸变性、SDS-PAGE电泳后加入兔抗人锰超氧化物歧化酶(MnSOD)和肌动蛋白(β-actin)多克隆抗体(1∶1500稀释),蛋白质湿法转移至硝基纤维素膜,羊抗兔二抗(1∶2000稀释)室温孵育2 h,用ECL化学发光法显色,Image J凝胶成像分析系统扫描成像,测定目标条带的A值,以β-actin为内参比较同一条带与β-actin的灰度值进行分析。
2.9 ELISA检测EPCs上清液中TSP-1蛋白水平
收集3组EPCs上清液,按照说明书步骤进行操作。取各组细胞上清液各100 μl加入酶标板中37 ℃孵育90 min,然后加入100 μl生物素标记抗体工作液37 ℃孵育60 min。洗涤3次,加入100 μl酶结合物工作液在37 ℃孵育30 min后,洗涤5次。加入90 μl底物溶液37 ℃孵育15 min,最后加入终止液50 μl后,立即在450 nm波长处测量A值。
2.10 统计学处理
实验数据以(
$ \overline{\text{x}}\text{±}\text{s} $ )表示,采用GraghPad Prism软件进行统计处理,采用单因素方差分析(One-way ANOVA)检验差异的显著性。以P<0.05为差异有统计学意义。3. 实验结果
3.1 各组大鼠体重和血糖的变化
正常组大鼠,精神状态良好,动作自如,反应灵敏,毛发平伏有光泽。而糖尿病大鼠体重变轻,精神萎靡,反应迟钝,毛杂乱无光泽,动作迟缓,弓背捲体,尿量显著增加。在建模4周后,糖尿病模型组空腹血糖值(23.33±3.61)mmol/L明显高于对照组(16.91±2.30)mmol/L,组间差异有统计学意义(P<0.01) (图1A) 。对照组动物体重随时间增加而增加,模型组在前3周随时间缓慢增加,但最后1周出现体重下降趋势。在第3、4周,两组动物体重有显著差异(P<0.01) (图1B) 。
3.2 大鼠骨髓来源EPCs的分离培养和鉴定
以EGM-2培养基定向诱导培养5 d后,镜下观察可见贴壁细胞形态由圆形逐渐向梭形转化(图2A)。流式细胞仪检测结果显示,CD34与CD133双阳性细胞占比不小于总细胞数的85.63%(图2B)。采用Dil-Ac-LDL和TITC-UEA-1双染细胞,显示红绿荧光双阳性细胞占视野中细胞的绝大多数(图2C)。上述结果表明,我们成功地制备了大鼠骨髓来源EPCs细胞,且细胞具有较高纯度。
3.3 雌激素孵育改善糖尿病大鼠EPCs的增殖和功能
与对照组比较,糖尿病大鼠EPCs的对数期增殖活性明显降低(P<0.01),而雌激素孵育后其增殖活性明显改善(P<0.01)(图3A)。与对照组比较,糖尿病大鼠EPCs的小管形成功能明显下降(P<0.01),而雌激素能够明显改善其小管形成功能(P<0.01)(图3B)。细胞迁移实验表明,与对照组比较,糖尿病大鼠EPCs细胞迁移能力明显受损(P<0.01),而雌激素孵育能够明显改善受损的细胞迁移能力(P<0.01)(图3C)。
3.4 雌激素孵育上调糖尿病EPCs细胞内NO含量
糖尿病大鼠EPCs细胞中NO含量较对照组明显降低(P<0.05),而雌激素孵育能够明显上调NO的含量(P<0.01)(图4A)。
3.5 雌激素孵育上调EPCs细胞中MnSOD的表达并下调上清液中TSP-1的表达
糖尿病组大鼠EPCs细胞上清液中TSP-1含量明显高于对照组(P<0.01),而给与雌激素孵育后细胞上清液中TSP-1含量明显降低(P<0.05,图4C)。Western blotting检测显示,糖尿病组大鼠EPCs中MnSOD蛋白表达较对照组明显降低(P<0.01),而雌激素孵育能够明显上调细胞中MnSOD蛋白表达(P<0.01)(图4D)。
4. 讨论
EPCs主要来源于骨髓,在血管新生中具有重要作用。1997年Asahara等[1]首次将其命名为内皮祖细胞,而后其在心血管方面的研究越来越多。EPCs与心血管疾病存在密不可分的联系。研究表明,EPCs的数量和功能与高血压、糖尿病、血脂等呈负相关[8-11]。此外,EPCs在脑缺血、阿尔茨海默症治疗中表现出较好的疗效[12-13]。故改善EPCs功能及增加其数量可作为改善糖尿病血管并发症的新靶点。并将能表达CD34、CD133 等干细胞特征或血管内皮生长因子受体2 (VEGFR2)等细胞标志物定义为EPCs[14]。用密度梯度离心法获取骨髓单核细胞,经培养并采用流式细胞术和荧光显微镜法对细胞进行鉴定,结果表明本实验成功制备大鼠骨髓来源EPCs细胞,且细胞具有较高纯度。
流行病学提示雌激素为心脑血管疾病的保护因素。绝经后女性卒中发生风险明显高于绝经前女性[15]。美国的调查数据显示,年龄在18~39岁的成年男性发生高血压的风险高于同龄女性,但是在年龄大于60岁成人中男性风险低于女性[16]。不同性别发生高血压及靶器官损伤的差异与雌激素相关[17]。此外,杨莹莹[18]等的研究表明雌激素对急性期高血压脑出血患者的EPCs功能有改善作用且与雌激素浓度呈正相关。但是雌激素对糖尿病EPCs功能改善作用及相关机制的研究较少。
本实验采用腹腔注射链脲佐菌素制作大鼠糖尿病模型,提取其骨髓来源的EPCs进行实验,结果发现糖尿病大鼠骨髓EPC的增殖能力较正常EPCs明显下降,而雌激素体外孵育能明显促进其增殖活力;除增殖活力受到抑制外,糖尿病大鼠EPCs的迁移能力和小管形成功能也明显受损,而在体外给予雌激素10 nmol/L孵育后细胞功能得到明显改善。说明雌激素能促进糖尿病大鼠EPCs的增殖并改善其功能。
研究表明氧化应激可导致血管内皮损伤。MnSOD是一种存在于线粒体并能清除机体新陈代谢中产生的过多的氧自由基(O2·−)等有害物质的一种酶[19,]。MnSOD在一定程度上能抵抗高糖所增加线粒体中的活性氧(ROS)细胞的伤害[20]。本实验采用Western blotting检测EPCs细胞中MnSOD蛋白表达,糖尿病组大鼠EPCs细胞中MnSOD蛋白表达明显低于对照组,而给与雌激素体外孵育后能明显上调细胞中MnSOD蛋白表达,说明雌激素通过上调EPCs中MnSOD水平而改善细胞功能。
一氧化氮(NO)与血管健康密切相关。NO通过促进EPCs向内皮细胞分化进而修复受伤的内皮,而抑制内源性NO的合成对EPCs的迁移能力有不良影响[21]。有文献报道,高血糖通过增加活性氧积聚、降低一氧化氮生物利用度及抑制内皮依赖性血管舒张功能,导致严重的血管内皮功能障碍[22-23]。凝血酶敏感蛋白-1(TSP-1)属细胞外基质糖蛋白,其过表达能抑制EPCs的功能[24],而NO含量降低能诱导TSP-1的表达[25]。本实验发现,糖尿病组大鼠EPCs中NO含量明显降低而细胞上清中TSP-1含量明显升高,雌激素处理能够逆转上述改变,说明雌激素通过增加EPC内NO水平并降低TSP-1含量进而改善糖尿病EPCs功能。
综上所述,本研究证实雌激素能改善受损的糖尿病大鼠EPCs功能并促进其增殖,作用机制可能与其降低细胞内的氧化应激及下调TSP-1的表达相关。本研究为将来进一步进行雌激素在糖尿病血管并发症方面的研究提供了深入的实验与理论依据。
-
-
[1] ASAHARA T, MUROHARA T, SULLIVAN A, et al. Isolation of putative progenitor endothelial cells for angiogenesis[J]. Science,1997,275(5302):964-967. doi: 10.1126/science.275.5302.964 [2] ACKERMANN M, PABST A M, HOUDEK J P, et al. Priming with proangiogenic growth factors and endothelial progenitor cells improves revascularization in linear diabetic wounds[J]. Int J Mol Med,2014,33(4):833-839. doi: 10.3892/ijmm.2014.1630 [3] 董雅芬, 舒菁菁, 马靖, 等. 天然药物及其活性成分保护内皮祖细胞机制的研究进展[J]. 药学实践杂志, 2013, 31 1): 11-13, 31. [4] TAN Q, LI Y, LI X, et al. Hyperinsulinemia impairs functions of circulating endothelial progenitor cells[J]. Acta Diabetol, 2019, 56(7): 785-795. [5] WEI H J, LIU L, CHEN F L, et al. Decreased numbers of circulating endothelial progenitor cells are associated with hyperglycemia in patients with traumatic brain injury[J]. Neural Regen Res,2019,14(6):984-990. doi: 10.4103/1673-5374.250577 [6] 赵小亚, 江如钞, 肖青. MMP2介导Sonic hedgehog信号通路对糖尿病内皮祖细胞功能的影响[J]. 广州医科大学学报, 2021, 49(1):1-6. doi: 10.3969/j.issn.2095-9664.2021.01.01 [7] 尚刘文心, 孙昕, 彭程, 等. 药物体外干预改善盐敏感性高血压小鼠内皮祖细胞功能研究[J]. 药学实践杂志, 2020, 38(3):221-226. doi: 10.12206/j.issn.1006-0111.201912074 [8] PIRES A, MARTINS P, PAIVA A, et al. Circulating endothelial progenitor cells in obese children and adolescents[J]. J Pediatr (Rio J),2015,91(6):560-566. doi: 10.1016/j.jped.2015.01.011 [9] ARAGONA C O, IMBALZANO E, MAMONE F, et al. Endothelial progenitor cells for diagnosis and prognosis in cardiovascular disease[J]. Stem Cells Int,2016,2016:8043792. [10] YUE D D, WEI Z Y, CHEN X, et al. Predictive value of circulating endothelial progenitor cells in prognosis of acute ischemic stroke[J]. J Shanghai Jiaotong Univ Med Sci,2017,37(7):964-968.[LinkOut [11] KANG H, MA X, LIU J, et al. High glucose-induced endothelial progenitor cell dysfunction[J]. Diab Vasc Dis Res,2017,14(5):381-394. doi: 10.1177/1479164117719058 [12] HECHT N, SCHNEIDER U C, CZABANKA M, et al. Endothelial progenitor cells augment collateralization and hemodynamic rescue in a model of chronic cerebral ischemia[J]. J Cereb Blood Flow Metab,2014,34(8):1297-1305. doi: 10.1038/jcbfm.2014.78 [13] SAFAR M M, ARAB H H, RIZK S M, et al. Bone marrow-derived endothelial progenitor cells protect against scopolamine-induced alzheimer-like pathological aberrations[J]. Mol Neurobiol,2016,53(3):1403-1418. doi: 10.1007/s12035-014-9051-8 [14] LANUTI P, ROTTA G, ALMICI C, et al. Endothelial progenitor cells, defined by the simultaneous surface expression of VEGFR2 and CD133, are not detectable in healthy peripheral and cord blood[J]. Cytometry A,2016,89(3):259-270. doi: 10.1002/cyto.a.22730 [15] 康鑫, 李光勤, 刘明苏, 等. 绝经后女性雌激素水平与缺血性脑卒中相关性的meta分析[J]. 当代医学, 2019, 25(1):14-18. doi: 10.3969/j.issn.1009-4393.2019.01.006 [16] FRYAR C D, OSTCHEGA Y, HALES C M, et al. Hypertension prevalence and control among adults: United States, 2015-2016[J]. NCHS Data Brief,2017(289):1-8. [17] SABBATINI A R, KARARIGAS G. Estrogen-related mechanisms in sex differences of hypertension and target organ damage[J]. Biol Sex Differ,2020,11(1):31. doi: 10.1186/s13293-020-00306-7 [18] 杨莹莹, 陈秋娟, 袁文, 等. 不同浓度雌激素对急性期高血压脑出血患者的内皮祖细胞功能及衰老的影响[J]. 山西医药杂志, 2017, 46(3):250-253. [19] BRESCIANI G, DA CRUZ I B M, GONZÁLEZ-GALLEGO J. Manganese superoxide dismutase and oxidative stress modulation[J]. Adv Clin Chem,2015,68:87-130. doi: 10.1016/bs.acc.2014.11.001 [20] 张鹏, 刘琰, 李萌, 等. 高糖环境下锰超氧化物歧化酶转染间充质干细胞促进抗氧化酶类表达[J]. 中国现代医学杂志, 2014, 24(20):27-30. doi: 10.3969/j.issn.1005-8982.2014.20.006 [21] LEMARIÉ C A, SHBAT L, MARCHESI C, et al. Mthfr deficiency induces endothelial progenitor cell senescence via uncoupling of ENOS and downregulation of SIRT1[J]. Am J Physiol Heart Circ Physiol,2011,300(3):H745-H753. doi: 10.1152/ajpheart.00321.2010 [22] 马春霞, 王利, 王萍, 等. 内毒素、NO及NOS在危重病应激性高血糖中的作用研究[J]. 宁夏医学杂志, 2016, 38(1):21-23. doi: 10.13621/j.1001-5949.2016.01.0021 [23] CERIELLO A, NOVIALS A, ORTEGA E, et al. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes[J]. Diabetes,2012,61(11):2993-2997. doi: 10.2337/db12-0224 [24] HU H, WANG B S, JIANG C Y, et al. Endothelial progenitor cell-derived exosomes facilitate vascular endothelial cell repair through shuttling miR-21-5p to modulate Thrombospondin-1 expression[J]. Clin Sci,2019,133(14):1629-1644. doi: 10.1042/CS20190188 [25] XIE H H, ZHOU S, CHEN D D, et al. GTP cyclohydrolase I/BH4 pathway protects EPCs via suppressing oxidative stress and thrombospondin-1 in salt-sensitive hypertension[J]. Hypertension,2010,56(6):1137-1144. doi: 10.1161/HYPERTENSIONAHA.110.160622 -






 下载:
下载:

 下载:
下载:


